Abstract
Objectives. We estimated the burden of disease in the United States attributable to obesity by gender, with life expectancy, quality-adjusted life expectancy, years of life lost annually, and quality-adjusted life years lost annually as outcome measures.
Methods. We obtained burden of disease estimates for adults falling into the following body-mass index categories: normal weight (23 to <25), overweight (25 to <30), and obese (≥ 30). We analyzed the 2000 Medical Expenditure Panel Survey to obtain health-related quality-of-life scores and the 1990–1992 National Health Interview Survey linked to National Death Index data through the end of 1995 for mortality.
Results. Overweight men and women lost 270 000 and 1.8 million quality-adjusted life years, respectively, relative to their normal-weight counterparts. Obese men and women lost 1.9 million and 3.4 million quality-adjusted life years, respectively, per year. Much of the burden of disease among overweight and obese women arose from lower health-related quality of life and late life mortality.
Conclusions. Relative to men, women suffer a disproportionate burden of disease attributable to overweight and obesity, mostly because of differences in health-related quality of life.
Between 1990 and 2000, the age-adjusted prevalence of obesity increased from 22.9% to 30.5% and the age-adjusted prevalence of overweight increased from 55.9% to 64.5%.1 If similar trends continue, obesity may result in a decline in life expectancy in the United States.2 This mortality risk arises from a higher risk of numerous comorbidities, including type 2 diabetes, hypertension, hypercholesterolemia, osteoarthritis, gallbladder disease, and some cancers.3 However, obesity may also produce psychological morbidity, especially among women.4
A number of studies have examined the burden of disease attributable to obesity and overweight with measures of mortality, such as annual years of life lost.5 Although mortality data provide a common comparable endpoint for all diseases, they provide little information about the suffering caused by diseases while people are alive. Morbidity studies typically capture the association between obesity and a subset of conditions with which it is associated.6–11 For instance, the burden of disease has been measured with the prevalence of high blood pressure, heart disease, and other conditions thought to be associated with obesity.9 However, analyses based on attributable risk typically exclude diseases with a psychological dimension and those that are less prevalent.4
To provide a more comprehensive assessment of morbidity, recent analyses have been conducted using preference-based health-related quality-of-life (HRQL) measures of overweight and obese people.12–14 The advantage of preference-based measures over other quality-of-life measures is that they can be used to calculate quality-adjusted life years (QALYs).15 The recent inclusion of an HRQL measure (the EuroQoL [EQ-5D]) into a large national survey makes it possible to capture the health states of people in the United States and convert them into QALYs.
We examined the burden of disease in the US adult general population by body mass index (BMI). Specifically, we examined: (1) the distribution of sociodemographic variables and selected chronic conditions; (2) the distribution of average HRQL scores by sociodemographic variables and conditions; (3) the annual number of deaths, years of life lost to death, and QALYs in men and women; and (4) life expectancy and quality-adjusted life expectancy for men and women.
METHODS
Overview and Definitions
We calculated BMI from self-reported height and weight. Persons with a BMI of less than 23 kg/m2 were excluded to avoid confounding by underlying medical conditions unrelated to body weight.16,17 Although the ideal comparison group has not been defined, we chose the group with the lowest morbidity and mortality to form a “normal-weight” comparator. The use of the group with the lowest morbidity and mortality maximizes the burden of disease estimated with national datasets, but does not completely eliminate confounding by diseases and conditions unrelated to overweight and obesity. We used the following definitions: BMI 23 to <25 kg/m2= normal weight; BMI 25 to < 30 kg/m2 = overweight; BMI ≥ 30 kg/m2 = obese.
Datasets
We obtained HRQL values from the Household Component of the 2000 Medical Expenditure Panel Survey (MEPS), and mortality ratios from the 1990–1992 National Health Interview Surveys (NHIS) linked to the National Death Index through the end of 1995.18–20 Both the MEPS and the NHIS are nationally representative samples of the civilian noninstitutionalized population.
The MEPS includes approximately 25 000 persons.18 Although 15438 adults provided questionnaire data for the EQ-5D, 12% were proxy responders and were excluded. Also excluded from our sample were adults with missing height or weight information (4.3% of the sample). These persons had slightly lower self-rated health than those with BMI information, but were otherwise similar sociodemographically. The final sample consisted of 13646 subjects. All EQ-5D scores were generated with recently published US preference weights.21 The EQ-5D self-classifier included in the MEPS enables the respondent to categorize his/her health according to 3 levels (no problem, moderate, severe) for 5 dimensions of health.22
The 1990–1992 NHIS included similar sociodemographic, height, and weight variables to those in MEPS.19 The NHIS can be linked to the National Death Index through the end of 1995, allowing for prospective mortality analyses of subjects in the original sample. The sample included 256 900 persons whose vital statuses were obtained during the 6 years of follow-up, over which time 11 214 persons died. We eliminated the subjects missing height or weight information (11.7% of the sample); these persons were older and tended to have higher mortality (6% vs 4% died) than those with complete BMI information. After also excluding persons aged younger than 18 years and those with a BMI < 23, 84 375 subjects remained in the analysis.
Calculations
Analyses were conducted using SAS version 8.2 (SAS Institute Inc, Gary, NC) and SUDAAN version 8.0.1 (Research Triangle Institute, Research Triangle Park, NC). These statistical packages permit adjustment for the complex sampling design used in the MEPS and NHIS. Both MEPS and NHIS data incorporated sampling weights and poststratification weights.
Spline regressions were employed to derive smoothed age-specific EQ-5D scores for persons aged 18 years and older.23 Spline regressions correct for bias, particularly at boundary regions, when independent variables are skewed or have outliers. We generated HRQL values for persons aged younger than 25 years, 25 to 44 years, 45 to 64 years, 65 to 74 years, and 75 years and older.
Cox proportional hazard survival models were used to generate the hazard ratios for overweight and obese relative to normal-weight individuals. Hazard ratios were generated from NHIS for the same intervals used to generate HRQL scores. Each analysis was adjusted for age and age squared.
Abridged life tables were generated for the general US population for the year 2000 with age intervals of 5 years (or fewer) to age 90 and older, and mortality data obtained from the National Center for Health Statistics.24 We calculated quality-adjusted life expectancy for each subgroup by first generating reference abridged life tables for normal-weight persons of each gender and then by multiplying age- and gender-specific mortality probabilities in the table by age-specific HRQL scores. Further details pertaining to the general construction of our life tables have been published elsewhere.25,26
Overweight- and obesity-related deaths were calculated as:
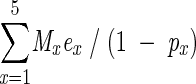 |
(1) |
where M= the total number of deaths in age interval x, e= the proportion of M excess deaths because of overweight and obesity in age interval x, and p= the proportion overweight or obese in age interval x. Total deaths were obtained from death certificate data.24 In 2000, there were 2 403 351 deaths, of which 356 (0.01%) were excluded because no information on subjects’ ages was available. The derivation of this formula is available from the corresponding author.
Total years of life lost were calculated as:
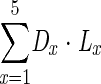 |
(2) |
where x= the age interval (< 25, 25–44, 45–64, 65–74, and ≥ 75 years), DX is the number of weight-related deaths within age interval x, and LX is the life expectancy for persons above the 2 thresholds at the midpoint of age interval x. LX was obtained from life table values for the reference group (e.g., 23.0 kg/m2 ≤ BMI < 25) to reflect the full potential life lost.
The QALYs lost to morbidity were calculated as:
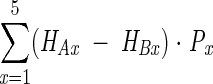 |
(3) |
where HAx is the HRQL score for normal-weight persons in age interval x, HBx is the HRQL score for persons either overweight or obese in age interval x, and Px is the population that is either overweight or obese in age interval x.
Total QALYs (because of both morbidity and mortality) were calculated as:
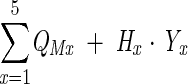 |
(4) |
where QMx is the total number of QALYs because of morbidity in age interval x, Hx is the HRQL score for persons in the BMI category of interest in age interval x, and Yx is the number of years of life lost in age interval x.
RESULTS
Table 1 ▶ highlights the sociodemographic and clinical profile of adults in the MEPS sample according to the 3 categories of BMI. Although there were more overweight men (57%) than women (43%), there were more obese women (54%) than men (46%). Adults who were obese compared with normal-weight persons were more likely to report fair or poor health, having diabetes, and having hypertension. All comparisons across rows are statistically significant at P< .05.
TABLE 1—
Basic Sociodemographic and Clinical Characteristics of the Total MEPS Sample of US Adults, by Body Mass Index (BMI) Category
| No. (%) | ||||
| n | BMI 23.0 to < 25 | BMI 25.0 to < 30 | BMI ≥ 30.0 | |
| Total sample | 10 301 (100) | 2174 (16.8) | 4798 (35.5) | 3329 (23.1) |
| Age | ||||
| 18–44 | 5058 (52.3) | 1125 (51.9) | 2342 (48.7) | 1591 (48.4) |
| 45–64 | 3465 (30.8) | 639 (29.5) | 1184 (32.7) | 1242 (36.7) |
| ≥ 65 | 1778 (16.9) | 410 (18.6) | 872 (18.6) | 492 (14.8) |
| Race/ethnicity | ||||
| White, non-Hispanic | 6266 (74.5) | 1417 (75.9) | 2951 (74.9) | 1898 (70.6) |
| Black, non-Hispanic | 1488 (11.1) | 245 (9.6) | 617 (11.1) | 625 (15.7) |
| Asian, non-Hispanic | 183 (3.0) | 70 (4.1) | 88 (2.3) | 25 (1.2) |
| AIAN, non-Hispanic | 53 (0.6) | 8 (0.5) | 21 (0.5) | 24 (0.9) |
| Hispanic | 2311 (10.5) | 434 (10.0) | 1121 (11.2) | 756 (11.7) |
| Gender | ||||
| Male | 5099 (47.3) | 971 (46.0) | 2682 (57.3) | 1444 (46.2) |
| Female | 5202 (52.7) | 1203 (54.1) | 2114 (42.7) | 1885 (53.8) |
| Marital status | ||||
| Married | 6309 (56.6) | 1258 (55.5) | 3037 (61.1) | 2014 (58.7) |
| Widowed | 757 (7.2) | 157 (7.2) | 331 (6.9) | 269 (7.7) |
| Divorced | 1143 (11.3) | 250 (12.2) | 522 (11.9) | 371 (11.8) |
| Separated | 224 (1.7) | 38 (1.3) | 103 (1.8) | 83 (2.1) |
| Never married | 1868 (23.3) | 471 (23.8) | 805 (18.3) | 592 (19.7) |
| Insurance | ||||
| Any private | 7237 (74.4) | 1556 (75.3) | 3471 (76.5) | 2210 (71.4) |
| Public only | 1561 (13.5) | 327 (14.7) | 664 (12.8) | 570 (15.0) |
| Uninsured | 1503 (12.1) | 291 (10.0) | 663 (10.8) | 549 (13.6) |
| Self-reported health/condition | ||||
| Fair or poor health | 1798 (15.0) | 302 (12.3) | 731 (13.3) | 765 (21.1) |
| Diabetes | 869 (6.3) | 86 (3.2) | 352 (6.7) | 431 (11.6) |
| Asthma | 938 (9.2) | 166 (8.3) | 403 (9.1) | 370 (11.3) |
| Hypertension | 2396 (19.8) | 338 (15.2) | 1010 (21.1) | 1048 (31.2) |
| Heart disease | 1066 (10.2) | 204 (9.6) | 456 (10.2) | 406 (12.5) |
| Number of conditionsa | ||||
| 0 | 6580 (67.2) | 1561 (72.2) | 3192 (66.1) | 1827 (55.3) |
| 1 | 2482 (22.8) | 463 (21.0) | 1095 (23.2) | 924 (28.0) |
| 2 or more | 1238 (10.1) | 149 (6.8) | 571 (10.8) | 578 (16.8) |
Note. MEPS = 2000 Medical Expenditure Panel Survey; AIAN = American Indian/Alaska Native. Column values add to 100% for each sociodemographic category. Subgroup comparisons all differ at P < .05; larger differences are highlighted in the text.
a Total number of self-reported medical conditions.
We found that HRQL scores declined with increasing category of weight with a few notable exceptions (data not shown). In particular, overweight non-Hispanic African Americans and overweight Hispanics had scores that were similar to the scores of normal-weight non-Hispanic African Americans and Hispanics. Overweight men had scores that were similar to the scores of normal-weight men.
Table 2 ▶ presents weight-related deaths, years of life lost, QALYs because of morbidity, and overall QALYs for overweight and obese persons by age. In the United States, relative to the normal-weight persons, there were 15000 additional deaths for overweight men and 37000 additional deaths for overweight women. Similarly, relative to normal-weight persons, there were 42 000 additional deaths for obese men and 70000 additional deaths for obese women. Young, overweight women had fewer deaths than young, overweight men.
TABLE 2—
Annual Overweight- and Obesity-Associated Deaths, Years of Life Lost (YLLs), and Quality-Adjusted Life Years (QALYs) Lost Relative to the Normal-Weight (BMI 23 to < 25) Group, by Age Group and Gender in US Adults
| Men | Women | |||
| Age Group | BMI 25 to < 30 | BMI ≥ 30 | BMI 25 to < 30 | BMI ≥ 30 |
| Deathsa | ||||
| < 25 | –290 | 1463 | –29 | 85 |
| 25–44 | 4673 | 10 430 | –750 | 3276 |
| 45–64 | –12 204 | 12 187 | 18 587 | 27 243 |
| 65–74 | 6904 | 8561 | 10 952 | 23 180 |
| ≥ 75 | 16 028 | 8910 | 7982 | 16 406 |
| Total | 15 111 | 41 550 | 36 742 | 70 190 |
| YLLsa | ||||
| < 25 | –18 773 | 94 850 | –2015 | 5962 |
| 25–44 | 203 101 | 453 258 | –36 145 | 157 897 |
| 45–64 | –422 780 | 422 169 | 718 620 | 1 053 277 |
| 65–74 | 120 237 | 149 093 | 228 580 | 483 801 |
| ≥ 75 | 165 161 | 91 814 | 93 298 | 191 755 |
| Total | 46 946 | 1 211 185 | 1 002 337 | 1 892 692 |
| QALYs (morbidity)b | ||||
| < 25 | 59 435 | 84 543 | 40 573 | 90 175 |
| 25–44 | 184 175 | 387 308 | 332 841 | 627 899 |
| 45–64 | 39 676 | 330 701 | 359 067 | 769 054 |
| 65–74 | –17 394 | 74 246 | 138 102 | 274 356 |
| ≥ 75 | –23 245 | 35 545 | 88 999 | 185 140 |
| Total | 242 647 | 912 343 | 959 583 | 1 946 624 |
| QALYs (total)a,b | ||||
| < 25 | 41 774 | 171 673 | 38 744 | 95 440 |
| 25–44 | 370 763 | 790 373 | 300 960 | 761 284 |
| 45–64 | –329 656 | 683 947 | 959 618 | 1 597 952 |
| 65–74 | 81 452 | 190 207 | 319 260 | 627 206 |
| ≥ 75 | 105 726 | 102 488 | 161 401 | 317 708 |
| Total | 270 059 | 1 938 689 | 1 779 983 | 3 399 590 |
Overweight men in the United States had 47000 additional years of life lost annually whereas overweight women had 1 million additional years of life lost annually relative to normal-weight persons. Obese men had 1.21 million years of life lost to disease annually whereas obese women had an additional 1.89 million years of life lost to disease annually relative to normal-weight persons.
In addition to measuring QALYs as a summary measure capturing both HRQL and mortality, we examined QALYs attributable to HRQL decrements alone. Health-related quality-of-life decrements because of being overweight were nearly 4 times higher among women than among men (960000 QALYs and 243 000 QALYs, respectively). Differences in HRQL among obese women were slightly greater than 2 times higher than among obese men (1.95 million QALYs and 912000 QALYs, respectively).
Overweight women had a 6.6-times higher burden of disease in total QALYs relative to overweight men (1.78 million QALYs relative to 270000 QALYs). The burden of disease among obese women was 1.8 times higher than among obese men (3.4 million QALYs relative to 1.94 million QALYs).
Figure 1 ▶ shows the total years of life and QALYs lost to overweight and obesity by gender per 100 000 persons. Here, we see that the rate (per 100 000 persons) of QALYs lost to morbidity for obese and overweight men remained relatively steady by age, whereas years of life lost by men generally increased with age. For both obese and overweight women, the rate of years of life lost was lower than the rate of QALYs lost to morbidity at young ages and then crossed around age 35 to 45 years before converging again later in life.
FIGURE 1—
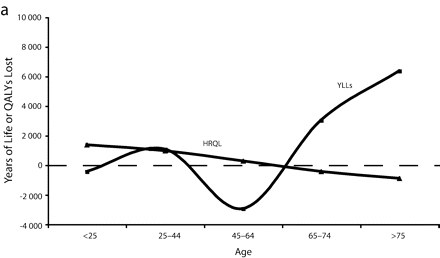
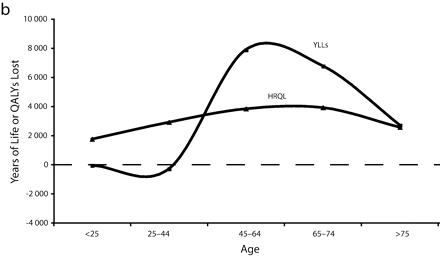
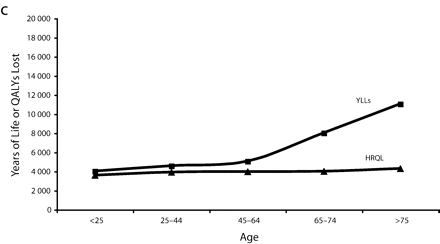
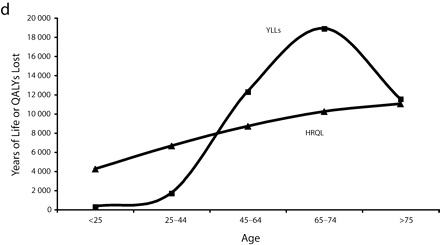
Total years of life lost and quality-adjusted life years (QALYs) lost to overweight and obesity by gender per 100 000 persons for overweight males (a), overweight females (b), obese males (c), and obese females (d).
Note. The measure of morbidity is the health-related quality of life (HRQL) score translated in QALYs lost to morbidity alone.
Table 3 ▶ shows the lifetime burden of disease for the 3 categories of BMI examined. Men aged 18 years in the normal-weight category had a life expectancy roughly equal to men of the same age in the overweight category—both 57 years. Obese men had a life expectancy of 54.3 years. By contrast, overweight women had a shorter lifespan than normal-weight women (62.4 vs 63.5 years). Obese women had a life expectancy of 60.7 years.
TABLE 3—
Years of Perfect Health, Life Expectancy, and Quality-Adjusted Life Expectancy Among US Adults, by Body Mass Index
| Men | Women | |||||
| Body mass index | 23 to < 25 | 25 to < 30 | ≥ 30 | 23 to < 25 | 25 to < 30 | ≥ 30 |
| Life expectancy at birth | 74.1 | 73.8 | 71.5 | 80.8 | 79.6 | 78 |
| Life expectancy at age 18 y | 57 | 56.7 | 54.3 | 63.5 | 62.4 | 60.7 |
| Quality-adjusted life expectancy at age 18 y | 50.5 | 50 | 46.1 | 55.6 | 52.7 | 48.4 |
Differences in quality-adjusted life expectancy demonstrated larger differences by gender. Normal-weight men lived 0.5 QALY more than overweight men, and 4.4 QALYs more than obese men. Women in the normal-weight range lived 2.9 QALYs more than overweight women and 7.2 QALYs more than obese women. (Differences between these values and those in Table 3 ▶ are because of rounding.)
DISCUSSION
When we looked at data representative of the US adult household population, we found that being overweight or obese had a profound impact on the length and quality of life, and that the interplay of morbidity and mortality produced disparate results for men and women. Being overweight had a small effect on both HRQL and mortality among men (270 000 QALYs lost), but a large impact on both outcomes for women (1.78 million QALYs lost). Likewise, obesity had a much greater impact on HRQL and mortality for obese women relative to obese men, producing a total of 1.94 million QALYs lost for men and 3.40 million QALYs lost for women.
However, this is not true across all age groups. Obese women aged younger than 45 years appeared to have lower excess mortality than younger obese men. After age 45, mortality for obese women far surpassed that of men (Figure 1 ▶). This pattern—a flip in male versus female mortality at age 45—has been observed before.17 This previous study used merged data from National Health and Nutrition Epidemiological Follow-Up Study and National Health and Nutrition II Mortality Study, but did not provide HRQL data to contextualize it.
Across all ages, HRQL was significantly lower among obese women than obese men; women aged younger than 45 years lost 1.5 times as many QALYs to morbidity than did men in the same age group. There are several possible overlapping explanations for these disparate gender findings. First, mortality by BMI may be confounded by muscle mass in very fit men. This hypothesis explains why overweight men have lower mortality than overweight women, but does not explain the distribution of years of life lost by age seen in Figure 1 ▶. Second, obesity in women may be more strongly associated with morbidity that translates into later mortality than in men. Third, psychological morbidity associated with obesity-related stigma might contribute to both the greater HRQL burden in women than men and the later increase in mortality in women.
This third hypothesis is consistent with other authors’ findings that there was a much stronger association between depression and BMI in women than in men.4,27 It is also consistent with our finding that obese and overweight women of all ages suffered disproportionately from lower HRQL relative to men. Stress and depression have been linked to increases in the release of cortisol, vasopressors, and oxidative chemicals, which in turn may lead to an increased risk of the metabolic syndrome and heart disease—a process that necessarily takes years to lead to increases in mortality.27–30 Conversely, it is plausible that stress, anxiety, and depression could be the cause of obesity and higher mortality in the first place, or that a 2-way causal relationship exists.
When we compared the overweight and obese categories within genders, we found that men experienced slightly less than a 3-fold increase in the number of excess deaths when moving from the overweight to the obese category and a 7-fold increase in QALYs lost. Women, on the other hand, experienced less than a 2-fold increase in deaths and QALYs when moving between these categories. It is possible that men catch up to women in total QALYs lost in the obese category because of the aforementioned physiological differences in body fat versus lean muscle mass distribution by gender. Alternatively, this might happen once BMI is high enough to affect labor market participation among men (thus affecting access to health insurance or other goods and services that might affect survival). Clearly, more research is needed to iron out the pieces of these differences in quality versus quantity of life by age and gender.
Whereas these annual losses are indicative of the burden of disease to society as a whole, changes in life expectancy and quality-adjusted life expectancy provide information on the burden of disease among individuals (Table 3 ▶). We found that being overweight had a modest impact on male quality-adjusted life expectancy: +0.5 QALY relative to normal-weight men. However, overweight had a relatively large impact on female quality-adjusted life expectancy: −2.9 fewer QALYs over a lifespan. Being obese had a large impact on quality-adjusted life expectancy for both sexes: −4 QALYs for men and −7 QALYs for women. Although no other authors have examined quality-adjusted life expectancy by BMI, other authors have examined differences in unadjusted life expectancy by BMI.2,31 These authors predict that, if trends continue, life expectancy in the United States will ultimately fall because of high rates of obesity among youngsters.
Likewise, although we did not conduct a trend analysis, it is conceivable that such trends will erase the gender gap in life expectancy. Were all women within the normal-weight category (BMI 23 to < 25), life expectancy for women would be 1.8 years longer and 3.9 QALYs longer than it is now.24 For men, there would be little change in life expectancy, and just 0.7 QALYs would be added to male quality-adjusted life expectancy.
There are, as of yet, few burden-of-disease analyses that also include quality-adjusted life expectancy or QALYs lost annually as outcome measures from which comparisons can be made to overweight and obesity. One exception is a recent study of poverty.32 Because the prevalence of obesity is higher than that of poverty, more QALYs are lost to obesity (5.3 million) every year than to poverty (3.1 million). However, the impact of obesity on quality-adjusted life expectancy is considerably lower than that for poverty. Whereas obese 18-year-olds in the general US population have approximately 47 QALYs ahead of them, those 18-year-olds living under the poverty threshold can expect to live just 43 QALYs. That obesity has a large impact on QALYs lost annually in the population, but a relatively smaller impact on QALYs lost among the average obese person underscores the fact that the burden of disease because of obesity is driven more by its high prevalence than its severity.
Flegal et al. recently released revised estimates of annual deaths because of overweight and obesity.5 They used subjects in the BMI from 18.5 to less-than-25 range as a comparison group rather than the BMI from 23 to less-than-25 group we employed. Inclusion of persons with a BMI less than 23 produces a comparison group that is less healthy than the BMI from 23 to less-than-25 group.17 This less-healthy reference group may therefore result in an underestimate of the burden of disease attributable to overweight and obesity. If so, the use of this reference group partly accounts for their finding that overweight persons are at lower risk of mortality than normal-weight persons.5,33 Subjects within any BMI group will have morbidity unrelated to their body weight. Nonetheless, we felt that the group with the lowest morbidity and mortality serves as the optimal “normal” comparison group and therefore provides a more accurate picture of the relationship between being overweight or obese and health outcomes.
Our study had a number of limitations. First, subanalyses by gender and age resulted in a good deal of random error in our parameter estimates. At the outset of this analysis, we decided to produce estimates that minimized non-random error. We thus used age-specific hazard ratios (rather than age-adjusted hazard ratios) and derived EQ-5D scores using spline regression. Though the use of age-specific hazard ratios and HRQL scores increased random error in the analysis, the use of age-adjusted summary values would have introduced non–random error.34 The use of age-specific estimates for both HRQL and mortality made the derivation of 95% confidence intervals around our summary outcome indicators computationally prohibitive. Our findings, however, were largely consistent with other estimates of HRQL and mortality.13,17
Second, we calculated age-specific risk ratios for mortality using mortality data from 1990 through 1995; newer data were not readily available via public access. There is evidence that BMI is increasing among persons already categorized as obese.33 Therefore, it is possible that our hazards ratios underestimate the burden of disease today.
Third, we assumed that all excess mortality by BMI is attributable to obesity. However, differences may have existed among the groups because of other sociodemographic factors that may have affected the results.
Fourth, height and weight were self-reported. Obese persons have been found to be more likely to underestimate their weights and heights than are nonobese persons, women may be more likely to underestimate their weight, and men may be more likely to overestimate their heights.35,36 Thus, the actual number of overweight and obese persons may be higher.
Fifth, because of the limited number of response categories in the EQ-5D for each question, a ceiling effect may occur when measuring the health status of the US general population, which may limit its sensitivity to mild morbidity effects. Sixth, we eliminated proxy responders to the EQ-5D. Proxy responders tended to be poorer, less educated, and more likely to be African American or Hispanic. Finally, we omitted those with missing height and weight information. While 4.3% of persons were omitted from the HRQL analysis, 11.7% of subjects were omitted from the mortality analysis. These subjects tended to be older and have higher mortality than those for whom height and weight were available.
In conclusion, with MEPS and NHIS data, we examined the burden of disease in the United States because of overweight and obesity separately for men and women. We found that the inclusion of morbidity greatly changed what we know about the distribution of the burden of disease attributable to obesity by gender. Further research is needed to elucidate the factors that drive the gender differences in morbidity and mortality. It is possible that the obesity epidemic will not only shape the overall mortality experience in the future, but also will affect differences in the total burden of disease by gender.
Acknowledgments
This study was supported by a grant from the Agency for Healthcare Research and Quality (R03 HS013770).
Human Participant Protection No human participants were used in this study and no approval was required.
Peer Reviewed
Contributors E. Lubetkin originated the study and contributed to the development of the article. P. Muennig calculated the burden-of-disease estimates, led the writing, and framed the analysis. P. Franks conducted mortality and health-related quality-of-life analyses and contributed to the development of the article. H. Jia conducted the initial statistical analyses of health-related quality-of-life estimates.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. New Engl J Med. 2005;352:1138–1145. [DOI] [PubMed] [Google Scholar]
- 3.Burton BT, Foster WR. Health implications of obesity: an NIH Consensus Development Conference. J Am Diet Assoc. 1985;85:1117–1121. [PubMed] [Google Scholar]
- 4.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54:330–337. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. [DOI] [PubMed] [Google Scholar]
- 6.Goya Wannamethee S, Gerald Shaper A, Whincup PH, Walker M. Overweight and obesity and the burden of disease and disability in elderly men. Int J Obes Relat Metab Disord. 2004;28:1374–1382. [DOI] [PubMed] [Google Scholar]
- 7.Aekplakorn W, Chaiyapong Y, Neal B, et al. Prevalence and determinants of overweight and obesity in Thai adults: results of the Second National Health Examination Survey. J Med Assoc Thai. 2004;87:685–693. [PubMed] [Google Scholar]
- 8.Nowicki EM, Billington CJ, Levine AS, Hoover H, Must A, Naumova E. Overweight, obesity, and associated disease burden in the Veterans Affairs ambulatory care population. Mil Med. 2003;168:252–256. [PubMed] [Google Scholar]
- 9.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. [DOI] [PubMed] [Google Scholar]
- 10.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. [DOI] [PubMed] [Google Scholar]
- 11.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. [DOI] [PubMed] [Google Scholar]
- 13.Groessl EJ, Kaplan RM, Barrett-Connor E, Ganiats TG. Body mass index and quality of well-being in a community of older adults. Am J Prev Med. 2004;26:126–129. [DOI] [PubMed] [Google Scholar]
- 14.Le Pen C, Levy E, Loos F, Banzet MN, Basdevant A. “Specific” scale compared with “generic” scale: a double measurement of the quality of life in a French community sample of obese subjects. J Epidemiol Community Health. 1998;52:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996.
- 16.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. WMJ. 1998;97:20–21, 24–25, 27–37. [PubMed] [Google Scholar]
- 17.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SB. Design strategies and innovations in the medical expenditure panel survey. Med Care. 2003; 41(7 suppl):III5–III12. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. National Health Interview Survey. Available at: http://www.cdc.gov/nchs. Accessed October 1, 2005.
- 20.National Center for Health Statistics. National Death Index. Available at: http://www.cdc.gov/nchs/r&d/ndi/ndi.htm. Accessed October 1, 2005.
- 21.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 22.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33: 337–343. [DOI] [PubMed] [Google Scholar]
- 23.Siminoff J. Smoothing Methods in Statistics. New York, NY: Springer-Verlag; 1996.
- 24.Minino A, Arias E, Kochanek KD, Murphy SL, Smith BL. Deaths: final data for 2000. Natl Vital Stat Rep. 2002;50:1–119. [PubMed] [Google Scholar]
- 25.Muennig P. Designing and Conducting Cost-Effectiveness Analyses in Medicine and Health Care. San Francisco, Calif: Jossey-Bass; 2002.
- 26.Anderson R. Method for constructing complete annual life tables. National Center for Health Statistics. Vital Health Stat. 1999;2:1–35. [PubMed] [Google Scholar]
- 27.Palinkas LA, Wingard DL, Barrett-Connor E. Depressive symptoms in overweight and obese older adults: a test of the “jolly fat” hypothesis. J Psychosom Res. 1996;40:59–66. [DOI] [PubMed] [Google Scholar]
- 28.Smith DE, Thompson JK, Raczynski JM, Hilner JE. Body image among men and women in a biracial cohort: the CARDIA Study. Int J Eat Disord. 1999;25:71–82. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. [DOI] [PubMed] [Google Scholar]
- 31.Koplan JP, Liverman CT, Kraak VA, eds. Preventing Childhood Obesity: Health in the Balance. Washington, DC: National Academy Press; 2005. [PubMed]
- 32.Muennig P, Franks P, Jia H, Lubetkin E, Gold MR. The income-associated burden of disease in the United States. Soc Sci Med. 2005;61:2018–2026. [DOI] [PubMed] [Google Scholar]
- 33.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005; 293:1868–1874. [DOI] [PubMed] [Google Scholar]
- 34.Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol. 2004;160:331–338. [DOI] [PubMed] [Google Scholar]
- 35.Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115:223–230. [DOI] [PubMed] [Google Scholar]
- 36.Strauss RS. Comparison of measured and self-reported weight and height in a cross-sectional sample of young adolescents. Int J Obes Relat Metab Disord. 1999;23:904–908. [DOI] [PubMed] [Google Scholar]


