Abstract
The diabetes and obesity epidemics are closely intertwined. International randomized controlled trials demonstrate that, in high-risk individuals, type 2 diabetes can be prevented or at least delayed through lifestyle modification and, to a lesser degree, medication. We explored the relative roles of science, surgery, service delivery, and social policy in preventing diabetes.
Although it is clear that there is a role for all, diabetes is a complex problem that demands commitment across a range of government and nongovernment agencies to be effectively controlled. Accordingly, we argue that social policy is the key to achieving and sustaining social and physical environments required to achieve widespread reductions in both the incidence and prevalence of diabetes.
In developed countries, chronic diseases are the leading cause of premature mortality and reduced quality of life. In developing countries, chronic diseases are increasingly matching, and in some cases overtaking, communicable diseases in contributing to a dual burden of ill health. Prevention is increasingly seen as a major strategy for combating chronic diseases. Type 2 diabetes, with its common risks and repercussions and complex relationship with sedentariness, obesity, cardiovascular disease, hypertension, dyslipidemia, stress, depression, and socioeconomic status, makes an ideal model for chronic disease prevention (Figure 1 ▶).1 We discuss the broad categories of prevention that are applicable to type 2 diabetes and related chronic diseases through common risk factors and comorbidities and acknowledge the need for comprehensive solutions across these areas.
FIGURE 1—
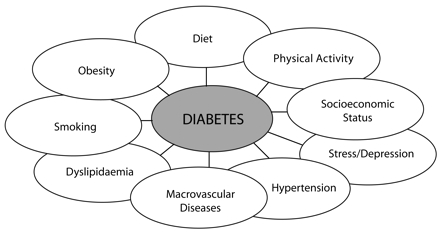
Factors associated with type 2 diabetes as a “composite” chronic disease.
Diabetes is now cited by many as a global epidemic alongside and intertwined with the obesity epidemic. The International Diabetes Federation estimates that there were 189 million people with diabetes in 2003 and predicts an increase to 324 million in 2025.2 Estimates from the World Health Organization (WHO) are similar, projecting an increase from 171 million in 2000 to 366 million in 2030.3 Approximately 70% of this growth is predicted to occur in the developing world and will increasingly affect people aged younger than 65 years who are still in the productive stages of their life cycle,4 thus posing an economic threat over and above the more direct disease cost to the public purse.
Type 2 diabetes is a complex metabolic disorder triggered by lifestyle factors superimposed on a genetic predisposition, is responsible for approximately 90% of all diabetes, and accounts for most of the public health and cost burden attributable to diabetes. Further, although type 2 diabetes is mainly a condition of adults, recent studies highlight its increasing prevalence in adolescents and children.5 The rapid rise of childhood obesity and its causal link to diabetes has led Olshansky et al. to forecast that type 2 diabetes has the potential to result in a decline in the overall life expectancy of the population within the first half of this century.6
The principal risk factors for type 2 diabetes include aging, obesity, and low levels of physical activity. There is also accumulating evidence that shows that sedentary behavior, particularly watching television, is an independent risk factor for obesity and type 2 diabetes.7,8 Some ethnic and cultural groups have an increased susceptibility to developing diabetes. In some Pacific Islander and Native American populations, type 2 diabetes affects up to 40% of adults.9,10 Increasing worldwide urbanization, with its attendant sedentary lifestyle and readily available energy-dense foods, elevates the prevalence of risk factors for type 2 diabetes. Beaglehole and Yach11 note positive effects of globalization on health but point out that these beneficial effects are offset by other, detrimental effects. Other recent reports that examine the interplay between macroeconomic forces and health also note the rising chronic disease risk attributable to urbanization.12
Prevalence of type 2 diabetes is also linked to socioeconomic status. In developed countries, diabetes prevalence is significantly higher in the lowest socioeconomic groups.13 However, in developing countries, this situation may be reversed because the lower socioeconomic sectors of society continue traditional lifestyles long after their more affluent counterparts have shifted to sedentary lifestyles and energy-dense diets. Given the increasing prevalence of depression worldwide, prospective data suggesting that depression is associated with twice the risk of future development of diabetes14 is also noteworthy.
The complications of diabetes include coronary heart disease, stroke, lower limb amputation, impotence, renal failure, and visual impairment up to and including blindness. People with diabetes also suffer a considerable psychological burden,15 including depression rates that are 2–3 times higher than their nondiabetic counterparts16; an increased likelihood of anxiety states17; high self-reported rates of poor quality of life, especially in the presence of diabetic complications18–20; and high rates of poor well-being.21 The World Health Report 22 cites diabetes as accounting for almost 1 million deaths annually, but these figures underestimate the true burden, because diabetes is known to be underreported on death certificates. Further, diabetes undoubtedly contributes to the burden of mortality from cardiovascular diseases, which accounted for some 17 million deaths globally in 2002.22
THE COST OF DIABETES IS ALREADY DISPROPORTIONATELY HIGH
The American Diabetes Association estimated that, in the United States in 2002, the national cost of diabetes was $132 billion—$92 billion in direct medical expenditures and $40 billion in indirect costs related to lost days of work, restricted activity, premature mortality, and permanent disability.23 This same organization estimated that these costs will increase to $192 billion in 2020.23 In addition, at present, total national costs in the US attributable to obesity approach $100 billion annually.24
The annual average total cost of health care for a person with type 2 diabetes in Australia in 2001 was $4290.19 In addition, each person with the disease incurs an average annual cost for a nonprofessional care-giver of $1505 and receives an average of $4430 in government benefits.19 Complications are the main source of all types of diabetes costs,25 increasing the annual cost from $3220 among people without complications to $7715 for people with both microvascular and macrovascular complications.19 These figures are similar to those outlined in the European CODE-2 (Cost of Diabetes in Europe—Type 2) study26 and the United Kingdom T2ARDIS (Type 2 Diabetes Accounting for a Major Resource Demand in Society) study.27
The Economic Argument for Preventing Diabetes
There is accumulating evidence that diabetes prevention is cost-effective. Herman et al. simulated the lifetime cost-effectiveness of interventions designed to prevent diabetes from a health system and a societal perspective.28 In comparison with placebo, costs per quality-adjusted life-year were approximately $1100 for lifestyle interventions and $31300 for treatment with metformin. From a societal perspective, the interventions cost approximately $8800 and $29900 per quality-adjusted life-year, respectively.
Lifestyle interventions that targeted extremely obese individuals and women with previous gestational diabetes were cost-effective.29 Using a population-based cost–benefit model to assess a diabetes early detection and prevention program, Walker et al.30 estimated that the program would produce cost savings after 8 years and would cost $18600 for each case of diabetes prevented. However, the program would require a budget of $1.4 billion over the 8 years to achieve the longer-term savings.
IS PREDIABETES THE WINDOW OF OPPORTUNITY?
In the United States, estimated lifetime risks of acquiring diabetes (for someone born in 2000) are 32.8% for males and 38.5% for females.31 The long preclinical phase of type 2 diabetes, increasingly referred to as prediabetes,32 provides an opportunity to intervene to prevent progression to overt diabetes and reduce the associated health and economic burdens. People with prediabetes can have impaired fasting glucose (IFG; detected via a fasting plasma glucose test), impaired glucose tolerance (IGT; detected via an oral glucose tolerance test), or both.33 Approximately 10% to 20% of the general population has prediabetes.34 Without intervention, approximately one third of individuals with IFG or IGT and two thirds of individuals with both will develop diabetes within 6 years.35
Type 2 diabetes has a long, asymptomatic preclinical phase, estimated to be up to 12 years,36 which frequently goes undetected. Consequently, at the time of diagnosis, many of those diagnosed have 1 or more diabetes complications.37 Prevalence data from countries as diverse as Mongolia38 and Australia39 demonstrate that for every other person with diagnosed diabetes, there is another who has undiagnosed diabetes. Other countries have even higher rates of undiagnosed diabetes—80% in Tonga40 and 60–80% in various African countries.41–43 In China, India, and many countries in sub-Saharan Africa, large-scale poverty and lack of access to even rudimentary health services cause many people to die as a result of having diabetes without ever having been diagnosed.
In type 2 diabetes, all interventions from the time of initial departure from good health are aimed at preventing, delaying, or reversing diabetic complications. Consequently, both prediabetes and undiagnosed diabetes, although representing different degrees of impaired glucose metabolism, are equally important prevention targets. Further, every population includes people across the spectrum of risk, from low to high risk, and people who already have evidence of disordered metabolism (e.g., prediabetes) or undiagnosed diabetes.
We reviewed the evidence that type 2 diabetes can be prevented or significantly delayed and explored opportunities for successful intervention, as well as the relative merits, potential contribution, and feasibility of science, surgery, service delivery, and social policy in the future prevention of diabetes. We believe that combining a high-risk approach with a population approach is likely to bring health gain across the continuum from preventing the development of risk factors in the general population to reducing or reversing established modifiable risks and preventing the development of diabetes (Figure 2 ▶). The complex nature of diabetes means that many organizations and agencies need to be engaged to effectively control the disease. In addition, policies are required to achieve an appropriate supporting and sustainable social and physical environment.
FIGURE 2—
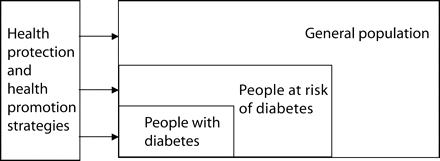
Population health protection and health promotion strategies bring benefit across the diabetes disease continuum.
SCIENCE
Well-designed, rigorous randomized controlled trials conducted at an international level have clearly demonstrated that type 2 diabetes is preventable or at least can be significantly delayed through lifestyle modifications and, to a lesser degree, medication.
Prevention or Delay of Type 2 Diabetes in People With IGT
The Chinese Da Qing study44 demonstrated the beneficial effects of lifestyle interventions in people with IGT; diet, exercise, and diet combined with exercise interventions reduced diabetes risks by 31%, 46%, and 42%, respectively. The Malmo Preventative Trial45 showed average annual conversion rates from IGT to diabetes of 0.3% among men with normal glucose tolerance, 1.7% among people with IGT receiving dietary and physical activity counseling, and 4.6% among people with IGT receiving routine treatment. Two recent studies have provided definitive confirmation of these findings. The Finnish Diabetes Prevention Study46 was the first well-designed randomized controlled trial to demonstrate that lifestyle changes could prevent or delay the onset of type 2 diabetes; such changes resulted in a 58% reduction in prevalence compared with a control group. The US Diabetes Prevention Program confirmed these findings with lifestyle interventions, also showing a 58% reduction in type 2 diabetes.47
Other studies have used pharmacotherapy to prevent or delay the development of diabetes.48 The landmark US Diabetes Prevention Program study included an arm in which participants were treated with metformin, which resulted in a 31% reduction in the incidence of diabetes.47 In the Study to Prevent Noninsulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial,49 acarbose reduced the development of diabetes in people with IGT by 25%. The TRIPOD (Troglitazone in the Prevention of Diabetes) study50 reported a 50% reduction in diabetes incidence when women who had gestational diabetes used troglitazone (an insulin sensitizer). The XENDOS (Xenical in the Prevention of Diabetes in Obese Subjects) Study51 focused on use of the weight loss medication Orlistat in combination with lifestyle interventions among obese (body mass index ≥ 30 kg/m2) people aged 30 to 60 years. Lifestyle intervention combined with Orlistat decreased diabetes by 37% compared with lifestyle intervention combined with placebo. This improvement was seen in people with IGT, not those with normal glucose tolerance. However, to date, evidence from randomized controlled trials of the effect of lifestyle interventions on those with normal glucose tolerance is lacking, although there is some equivocal evidence that diabetes may be preventable in this population.52
Other Emerging Scientific Contributions
Emerging contributions that may have an impact on diabetes and obesity in the longer term include research on “polypills,” cannabinoids, and human genomes. However, their potential benefits and harms in terms of treating established risk factors as compared with the wider potential benefits of changing the social and built environment to reduce the likelihood of risk factor development are untested.
There is continuing debate among public health officials over Wald and Law’s53 suggestion, on the basis of a review of the literature on pharmacotherapy for cardiovascular risk reduction, that a “polypill” containing small doses of 3 different antihypertensive medications, a statin, a small dose of aspirin, and folate, may reduce the incidence of death from heart attacks and strokes by up to 85% in people from high-risk nations and in those aged 55 years and older. Other researchers have extended this concept to diabetes prevention by including metformin as one of the potential ingredients. While its simplicity is seductive, the notion of a polypill is, in effect, yet another band-aid solution that does nothing to address determinants of chronic disease risks. Acting to prevent risk factors from developing is particularly relevant to diabetes prevention, where lifestyle intervention has been shown to be twice as effective as treatment with metformin.46,47
Recent attention also has focused on the role of the cannabinoid system in regulating food intake.54 Central cannabinoid receptors located in the brain respond to endocannabinoids and the psychoactive ingredient of marijuana in a variety of ways, including appetite stimulation and food reward, which influence the intake of palatable food and drink. This system appears to not “switch off” when eating excess amounts of food. Pharmacological agents, such as rimonabant, which is in the advanced stages of clinical trials and could possibly reach the market by 2006,55 have been developed to inhibit central cannabinoid receptors. Whether rimonabant proves to be more efficacious or cost-effective than currently available pharmacotherapy for weight control remains to be seen.
Now that the human genome has been mapped, attention is focusing on exploring the genetic and environmental influences on common diseases.56 Large-scale trials are being developed in an attempt to systematically examine the gene–environment–disease relationship. This research has the potential to improve the ability to target the individuals or groups most likely to benefit from prevention strategies. However, the possibility of preventative health gain as a result of human genome research is very much in the future.
SURGERY
Although a variety of options exist to help reduce overweight and obesity, including dietary therapy, changes in physical activity, behavior therapy techniques, and pharmacotherapy, in general their effectiveness is limited for achieving substantial and sustained weight control.57 By contrast, weight loss surgery can achieve substantial weight reductions.58 The Swedish Obese Subjects Study included 1879 patient pairs in which one member was surgically treated and the other received nonsurgical obesity treatment. The 2-year mean weight loss was 28 kg among obese patients who had undergone surgery compared with 0.5 kg among obese participants who had not. After 8 years, the mean weight loss was 20 kg in the surgical group, whereas the controls had gained 0.7 kg.59,60 In the surgical group, 8-year incidence rates of diabetes reduced 5-fold compared with the control group.61 Another retrospective study also indicated that diabetes could be prevented over the long term (14-year follow-up) with gastric bypass surgery.61 However, although some evaluations suggest that obesity surgery can be cost-effective,62,63 Segal et al.29 found that gastric surgery was less cost-effective per life year saved compared with lifestyle modification to prevent diabetes.
In assessing whether bariatric surgery should be offered more widely for the prevention of type 2 diabetes, it should be noted that just a modest weight loss of 5% to 10% has been shown to have health benefits.64 The feasibility and safety of offering invasive surgery as a prevention strategy on a broad scale poses problems, and there is an argument for channeling resources into the creation of environments that reduce the likelihood of obesity rather than waiting to act after becoming obese. This might include regulation of the food industry with regard to advertising, labelling, and the fat and sugar content of food, as well as urban planning and social policies that promote physical activity. In view of these considerations it seems unlikely that bariatric surgery will have a significant role as a prevention strategy for type 2 diabetes.
SERVICE DELIVERY
The evidence base from randomized controlled trials focusing on diabetes prevention is almost exclusively centered around IGT, with available evidence involving “at-risk” individuals. This situation raises a number of issues. IGT has not been traditionally “owned” by any particular health care provider group, although in some countries primary care physicians are beginning to express interest in claiming IGT as part of their area of clinical responsibility. Diabetes specialists have been a little slower in providing services for people at risk of diabetes, possibly as a result of the increasing numbers of people with overt diabetes requiring treatment, or possibly as a result of funding mechanisms.
There is also an obvious mismatch between the most prevalent health problems (i.e., chronic conditions) and the preparedness of the workforce to deal with these problems. Pruitt and Epping-Jordan have called for a restructuring of training to include a new set of core competencies, including knowledge, skills, and abilities, that are designed to prepare 21st-century health workers to manage today’s most prevalent health problems.65 The World Health Organization (WHO) Global Strategy on Diet, Physical Activity and Health suggests that patients’ and families’ routine contacts with health service staff should include practical advice on the benefits of healthy diets and increased levels of physical activity in combination with support to help patients initiate and maintain healthy behaviors. The WHO Global Strategy also proposes that a large part of the population can be reached through routine inquiries into key dietary habits and physical activity, along with provision of simple information and skill building techniques designed to produce behavior change, and that such a strategy can be cost-effective.66
Among clinicians, funders, planners, and policymakers, the prominence of IGT in the prevention evidence has served to create an emphasis on the high-risk approach. This emphasis is no doubt because of the weight and quality of the evidence, but such an approach is also attractive because it is an easier option than dealing with the multisystem, multisector, and multisetting complexities associated with creating healthier antiobesogenic, antidiabetogenic environments. Glascow et al.67 have pointed out that the high-risk approach must complement a wider public health approach to chronic disease prevention. Geoffrey Rose neatly conceptualized this notion, saying, “It makes little sense to expect individuals to behave differently from their peers; it is more appropriate to seek a general change in behavioral norms and in the circumstances which facilitate their adoption.” 68(p164)
Satterfield et al.69 conducted a systematic review of 16 studies on community-based interventions designed to prevent or modify risk factors for type 2 diabetes. The interventions essentially targeted ethnic populations that had a high prevalence of diabetes. Strategies used invariably combined diet and exercise. The results of this review were inconclusive owing to lack of data on reductions in blood glucose levels or other diabetes risk factors, as well as study design limitations. Satterfield et al. highlighted the need for further research in this area, but suggested a combined population and high-risk approach.
In Sweden, the Stockholm Diabetes Prevention Program aims to reduce Sweden’s incidence of type 2 diabetes by 25% over 10 years by combining community-wide strategies with more focused activities, such as interventions in the workplace and in schools. The Finnish Prevention Programme exemplifies a nationally coordinated and integrated comprehensive approach to diabetes prevention.71
Combining a high-risk approach with a population approach means that any investment in primary prevention has the capacity to bring gains across the continuum of care. Figure 1 ▶ illustrates how broader changes in the social environment can affect people who already have diabetes as well as those identified as at risk and those in the population who do not yet have any manifestation of disease or disease risk and who need to be kept healthy.
SOCIAL POLICY
Although we recognize the benefits of science, surgery, and service delivery in relation to certain aspects of chronic disease prevention, it is clear that, either independently or in concert, none can achieve the broad scale changes required to prevent diabetes and obesity on a population basis. Beaglehole and Yach11 pointed out that the increasing burden of noncommunicable diseases in poor countries and poor populations has been neglected by policymakers as well as major multilateral and bilateral aid donors and academics. They call for a comprehensive policy and action response on the part of governmental and nongovernmental agencies. The Global Strategy on Diet, Physical Activity and Health66 recently reinforced the idea that there is a need for a combined health, fiscal, and social policy approach, as outlined in the box on this page. Similarly, Wanless72 called for the government of the United Kingdom to adopt an intersectoral approach to bring about the full engagement of all levels of the health system, all sectors of government, and the public to address determinants of disease and transform the system into a health system rather than a sickness system. Others conceptualize this as a “sectors and settings” approach,73 sectors being the parts of community and social governance that regulate various areas such as health, education, and transportation and settings being the locations where individuals live, work, pray, and play.
Key Policy Recommendations of the World Health Organization’s Global Strategy on Diet, Physical Activity and Health.
National dietary and physical activity guidelines
Fiscal and agriculture policies designed to influence food availability and food choices
Transportation and environmental policies designed to promote physical activity
School policies
Changes in marketing practices, especially marketing targeted toward children
Stronger prevention elements within health services
Public awareness campaigns
Clear, simplified messages (reduce salt, sugar, fat; increase fruits and vegetables, physical activity)
Accurate nutrition labeling and monitored nutrition and health claims
With regard to changing individual and overall dietary behaviors, some propose to emulate the methods adopted by the anti-smoking lobby. The implications of the Tobacco Framework Convention in terms of addressing determinants of food-related morbidity and mortality are set out by Yach et al.74 However, although credit is unreservedly due to the public health effort that brought about the policy and public mind-set that smoking is unhealthy, uncool, and even uncouth, it must be noted that the antismoking lobby had the advantage of a very clear-cut and simple message: “Smoking kills—don’t smoke.” The diabetes and obesity message is not so straightforward. Yach et al.75 identified 3 pivotal levers for change: (1) raising the profile of chronic diseases in the minds and on the agenda of policymakers, (2) providing policymakers with the necessary evidence to support the case for prevention, and (3) advocating the need for widespread heath system changes. This strategy should be expanded to include macroeconomic policies and entire government systems, not just health systems. Others have argued that the policy approach in government alone is not sufficient in a world where power and authority often rests with global corporations. These researchers have called for an “expansion of the public health table” to include big business, labor unions, and other organs of civil society in order to discuss health challenges and agree on a way to move forward.76 The question is, “how can such a plan be achieved, and who can influence the process?”
Governments
Clearly, governments, in cooperation with other stakeholders (e.g., industry, nongovernmental organizations, and health professionals), play a central role in creating an environment that empowers and encourages individuals, families, and communities to make positive, life-enhancing behavior changes in terms of diet and patterns of physical activity.75 In addition to direct health policy and services, the responsibility of governments includes sectors that have a pivotal influence on health, such as agriculture, education, and transportation. Social determinants of health are also mediated by fiscal policy and employment opportunities. Consequently, it is imperative that the executive of the government, especially the head of the government, and the finance minister be involved in discussions that traditionally have been limited to matters of microeconomic reform inside the health portfolio. Commerce, industry, and labor traditionally have not been invited to the discussion, but should also be involved.
The growing awareness and action of the United Kingdom and Finnish governments have already been described, and the United States has recently joined the ranks of countries that have formal diabetes action plans in place.77 Other governments, such as those of Canada, Brazil, and Thailand, are also demonstrating their commitment to prevention of diabetes or other chronic diseases, or both, through policies, programs, and legislation aimed at promoting health and reducing lifestyle risks. Although these initiatives alone will not effect change, they provide a focus and direction for action.
In addition, a new advocacy model, “parliamentary diabetes support groups,” is emerging from within governments in the US, Australia,78 and, more recently, the United Kingdom. In this model, parliamentarians of all political persuasions collaborate in promoting attention to diabetes at a national level. This advocacy strategy adds new meaning to the term political will, reflects an unprecedented level of political and public awareness of diabetes, and signals that alarm bells at the growing tide of diabetes are ringing loud and clear in the corridors of political power.
Nongovernmental Organizations
Beaglehole and Yach11 noted that advocacy at the global level in the area of non-communicable diseases is weak and disease specific rather than integrated and coordinated across diseases. However, there are formal “chronic disease alliances” (e.g., for heart, kidney, and diabetes) between nongovernmental organizations (in the United States, Canada, and Australia), and these alliances are becoming increasingly influential. The Oxford Health Alliance is an example of an industry–academic global anti–chronic disease partnership that brings together important global players to advocate for and to act on chronic diseases.79
Business
Industry and business are also acting to influence health though workplace policies aimed at protecting and promoting health and offering and rewarding preventive activities. Organizations such as the Leapfrog Group,80 General Electric, Ford, and Proctor and Gamble are initiating provider reward programs for quality and effective care, and Johnson & Johnson has a Health and Wellness Program that covers more than 47 000 domestic employees. Although these and many emerging workplace programs are not necessarily specific to diabetes and obesity, they all aim to improve health. They also signal a corporate mindset that is coinciding with another significant, potential driver of social policy: the rising voices of consumers and civil society demanding attention to the determinants of health.
Civil Society
The WHO states that civil society and nongovernmental organizations can help to ensure that consumers ask governments to provide support for healthy lifestyles and ask the food industry to provide healthy products.66 Raymond et al.76 took this notion further by proposing that civil society is the key platform for mobilizing and actualizing associative behaviors designed to promote awareness, education, and advocacy for health. They advocate for representatives of business and commerce becoming involved in defining the problem, proposing solutions, and implementing those solutions, because a healthy work-force and market are central to these representatives’ core business. Raymond et al. also noted the power of the Internet in promoting what they called the “globalization of associative behavior.”76
Parents Jury81 is a case in point. It is an Internet-based initiative that offers parents information and a say in matters that affect their children’s physical activity and nutrition (e.g., advertising of junk food during prime time television hours). GLOBALink,82 another Web site, passes on lessons from one generation of tobacco control advocates to the next, and Patient View,83 a group that monitors and analyzes developments in health, communicates its findings with health and social campaigners via its electronic publication, HSCNews.
The People’s Health Movement84 is another “free association” that is working to influence social policy. Guided by a vision of “a world in which people’s voices guide the decisions that shape our lives,”84 The Peoples’ Health Movement leads the production of Global Health Watch,85 the first alternative health report. This alternative report, which was started on the basis that civil society needs to produce its own global health report unfettered by political restrictions, challenges the relevance of the WHO World Health Reports.
Although there is a need to invest in building the evidence base around the role of policy, and, in particular, finding the appropriate tools for evaluating a policy’s impact, there is clearly a convergence of opinion that it is time to enact policies aimed at creating healthier social and physical environments. This opinion is accompanied by an emerging trend to return to not only the concept but the reality of community: where we live and the types of societies we want. There are also signs that we are returning to the notion of community as a “new” public health concept. This trend will see greater engagement by consumers and civil society in health care policy and decisionmaking at all levels. Although the benefits of such a “fully engaged scenario”72(p3) remain to be rigorously tested, modeling has indicated that increased investment in these areas will bring both health and financial gains in the longer term. In the meantime, lobbying groups are increasingly calling governments and industry, particularly the food industry, into account. Consumers will increasingly sit on high-level government policy, strategic planning, monitoring, and research committees, and communities will have greater input into town planning and environmental issues that have an impact on health.
Governments have the classic tools of legislation, regulation, and taxation at their disposal to enact social policies that can serve to turn the tide of diabetes, obesity, and other chronic conditions. Thanks to the growing and increasingly concerted voices of lobby groups, governments are beginning to take this role more seriously. A tipping point seems to be approaching, and, if we all add our voices to those already calling for the creation of healthier environments, the reduction of the disease and cost burdens of diabetes, obesity, and other chronic diseases will become reality.
Peer Reviewed
Contributors R. Colagiuri developed the idea for the article and, together with S. Colagiuri, undertook the majority of the writing. D. Yach provided input and references and reviewed the various drafts. S. Pramming provided input and reviewed progressive drafts of the article.
References
- 1.Colagiuri R. Diabetes as a health promotion focus: a disease for all reasons. Health Promotion J Aust. 2004;15:95–99. [Google Scholar]
- 2.International Diabetes Federation. Diabetes atlas. Available at: www.idf.org/e-atlas. Accessed March 15, 2006.
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 4.King H, Aubert R, Herman W. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diabetes Care. 1998;21:1414–1431. [DOI] [PubMed] [Google Scholar]
- 5.Reinehr T, Wabitsch M. Type 2 diabetes mellitus in children and adolescents. In: Ganz M, ed. Prevention of Type 2 Diabetes. West Sussex, England: John Wiley & Sons Inc; 2005:21–40.
- 6.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. [DOI] [PubMed] [Google Scholar]
- 8.Cameron AJ, Welborn TA, Zimmet PZ, et al. Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (Aus-Diab). Med J Aus. 2003;178:427–432. [DOI] [PubMed] [Google Scholar]
- 9.Dowse GK, Zimmet PZ, Finch CF, et al. Decline in incidence of epidemic glucose intolerance in Nauruans: implications for the “thrifty genotype.” Am J Epidemiol. 1991;133:1093–1104. [DOI] [PubMed] [Google Scholar]
- 10.Knowler W. Diabetes mellitus in Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990;6:1–27. [DOI] [PubMed] [Google Scholar]
- 11.Beaglehole R, Yach D. Globalisation and the prevention and control of non-communicable disease: the neglected chronic diseases of adults. Lancet. 2003; 362:903–908. [DOI] [PubMed] [Google Scholar]
- 12.Leeder S, Raymond S, Greenberg H, Liu H, Esson K. Race Against Time: The Challenge of Cardiovascular Disease in Developing Economies. New York, NY: Columbia University; 2004.
- 13.Chronic Diseases and Associated Risk Factors in Australia, 2001. Canberra, Australian Capital Territory, Australia: Australian Institute of Health and Welfare; 2002.
- 14.Eaton WW. Epidemiological evidence on the co-morbidity of depression and diabetes. J Psychosom Res. 2002;53:903–906. [DOI] [PubMed] [Google Scholar]
- 15.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. [DOI] [PubMed] [Google Scholar]
- 17.Kruse J, Schmitz N, Thefeld W. On the association between diabetes and mental disorders in a community sample: results from the German National Health Interview and Examination Survey. Diabetes Care. 2003;26:1841–1846. [DOI] [PubMed] [Google Scholar]
- 18.Holmes J, McGill S, Kind P, Bottomley J, Gillam S, Murphy M. Health-related quality of life in type 2 diabetes (T2ARDIS-2). Value Health. 2000;3(suppl 1): S47–S51. [DOI] [PubMed] [Google Scholar]
- 19.Colagiuri S, Colagiuri R, Conway B, Grainger D, Davey P. DiabCo$t Australia: Assessing the Burden of Type 2 Diabetes in Australia. Canberra, Australian Capital Territory, Australia: Diabetes Australia; 2003.
- 20.Koopmanschap M. Coping with type II diabetes: the patient’s perspective. Diabetologia. 2002;45: S18–S22. [DOI] [PubMed] [Google Scholar]
- 21.Peyrot M, Rubin RR, Lauritzen T, et al., for the International DAWN Advisory Panel. Psychosocial problems and barriers to improved diabetes management: results of the Cross-national Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. In press. [DOI] [PubMed]
- 22.The World Health Report 2003—Shaping the Future. Geneva, Switzerland: World Health Organization; 2003.
- 23.American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. [DOI] [PubMed] [Google Scholar]
- 24.Identification, Evaluation and Treatment of Overweight and Obesity in Adults. Bethesda, Md: National Institutes of Health; 1998.
- 25.Williams R, Van Gaal L, Lucioni C. Assessing the impact of complications on the costs of type II diabetes. Diabetologia. 2002;45:S13–S17. [DOI] [PubMed] [Google Scholar]
- 26.Jonsson B. Revealing the cost of type II diabetes in Europe. Diabetologia. 2002;45:S5–S12. [DOI] [PubMed] [Google Scholar]
- 27.Williams R, Gillam S, Murphy M, et al. The True Costs of Type 2 Diabetes in the UK: Findings From T 2ARDIS and CODE-2 UK. Uxbridge, England: Glaxo-SmithKline; 2002.
- 28.Herman WH, Hoerger TJ, Brandle M, et al., for the Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal L, Dalton AC, Richardson J. Cost-effectiveness of the primary prevention of non-insulin-dependent diabetes mellitus. Health Promotion Int. 1998;13:197–209. [Google Scholar]
- 30.Walker A. Colagiuri S, McLennan M. Cost-benefit model for diabetes prevention and care, 2003. Available at: http://www.natsem.canberra.edu.au. Accessed March 15, 2006.
- 31.Venkat Narayan KM, Boyle JP, Thompson, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. [DOI] [PubMed] [Google Scholar]
- 32.Vinicor F, Bowman B, Engelgau M. Diabetes: prevention needed. Lancet. 2003;361:544. [DOI] [PubMed] [Google Scholar]
- 33.Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 1999.
- 34.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. [DOI] [PubMed] [Google Scholar]
- 35.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA. 2001;285:2109–2113. [DOI] [PubMed] [Google Scholar]
- 36.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 years before clinical diagnosis. Diabetes Care. 1992;15:815–819. [DOI] [PubMed] [Google Scholar]
- 37.UK Prospective Diabetes Study Group. Complications in newly diagnosed type 2 diabetic patients and their association with different clinical and biochemical risk factors. Diabetologia. 1990;13:1–11. [PubMed] [Google Scholar]
- 38.Suvd B, Gerel H, Otgooloi D, et al. Glucose intolerance and associated factors in Mongolia: results of a national survey. Diabet Med. 2002;19:502–508. [DOI] [PubMed] [Google Scholar]
- 39.Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25:829–834. [DOI] [PubMed] [Google Scholar]
- 40.Colagiuri S, Colagiuri R, Na’ati S, Muimuiheata S, Hussain Z, Palu T. The prevalence of diabetes in the Kingdom of Tonga. Diabetes Care. 2002;25:1378–1383. [DOI] [PubMed] [Google Scholar]
- 41.Aspray TJ, Mugusi F, Rashid S, et al. Rural and urban differences in diabetes prevalence in Tanzania: the role of obesity, physical inactivity and urban living. Trans R Soc Trop Med Hyg. 2000;94:637–644. [DOI] [PubMed] [Google Scholar]
- 42.Amoah AG, Owusu SK, Adjei S. Diabetes in Ghana: a community based prevalence study in Greater Accra. Diabetes Res Clin Pract. 2002;56:197–205. [DOI] [PubMed] [Google Scholar]
- 43.Mbanya JC, Ngogang J, Salah JN, Minkoulou E, Balkau B. Prevalence of NIDDM and impaired glucose tolerance in a rural and an urban population in Cameroon. Diabetologia. 1997;40:824–829. [DOI] [PubMed] [Google Scholar]
- 44.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson KF, Lindgarde F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmo Preventative Trial with diet and exercise. Diabetologia. 1998;41:1010–1016. [DOI] [PubMed] [Google Scholar]
- 46.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 47.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padwal R, Majumbar SR, Johnson JA, Varney J, McAlister FA. A systematic review of drug therapy to delay or prevent type 2 diabetes. Diabetes Care. 2005; 28:736–744. [DOI] [PubMed] [Google Scholar]
- 49.Chiasson J, Josse R, Gomis G, Hanefield R, Karasik M, Laakso A. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. [DOI] [PubMed] [Google Scholar]
- 50.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. [DOI] [PubMed] [Google Scholar]
- 51.Torgerson J, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study. Diabetes Care. 2004;27:155–161. [DOI] [PubMed] [Google Scholar]
- 52.Davey Smith G, Bracha Y, Svendsen KH, Neaton JD, Haffner SM, Kuller LH, for the Multiple Risk Factor Intervention Trial Research Group. Incidence of type 2 diabetes in the randomized Multiple Risk Factor Intervention Trial. Ann Intern Med. 2005;142:313–322. [DOI] [PubMed] [Google Scholar]
- 53.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrold JA, Williams G. The cannabinoid system: a role in both homeostatic and hedonic control of eating. Br J Nutr. 2003;90:729–734. [DOI] [PubMed] [Google Scholar]
- 55.Acomplia (rimonabant)—investigational agent for the management of obesity. Available at: http://www.drugdevelopment-technology.com/projects/rimonabant. Accessed March 15, 2006.
- 56.Design considerations for a potential United States population-based cohort to determine the relationships among genes, environment, and health: recommendations of an expert panel. Available at: http://www.genome.gov/Pages/About/OD/ReportsPublications/PotentialUSCohort.pdf. Accessed March 15, 2006.
- 57.Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults. Canberra, Australian Capital Territory, Australia: National Health and Medical Research Council, Commonwealth of Australia; 2003.
- 58.Monteforte MJ, Turkelson CM. Bariatric surgery for morbid obesity. Obes Surg. 2000;10:391–401. [DOI] [PubMed] [Google Scholar]
- 59.Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–484. [DOI] [PubMed] [Google Scholar]
- 60.Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000; 36:20–25. [DOI] [PubMed] [Google Scholar]
- 61.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sjostrom L, Nabro K, Sjostrom D. Costs and benefits when treating obesity. Int J Obes. 1995;19(suppl 6): S9–S12. [PubMed] [Google Scholar]
- 63.Narbro K, Agren G, Naslund I, Sjostrom L, Peltonen M. Decreased medication for diabetes and cardiovascular disease after weight loss. Int J Obes. 2000;24(suppl 1):S42. [Google Scholar]
- 64.Goldstein D. Beneficial health effects of modest weight loss. Int J Obes. 1992;16:397–415. [PubMed] [Google Scholar]
- 65.Pruitt SD, Epping-Jordan JE. Preparing the 21st century global healthcare workforce. BMJ. 2005;330:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. Global strategy on diet, physical activity and health, 2004. Available at: http://www.who.int/dietphysicalactivity/en/. Accessed March 20, 2006.
- 67.Glascow RE, Wagner EH, Kaplan RM, Vinicor F, Smith L, Norman J. If diabetes is a public health problem, why not treat it as one? A population-based approach to chronic diseases. Ann Behav Med. 1999;21:159–170. [DOI] [PubMed] [Google Scholar]
- 68.Rose G. The Strategy of Preventive Medicine. Oxford, England: Oxford University Press; 1992. Cited By: World Health Organization. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/ FAO expert consultation. Available at: http://www.who.int. Accessed March 12, 2006.
- 69.Satterfield DW, Volansky M, Caspersen CJ, et al. Community-based lifestyle interventions to prevent type 2 diabetes. Diabetes Care. 2003;26:2643–2652. [DOI] [PubMed] [Google Scholar]
- 70.Bjaras G, Ahlbom A, Alvarsson M, et al. Strategies and methods for implementing a community-based diabetes primary prevention program in Sweden. Health Promotion Int. 1997;12:151–160. [Google Scholar]
- 71.Development Programme for the Prevention and Care of Diabetes in Finland 2000–2010. Tampere, Finland: Finnish Diabetes Association; 2001.
- 72.Wanless D. Securing Good Health for the Whole Population. London, England: HM Treasury; 2004.
- 73.Swinburn B, Egger G, Raza F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med. 1999;29:563–570. [DOI] [PubMed] [Google Scholar]
- 74.Yach D, Hawkes C, Epping-Jordan JE, Hofman KJ. The World Health Organization’s Framework Convention on Tobacco Control: implications for global epidemics of food related deaths and disease. J Public Health Policy. 2003;24:274–290. [PubMed] [Google Scholar]
- 75.Yach D, Hawkes C, Linn Gould C, Galbraith S. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA. 2004; 291:2616–2626. [DOI] [PubMed] [Google Scholar]
- 76.Raymond S, Greenberg HM, Hui L, Leeder S. Civil society confronts the challenge of chronic Illness. Development. 2004;47:97–103. [Google Scholar]
- 77.Diabetes: A National Plan for Action: Steps to a Healthier US. Washington, DC: US Dept of Health and Human Services; 2004.
- 78.Diabetes parliamentary support group. Available at: http://www.diabetesaustralia.com.au/conquest/04autumn. Available at: www.diabetesaustralia.com.auconquest/04autumn. Accessed March 15, 2006.
- 79.Oxford Vision 2020: towards a healthier future. Available at: http://www.oxfordvision2020.org. Accessed March 10, 2006.
- 80.Leapfrog Group Web site. Available at: http://www.leapfroggroup.org. Accessed March 7, 2006.
- 81.Parents Jury Web site. Available at: http://www.parentsjury.org. Accessed March 2, 2006.
- 82.GLOBALink Web site. Available at: http://www.globalink.org. Accessed March 14, 2006.
- 83.Patient View Web site. Available at: http://www.patient-view.com. Accessed March 3, 2006.
- 84.People’s Health Movement Web site. Available at: http://www.phmovement.org/. Accessed March 10, 2006.
- 85.Global Health Watch Report. Available at http://www.ghwatch.org/2005_report.php Accessed June 14, 2006.


