Abstract
Background
Exposure to (±)3,4-methylenedioxymethamphetamine ((±)MDMA) results in lasting reductions of many markers for serotonin terminals in a range of species. In rodents, the severity of insult depends in large part on the generation of hyperthermia in the subject. (±)MDMA can produce either hyperthermia or hypothermia in rodents depending on the ambient temperature and these effects may be limited to the S(+) enantiomer. Limited prior evidence suggests (±)MDMA does not produce hyperthermia in chair-restrained monkeys [Bowyer et al., 2003, Neurotoxicology 24(3):379–390]. This study was therefore conducted to determine if racemic MDMA and its enantiomers induce hyperthermia and increase spontaneous locomotor activity in unrestrained rhesus monkeys.
Methods
Body temperature and spontaneous home cage activity were monitored continuously in four monkeys via radiotelemetric devices. The subjects were challenged with 1.7 mg/kg, i.m., (±)MDMA, S(+)MDMA and R(−)MDMA in pseudorandomized order.
Results
Maximum and average temperature in the four hour interval post-dosing was elevated 0.7–0.9°C by (±)MDMA and each enantiomer. Reductions in locomotor activity following dosing did not reliably differ from vehicle effects.
Conclusions
MDMA produces an acute hyperthermia in unrestrained rhesus monkeys, much as it does with rats, mice, pigs, rabbits and humans. Hyperthermia occurs despite no increase in locomotor activity thus the effect does not depend on motor activation. Each enantiomer appears to be equivalently active thus primates may differ from rodents in thermoregulatory sensitivity to the R(−) enantiomer. Significant differences in outcome between this and a prior study in monkeys indicate a need for additional study of the thermoregulatory impact of MDMA in nonhuman primates.
Keywords: MDMA, Macaca mulatta, circadian, thermoregulation, serotonin
1. Introduction
The Drug Abuse Warning Network estimates some 4,000 annual emergency department (ED) visits in which Ecstasy/MDMA is involved (Ball et al. 2003; Ball et al. 2004). ED surveys often report significant and malignant elevations in body temperature (Dams et al. 2003; Gillman 1997; Greene et al. 2003; Mallick and Bodenham 1997) but likely underrepresent the true prevalence of thermoregulatory distress. Many incidents may not appear serious enough to invoke emergency medical services, particularly given that MDMA use is illegal in most jurisdictions. It is challenging to identify the critical contributing factors to the relatively rare Ecstasy fatality from ED reports. In fatal cases, tissue levels of MDMA are usually quite high, but not exclusively so since case reports associate fatalities with a wide range of plasma levels of MDMA, i.e., 40–7000 ng/ml (Brown and Osterloh 1987; Dowling et al. 1987; Garcia-Repetto et al. 2003; Greene et al. 2003; Karlovsek 2004; Karlovsek et al. 2005). A survey of 102 medical examiner death reports (Patel et al. 2004) reported a mean peripheral blood level of 1120 ng/ml (SD= 1230) of MDMA. Furthermore, although the majority of street “Ecstasy” pills contain some MDMA (Parrott 2004), they are often contaminated with other psychoactive compounds with thermomodulatory properties (Baggott et al. 2000; Cheng et al. 2003; Palhol et al. 2002), making it difficult to determine the specific contribution of MDMA to thermoregulation. Thus, animal models are required to determine the circumstances under which MDMA can disrupt thermoregulation to further understanding of how such disruption may result in ED visits (and potential fatality) for the recreational user.
MDMA intake results in an acute elevation of body temperature in human laboratory studies following doses (1.5–2.0 mg/kg, p.o.) described as within the range of common recreational doses (Freedman et al. 2005; Liechti et al. 2000). MDMA also produces an acute hyperthermia in rats (Brown and Kiyatkin 2004; Dafters 1994; Malberg and Seiden 1998), mice (Carvalho et al. 2002; Fantegrossi et al. 2003), guinea pigs (Saadat et al. 2004), pigs (Fiege et al. 2003; Rosa-Neto et al. 2004) and rabbits (Pedersen and Blessing 2001). Body temperature data have not generally been included in monkey studies of lasting serotonergic alterations (Insel et al. 1989; Ricaurte et al. 1988; Taffe et al. 2002a; Taffe et al. 2001). It is surprising that MDMA apparently reduced body temperature in chair-restrained rhesus monkeys (Bowyer et al. 2003) and therefore the present study was undertaken to test the hypothesis that MDMA elevates temperature in unrestrained rhesus monkeys. A second goal was to determine if MDMA stimulates locomotor activity in monkeys as it does in rodents (Dafters 1994; Fantegrossi et al. 2003; Gold and Koob 1988). Increases in locomotor activity would complicate the comparison with the human laboratory studies in which volunteers were not engaging in significant levels of activity (Freedman et al. 2005; Liechti et al. 2000).
The enantiomers of MDMA may differ in effect with the S or (+) enantiomer reported to be more potent in terms of human subjective response (Shulgin and Shulgin 2000) and in producing stereotyped behaviors in rats (Hiramatsu et al. 1989). The S(+)-MDMA enantiomer produces hyperthermia with equivalent efficacy as the racemate in mice, while R(−)MDMA appears ineffective in elevating temperature (Fantegrossi et al. 2003). Thus the final goal was to test the hypothesis that racemic and S(+)-MDMA would stimulate hyperthermia in monkeys whereas R(−)-MDMA would not.
2. Methods
2.1 Animals
Four male rhesus monkeys (Macaca mulatta; Chinese origin) were employed in this study. Animals were 6–7 years of age, weighed 8.5–12.5 kg at the start of the study and exhibited body condition scores (Clingerman and Summers 2005) of 2.5 (N=2) or 3.5 (N=2) out of 5 at the nearest quarterly exam. Daily chow (Lab Diet 5038, PMI Nutrition International; 3.22 kcal of metabolizable energy (ME) per gram) allocations were determined by a power function (Taffe 2004a; 2004b) fit to data provided in a National Research Council recommendation (NRC/NAS 2003) and modified individually by the veterinary weight management plan. Daily chow ranged from 140 to 224 g per day for the animals in this study. Animals were individually housed in a controlled temperature environment (23.5°C) and fed in the home cage. The animals’ normal diet was supplemented with fruit or vegetables seven days per week and water was available ad libitum in the home cage at all times. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al. 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.2 Apparatus
Radio telemetric transmitters (TA10TA-D70; Transoma/Data Sciences International; “DSI”) were implanted subcutaneously in the flank by, or under supervision of, the TSRI veterinary staff using sterile techniques. Animals were allowed to recover for 6 weeks prior to initiating the drug studies. Temperature and gross locomotor activity recordings were recorded every 10 minutes. The raw data were collected as a single temperature value every 10 minutes and a per-minute average of activity over each 10 minute interval. An activity “count” requires a movement across approximately half of the cage in any direction in this system. Ambient room temperature (TA) was also recorded every 10 minutes by the system.
2.3 Drug challenge studies
(±)3,4-methylenedioxymethamphetamine HCl, S(+)3,4-methylenedioxymethamphetamine HCl and R(−)3,4-methylenedioxymethamphetamine HCl were administered intramuscularly with a constant dose of 1.7 mg/kg and an injection volume of 0.1 ml/kg. Drugs were provided by the National Institute on Drug Abuse. Challenges were administered at 1030 with active doses separated by 1–2 weeks in a pseudorandomized order. Vehicle challenges were performed the day prior to active challenge. Ambient temperature averaged 23.5°C with a daily range of 23–24°C throughout the study. The room temperature range in the four hour interval post-dosing was 23.25–23.75°C.
Animals were monitored directly by the investigators for a minimum of two hours post-challenge via remote video feed. Animals were housed normally with 4 monkeys directly across from them and 6 additional animals within sight to their left. No personnel entered the room for 2 hrs post-injection on vehicle and active days in variation from the usual M-F routine in which staff move monkeys in and out of the room for behavioral testing purposes (e.g., (Taffe et al. 2002b; 2004; Weed et al. 1999) throughout the day.
2.4 Data Analysis
Temperature and activity data were averaged over 30 minute bins (i.e., average of 3 sequential samples). Two way randomized block analysis of variance (ANOVA) was employed to evaluate acute treatment related effects starting 30 min prior to injection and continuing a total of 480 min post-injection. This interval was selected because the lights were turned off for the evening at the conclusion of this interval. The circadian temperature pattern is sensitive to this, as can be observed in Figure 1. Thus the two within subjects factors for ANOVA were time relative to injection (−30 to 450) and drug treatment condition (Vehicle, MDMA) for (±)MDMA, S(+)MDMA and R(−)MDMA. In addition, the average and maximum (of all 10-min samples) temperature observed in the interval 4 hrs post-injection following all three compounds (and related vehicle challenges) was analyzed in a two way randomized block ANOVA. Average and maximum activity were derived from the summed activity in each 10-minute interval. For all ANOVAs, significant main effects were followed up with Fisher’s LSD posthoc test. All statistical analyses were conducted using GB-STAT v7.0 for Windows (Dynamic Microsystems, Inc., Silver Spring MD) and the criterion for significance in all tests was p < 0.05.
Figure 1.
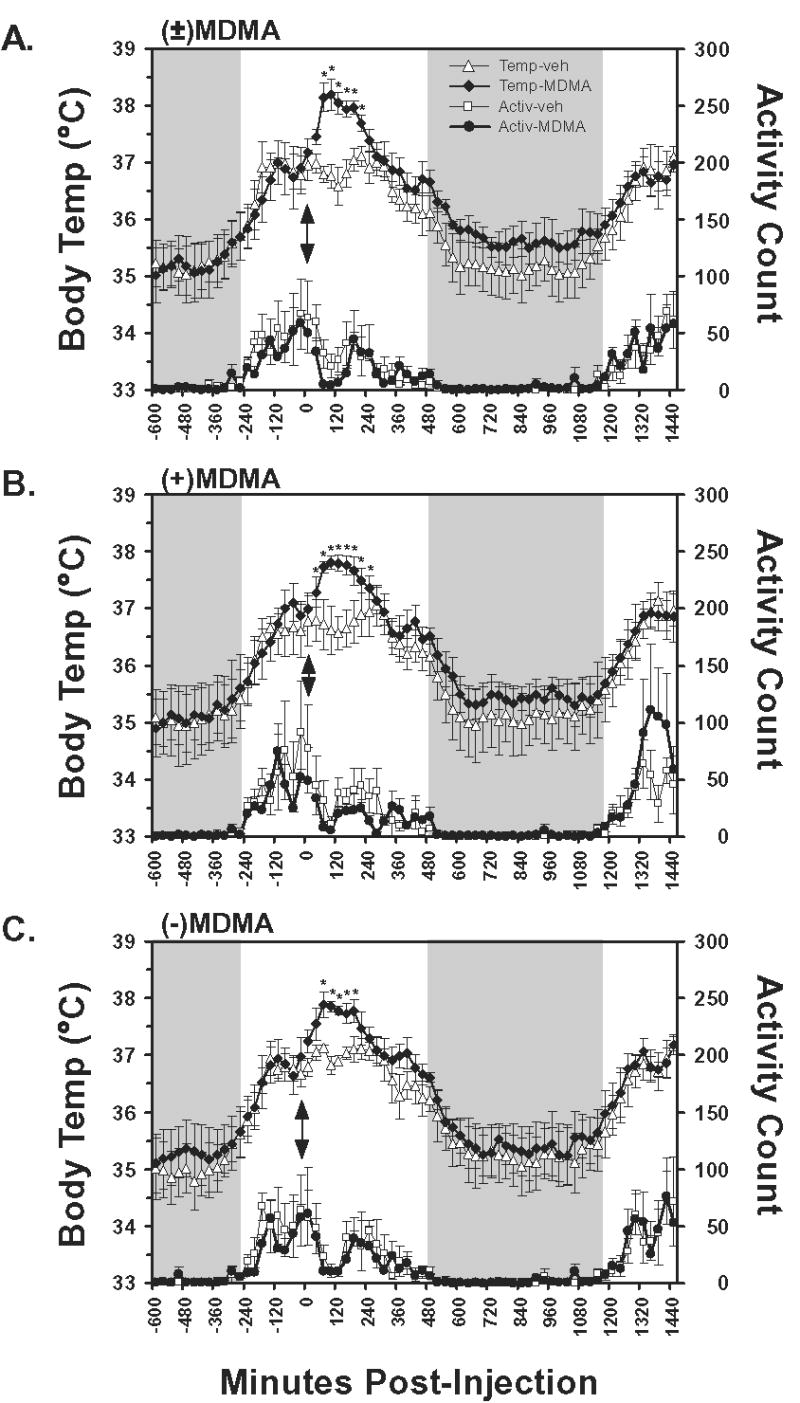
The mean (N=4, bars indicate SEM) subcutaneous temperature and activity values following A) racemic MDMA, B) S(+)MDMA and C) R(−)MDMA, as well as the respective vehicle challenges, are presented. Temperature values are an average of three sequential 10-min interval samples and activity data represent the average of summed activity counts across three sequential 10-min intervals. Injections were made at 1030 for all conditions, thus the data represent the interval from midnight prior to injection to 1030 the following day. Shaded regions indicate the night period during which the room lights are off and arrows indicate the time of injection. A significant increase from both the 30-min timepoint preceding injection and the corresponding vehicle timepoint is indicated by *. Additional differences of significance are detailed in the Results.
3. Results
The temperature and activity measures exhibited a diurnal circadian pattern in which activity and temperature increased when the lights were turned on and decreased noticeably once lights were turned off (Figure 1). Mean temperature varied about 2°C across the day, consistent with a similar daily temperature pattern for intraperitoneal temperature in Japanese macaques or subcutaneous temperature in cynomolgous and rhesus macaques (Almirall et al. 2001; Horn et al. 1998; Takasu et al. 2002). Temperature dropped significantly across the 4 hour analysis period, as confirmed by main effects of time post-injection for each of the (±)MDMA [F16,48 = 14.93; p < 0.0001], S(+)MDMA [F16,48 = 10.16; p < 0.0001] and R(−)MDMA [F16,48 = 10.76; p < 0.0001] studies.
The administration of (±)MDMA, S(+)MDMA or R(−)MDMA resulted in a significant elevation of temperature as is shown in Figure 1. Statistically reliable main effects of drug treatment condition were confirmed for the (±)MDMA [F1,3 = 19.88; p < 0.05] and R(−)MDMA [F1,3 = 8.4; p < 0.05] studies but not the S(+)MDMA [F1,3 = 4.75; p = 0.11] study. Reliable time × drug condition interactions were also confirmed in the (±)MDMA [F16,48 = 4.03; p < 0.0001], S(+)MDMA [F16,48 = 10.91; p < 0.0001] and R(−)MDMA [F16,48 = 2.43; p < 0.01] studies.
The posthoc analyses confirmed that temperature was significantly increased relative to the 30-min interval prior to injection 30–180 min after (±)MDMA, 0–210 min after S(+)MDMA and 30–150 min after R(−)MDMA. No differences were confirmed for these intervals following the respective vehicle conditions. Consistent with the main effect of time post-injection for all studies, multiple timepoints from ~300–450 min post-injection were significantly lower than pretreatment in all (drug or vehicle) conditions consistent with a circadian cooling that is typically observed at that time of day.
Locomotor activity was significantly reduced in the interval post-injection for the (±)MDMA [F16,48 = 4.77; p < 0.0001], S(+)MDMA [F16,48 = 2.63; p < 0.01] and R(−)MDMA [F16,48 = 2.94; p < 0.01] studies however no significant differences attributable to drug treatment condition, nor the interaction of factors were confirmed in any of the analyses.
The average temperature increase observed after (±)MDMA did not differ significantly from that observed after either enantiomer in the four hours following injection (Table 1). The ANOVA confirmed a significant effect of drug challenge over vehicle (main effect of treatment condition [F1,3 = 23.59; p < 0.05] and the posthoc test confirmed that average temperature was higher than the respective vehicle challenge day for each test compound. No differences were observed between active treatment conditions, nor between the three vehicle conditions. The maximum temperature following drug challenge was also significantly elevated relative to vehicle [F1,3 = 10.29; p < 0.05] and post hoc evaluation of this effect confirmed a significant increase for racemic MDMA and each enantiomer over the respective vehicle conditions. In addition, the maximum temperature observed after (±)MDMA was significantly higher than that observed after S(+)MDMA. Although both average and maximum 10-minute activity counts (Table 1) were lower relative to vehicle in the four hour interval post-MDMA, these effects were not statistically reliable, nor were any differences observed between the enantiomers and racemic MDMA.
Table 1.
Average and Maximum Temperature in Four Hours Post-Injection
| Temperature | Activity | |||||
|---|---|---|---|---|---|---|
| (±)-MDMA | (+)-MDMA | (−)-MDMA | (±)-MDMA | (+)-MDMA | (−)-MDMA | |
| Average (vehicle) | 36.9 (± 0.21) | 36.8 (± 0.38) | 37.0 (± 0.13) | 115 (± 29.6) | 119 (± 39.0) | 104 (± 21.2) |
| Average (MDMA) | *37.8 (± 0.07) | *37.6 (± 0.15) | *37.7 (± 0.12) | 73 (± 28.1) | 62 (± 9.8) | 83 (± 20.5) |
| Maximum (vehicle) | 37.2 (± 0.17) | 37.1 (± 0.42) | 37.4 (± 0.10) | 393 (± 69.7) | 410 (± 55.9) | 402 (± 72.3) |
| Maximum (MDMA) | *38.3 (± 0.28) | *§37.9 (± 0.10) | *38.1 (± 0.13) | 307 (± 96.4) | 278 (± 44.3) | 354 (± 75.9) |
A significant difference from the respective vehicle condition is indicated by * and a significant difference from the (±)MDMA active drug condition is indicated by §.
4. Discussion
This study demonstrates that rhesus monkeys develop elevated body temperature following an intramuscular injection of a moderate dose (1.7 mg/kg) of MDMA under normal (23.5°C) ambient temperature conditions. The finding is consistent with prior reports of MDMA-associated temperature increases in most species, including humans. Effects of (±)MDMA lasted about 4 hours after injection and resulted in a peak subcutaneous temperature 0.9°C higher than the maximum temperature observed after vehicle injection. The average temperatures in the 4-hour interval after injection also differed by about 0.9°C from the vehicle condition. These results suggest that a prior finding of decreased colonic temperature in chaired monkeys (Bowyer et al. 2003) was possibly a consequence of the experimental preparation.
Differences between the Bowyer et al. (2003) results and the present data are unlikely to be due to ambient temperature (TA). The Bowyer et al. studies were conducted under TA of 20.8–21.7°C (J. Bowyer, M. Paule, personal communication) which is close to the present 23.5°C and in both cases TA was below the wide thermoneutral range (24.7–30.6°C) estimated for rhesus monkeys (Johnson and Elizondo 1979). Thus the position of the TA relative to thermoneutral conditions cannot explain the discrepancy. In any case, primates likely differ considerably from rodents in thermoregulatory tolerance to MDMA since a recent study of humans exposed to 2 mg/kg (±)MDMA orally showed that core temperature is increased under both 18°C and 30°C TA conditions (Freedman et al. 2005). This is unlike rodents that become hypothermic following MDMA under sufficiently low TA conditions (Malberg and Seiden 1998).
Circadian factors are unlikely to explain the differences with the Bowyer et al. (2003) results either. Although body temperature declines in the late afternoon (e.g., Figure 1) the injections from which the temperature data derives in the Bowyer study were conducted 0800–0900 (J. Bowyer, M. Paule, personal communication) thus normal circadian effects would be to increase or maintain stable temperatures. One possible explanation is that the temperatures of the Bowyer animals were artificially elevated during the single pre-MDMA baseline observation due to the stress of the chairing procedure; however there are plausible alternative hypotheses. The present study used subcutaneous placement of the temperature probes that would theoretically make the measure more sensitive to hemodynamic effects of MDMA. However MDMA produces vasoconstriction (Mills et al. 2004; Ootsuka et al. 2004) that would be predicted to result in less pronounced elevation or even a reduction in a subcutaneous temperature measurement relative to “core” body temperature. Indeed, rat skin temperature can be unchanged following MDMA despite increases in core temperature (Mechan et al. 2002) at least under 20°C TA. Evidence from the Freedman et al. (2005) study is equivocal since apparent trends for MDMA-associated increases in skin temperature under both 18°C and 30°C TA were not found to be statistically reliable (Freedman et al. 2005). Interpretation of these findings is also facilitated by a body of work from Elizondo and colleagues suggesting skin and core temperatures are highly correlated in rhesus monkeys under a variety of manipulations (Elizondo et al. 1976; Johnson and Elizondo 1979; Oddershede and Elizondo 1980; Smiles et al. 1976). In total such considerations predict that subcutaneous temperature changes induced by MDMA should be equivalent to, or underpredict, core temperature changes and do not explain the discrepancy of the present data from Bowyer et al. 2003.
Exposure to racemic MDMA or either enantiomer was not associated with increases in spontaneous locomotion. This is unlike rodent studies in which MDMA appears to stimulate locomotor activity across a range of doses. The telemetry activity “counts” constitute a full-body movement across approximately half of the cage in any direction and may thus be insensitive to smaller movements. However, on direct observation the monkeys appeared clearly drug intoxicated but otherwise quietly resting and almost normally responsive to other animals in the room without evidence of repetitive stereotyped movement. Therefore with respect to potential confounds from motor activity, the present temperature data would appear to be most similar to temperature results from human studies (Freedman et al. 2005; Liechti et al. 2000). While locomotor activity increases (such as dancing at nightclubs or “rave” parties) may interact with MDMA to increase body temperature in some recreational consumption settings, reliable hyperthermia can occur independently of increased muscular activity, likely because of increases in metabolism (Freedman et al. 2005). Thus individuals who prefer to consume MDMA in a quiet environment are likely still at some risk for thermodysregulatory events.
The fact that (±)MDMA and S(+)MDMA were equipotent in producing hyperthermia is consistent with a report that S(+)MDMA and (±)MDMA have equipotent effects on temperature in mice (Fantegrossi et al. 2003). Interestingly, Fantegrossi and colleagues (2003) report no impact of R(−)MDMA on temperature in mice, even following doses close to the LD50. In a similar enantiomeric dissociation 10 or 20 mg/kg R(−)MDMA has no lasting impact on brain tissue serotonin (5HT) content, where similar doses of S(+)MDMA result in dose-dependent decreases of 5HT in brain of up to 50% (Schmidt 1987). The R(−) enantiomer is, however, active in rodents in other assays. Either S(+)MDMA or R(−)MDMA produces conditioned place preference (CPP) similar to twice the dose of (±)MDMA (Meyer et al. 2002). In addition, S(+)MDMA and R(−)MDMA have equipotent effects on head twitch in mice (Fantegrossi et al. 2005) where, surprisingly, (±)MDMA is inactive. Finally, Schmidt (1987) reports that the acute depletion of 5HT from brain is equivalent following S(+)MDMA and R(−)MDMA. Clearly a simple formulation of “active” versus “inactive” enantiomer is incorrect for MDMA since the effects of its enantiomers appear to depend on outcome assay (temperature, behavior, neurotoxicity, etc). The present results suggest that the impact of MDMA enantiomers on a given assay such as body temperature may depend on species differences as well. Therefore this study cautions that primate responses to MDMA may differ from that of rodents or other species on additional outcome measures, potentially in ways that are critical for translating information to the human condition.
Acknowledgments
This work was supported by USPHS grants DA013390 and DA018418. This is publication #17404-NP from The Scripps Research Institute.
References
- Almirall H, Bautista V, Sanchez-Bahillo A, Trinidad-Herrero M. Ultradian and circadian body temperature and activity rhythms in chronic MPTP treated monkeys. Neurophysiol Clin. 2001;31:161–70. doi: 10.1016/s0987-7053(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Baggott M, Heifets B, Jones RT, Mendelson J, Sferios E, Zehnder J. Chemical analysis of ecstasy pills. Jama. 2000;284:2190. doi: 10.1001/jama.284.17.2190. [DOI] [PubMed] [Google Scholar]
- Ball J, Garfield T, Morin C, Steele D (2003) Emergency Department Trends From the Drug Abuse Warning Network, Final Estimates 1995–2002. DAWN Series D-24, DHHS Publication No. (SMA) 03-3780. Substance Abuse and Mental Health Services Administration, Office of Applied Studies, Rockville, MD
- Ball J, Morin C, Cover E, Green J, Sonnefeld J, Steele D, Williams T, Mallonee E (2004) Drug Abuse Warning Network, 2003: Interim National Estimates of Drug-Related Emergency Department Visits. DAWN Series D-26, DHHS Publication No. (SMA) 04-3972. Substance Abuse and Mental Health Services Administration, Office of Applied Studies, Rockville, MD
- Bowyer JF, Young JF, Slikker W, Itzak Y, Mayorga AJ, Newport GD, Ali SF, Frederick DL, Paule MG. Plasma levels of parent compound and metabolites after doses of either d-fenfluramine or d-3,4-methylenedioxymethamphetamine (MDMA) that produce long-term serotonergic alterations. Neurotoxicology. 2003;24:379–90. doi: 10.1016/S0161-813X(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA (‘Ecstasy’) Jama. 1987;258:780–1. [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (ecstasy): modulation by environmental conditions. Eur J Neurosci. 2004;20:51–8. doi: 10.1111/j.0953-816X.2004.03453.x. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carvalho F, Remiao F, de Lourdes Pereira M, Piresdas-Neves R, de Lourdes Bastos M. Effect of 3,4-methylenedioxymethamphetamine (“ecstasy”) on body temperature and liver antioxidant status in mice: influence of ambient temperature. Arch Toxicol. 2002;76:166–72. doi: 10.1007/s00204-002-0324-z. [DOI] [PubMed] [Google Scholar]
- Cheng WC, Poon NL, Chan MF. Chemical profiling of 3,4-methylenedioxymethamphetamine (MDMA) tablets seized in Hong Kong. J Forensic Sci. 2003;48:1249–59. [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL (1996) Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources, National Research Council, Washington D.C., pp 125
- Clingerman KJ, Summers L. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 2005;34:31–6. doi: 10.1038/laban0505-31. [DOI] [PubMed] [Google Scholar]
- Dafters RI. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) in rats. Psychopharmacology (Berl) 1994;114:505–8. doi: 10.1007/BF02249342. [DOI] [PubMed] [Google Scholar]
- Dams R, De Letter EA, Mortier KA, Cordonnier JA, Lambert WE, Piette MH, Van Calenbergh S, De Leenheer AP. Fatality due to combined use of the designer drugs MDMA and PMA: a distribution study. J Anal Toxicol. 2003;27:318–22. doi: 10.1093/jat/27.5.318. [DOI] [PubMed] [Google Scholar]
- Dowling GP, McDonough ET, 3rd, Bost RO. ‘Eve’ and ‘Ecstasy’. A report of five deaths associated with the use of MDEA and MDMA. Jama. 1987;257:1615–7. doi: 10.1001/jama.257.12.1615. [DOI] [PubMed] [Google Scholar]
- Elizondo RS, Smiles KA, Barney CC. Effects of hypothalamic temperature changes on the sweat rate in Macaca mulatta. Isr J Med Sci. 1976;12:1026–8. [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–11. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, de la Garza R, Woods JH (2005) Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology (Berl) in press [DOI] [PubMed]
- Fiege M, Wappler F, Weisshorn R, Gerbershagen MU, Menge M, Schulte Am Esch J. Induction of Malignant Hyperthermia in Susceptible Swine by 3,4-Methylenedioxymethamphetamine (“Ecstasy”) Anesthesiology. 2003;99:1132–1136. doi: 10.1097/00000542-200311000-00020. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Johanson CE, Tancer ME (2005) Thermoregulatory effects of 3,4-Methylenedioxymethamphetamine (MDMA) in Humans. Psychopharmacology (Berl) in press [DOI] [PubMed]
- Garcia-Repetto R, Moreno E, Soriano T, Jurado C, Gimenez MP, Menendez M. Tissue concentrations of MDMA and its metabolite MDA in three fatal cases of overdose. Forensic Sci Int. 2003;135:110–4. doi: 10.1016/s0379-0738(03)00179-8. [DOI] [PubMed] [Google Scholar]
- Gillman PK. Ecstasy, serotonin syndrome and the treatment of hyperpyrexia. Med J Aust 167. 1997;109:111. doi: 10.5694/j.1326-5377.1997.tb138798.x. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF. Methysergide potentiates the hyperactivity produced by MDMA in rats. Pharmacol Biochem Behav. 1988;29:645–8. doi: 10.1016/0091-3057(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Greene SL, Dargan PI, O’Connor N, Jones AL, Kerins M. Multiple toxicity from 3,4-methylenedioxymethamphetamine (“ecstasy”) Am J Emerg Med. 2003;21:121–4. doi: 10.1053/ajem.2003.50028. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Nabeshima T, Kameyama T, Maeda Y, Cho AK. The effect of optical isomers of 3,4-methylenedioxymethamphetamine (MDMA) on stereotyped behavior in rats. Pharmacol Biochem Behav. 1989;33:343–7. doi: 10.1016/0091-3057(89)90511-x. [DOI] [PubMed] [Google Scholar]
- Horn TF, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Early physiological abnormalities after simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1998;95:15072–7. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Battaglia G, Johannessen JN, Marra S, De Souza EB. 3,4-Methylenedioxymethamphetamine (“ecstasy”) selectively destroys brain serotonin terminals in rhesus monkeys. J Pharmacol Exp Ther. 1989;249:713–20. [PubMed] [Google Scholar]
- Johnson GS, Elizondo RS. Thermoregulation in Macaca mulatta: a thermal balance study. J Appl Physiol. 1979;46:268–77. doi: 10.1152/jappl.1979.46.2.268. [DOI] [PubMed] [Google Scholar]
- Karlovsek MZ. Illegal drugs-related fatalities in Slovenia. Forensic Sci Int. 2004;146(Suppl):S71–5. doi: 10.1016/j.forsciint.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Karlovsek MZ, Alibegovic A, Balazic J. Our experiences with fatal ecstasy abuse (two case reports) Forensic Sci Int. 2005;147(Suppl):S77–80. doi: 10.1016/j.forsciint.2004.09.084. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–94. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick A, Bodenham AR. MDMA induced hyperthermia: a survivor with an initial body temperature of 42.9 degrees C. J Accid Emerg Med. 1997;14:336–8. doi: 10.1136/emj.14.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol. 2002;135:170–80. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Mayerhofer A, Kovar KA, Schmidt WJ. Rewarding effects of the optical isomers of 3,4-methylenedioxymethylamphetamine (‘Ecstasy’) and 3,4-methylenedioxyethylamphetamine (‘Eve’) measured by conditioned place preference in rats. Neurosci Lett. 2002;330:280–4. doi: 10.1016/s0304-3940(02)00821-2. [DOI] [PubMed] [Google Scholar]
- Mills EM, Rusyniak DE, Sprague JE. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J Mol Med. 2004;82:787–99. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- NRC/NAS (2003) Nutrient Requirements of Nonhuman Primates: Second Revised Edition. National Research Council of The National Academy of Sciences, Washington D.D.
- Oddershede IR, Elizondo RS. Body fluid and hematologic adjustments during resting heat acclimation in rhesus monkey. J Appl Physiol. 1980;49:431–7. doi: 10.1152/jappl.1980.49.3.431. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Res. 2004;1014:34–44. doi: 10.1016/j.brainres.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Palhol F, Boyer S, Naulet N, Chabrillat M. Impurity profiling of seized MDMA tablets by capillary gas chromatography. Anal Bioanal Chem. 2002;374:274–81. doi: 10.1007/s00216-002-1477-6. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology (Berl) 2004;173:234–41. doi: 10.1007/s00213-003-1712-7. [DOI] [PubMed] [Google Scholar]
- Patel MM, Wright DW, Ratcliff JJ, Miller MA. Shedding new light on the “safe” club drug: methylenedioxymethamphetamine (ecstasy)-related fatalities. Acad Emerg Med. 2004;11 :208–10. [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–54. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW. (+/−)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. Jama. 1988;260:51–5. [PubMed] [Google Scholar]
- Rosa-Neto P, Olsen AK, Gjedde A, Watanabe H, Cumming P. MDMA-evoked changes in cerebral blood flow in living porcine brain: correlation with hyperthermia. Synapse. 2004;53:214–21. doi: 10.1002/syn.20052. [DOI] [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Colado MI, Green AR. Hyperthermic and neurotoxic effect of 3,4-methylenedioxymethamphetamine (MDMA) in guinea pigs. Psychopharmacology (Berl) 2004;173:452–3. doi: 10.1007/s00213-003-1653-1. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- Shulgin A, Shulgin A (2000) PiHKAL: A Chemical Love Story, First edn. Transform Press, Transform Press
- Smiles KA, Elizondo RS, Barney CC. Sweating responses during changes of hypothalamic temperature in the rhesus monkey. J Appl Physiol. 1976;40:653–7. doi: 10.1152/jappl.1976.40.5.653. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004a;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Erratum: “Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004b;82:589. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Yuan J, Schroeder R, Hatzidimitriou G, Parsons LH, Ricaurte GA, Gold LH. Cognitive performance of MDMA-treated rhesus monkeys: sensitivity to serotonergic challenge. Neuropsychopharmacology. 2002a;27:993–1005. doi: 10.1016/S0893-133X(02)00380-9. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Davis S, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. Functional consequences of repeated (+/−)3,4-methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neuropsychopharmacology. 2001;24:230–9. doi: 10.1016/S0893-133X(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002b;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer’s type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–33. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Takasu N, Nigi H, Tokura H. Effects of diurnal bright/dim light intensity on circadian core temperature and activity rhythms in the Japanese macaque. Jpn J Physiol. 2002;52:573–8. doi: 10.2170/jjphysiol.52.573. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: Acquisition and long-term performance. Cogn Brain Res. 1999;8:184–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]


