Abstract
The biological functions of heterotrimeric G proteins and small GTPases are modulated by both extracellular stimuli and intracellular regulatory proteins. Using Saccharomyces cerevisiae two-hybrid screening, we identified tetratricopeptide repeat 1 (TPR1), a 292-amino-acid protein with three TPR motifs, as a Gα16-binding protein. The interaction was confirmed both in vitro and in transfected mammalian cells, where TPR1 also binds to several other Gα proteins. TPR1 was found to interact with Ha-Ras preferentially in its active form. Overexpression of TPR1 promotes accumulation of active Ras. TPR1 was found to compete with the Ras-binding domain (RBD) of Raf-1 for binding to the active Ras, suggesting that it may also compete with Ras GTPase-activating protein, thus contributing to the accumulation of GTP-bound Ras. Expression of Gα16 strongly enhances the interaction between TPR1 and Ras. Removal of the TPR1 N-terminal 112 residues abolishes potentiation by Gα16 while maintaining the interaction with Gα16 and the ability to discriminate active Ras from wild-type Ras. We have also observed that LGN, a Gαi-interacting protein with seven TPR motifs, binds Ha-Ras. Thus, TPR1 is a novel adaptor protein for Ras and selected Gα proteins that may be involved in protein-protein interaction relating to G-protein signaling.
Heterotrimeric guanine nucleotide-binding proteins (G proteins) are essential for the transduction of signals from a large number of receptors with seven transmembrane domains. These G proteins are composed of α, β, and γ subunits. The α subunit is responsible for binding of guanine nucleotides (10). Activation of G proteins involves exchange of Gα-bound GDP for GTP, whereas inactivation requires the hydrolysis of GTP to GDP, a process catalyzed by the intrinsic GTPase activity. It has been well established that agonist-bound receptors serve as guanine nucleotide exchange factors (GEFs) that promote G-protein activation. In recent years, the interaction of G proteins with a number of proteins affecting their activation state has been documented. The regulators of G-protein signaling (RGS) interact with G proteins and possess intrinsic GTPase-activating protein (GAP) activity that can greatly accelerate GTP hydrolysis (7, 30). On the other hand, a newly defined class of activators of G proteins (AGS) facilitates G-protein activation independently of receptor stimulation (3). These findings demonstrate that, in addition to G-protein-coupled receptors (GPCRs), intracellular molecules are involved in the regulation of G-protein signaling.
The Ras-like monomeric G proteins, also called small GTPases, are structurally and functionally related to Gα subunits. The Rho family of small GTPases is widely known for regulating the actin cytoskeleton and activating transcription (13, 29). An extensive list of proteins that bind small GTPases has been compiled. Recently, the tetratricopeptide repeat (TPR) located in the amino terminus of p67phox has been shown to interact with Rho small GTPases Rac1 and Rac2. This interaction is essential for activation of the NADPH oxidase (17). TPR is a degenerate 34-amino-acid sequence with a widespread phylogenetic distribution (2, 31). TPR motifs are often arranged in tandem, and they mediate protein-protein and protein-lipid interactions. Published studies indicate that TPR-containing proteins play important roles in a variety of cellular processes ranging from transcription and cell division to protein folding and transport (11).
Ras is a prototypic small GTPase and is critical for bridging a variety of cell surface receptors to nuclear signaling events that promote cell proliferation and differentiation (21). Ras is activated by the GEFs sos and GRF or GRP (28). Rapid turnoff of Ras is accomplished by GAPs including p120-GAP and neurofibromin (22). Although heterotrimeric G proteins are known to activate Ras, current models cannot fully explain the underlying mechanism, suggesting that there may be additional regulators of Ras function.
The Gα proteins Gα12 and Gα13 are known to activate the small GTPase RhoA. In recent reports, RhoGEFs p115-RhoGEF and PDZ-RhoGEF were shown to interact with both the Gα12 or Gα13 subunits and RhoA, acting as a RGS for the Gα proteins and a GEF for RhoA (9, 14, 18). Since activation of many GPCRs stimulates the mitogen-activated protein (MAP) kinase cascade downstream of Ras (4, 15), it is possible that Gα proteins physically and functionally interact with Ras through an intermediate protein. However, no such adaptor or scaffold protein has been described for Gα proteins and Ras.
In an attempt to identify novel proteins that interact with Gα proteins of the Gq family, we examined a cDNA library by S. cerevisiae two-hybrid screening using Gα16 as bait. Tetratricopeptide repeat 1 (TPR1), a 292-amino-acid protein of unknown function that contains three TPR motifs, was isolated as a binding partner for Gα16. Upon further characterization, it was found that TPR1 interacts with Ras, preferably Ras-GTP. Overexpression of TPR1 promoted accumulation of active Ras and phosphorylation of ERK1/2 (p44/p42). Furthermore, Ras interaction with TPR1 and accumulation of active Ras were facilitated by expression of Gα16, suggesting a potential link between Gα proteins and Ras signaling through TPR1.
MATERIALS AND METHODS
S. cerevisiae two-hybrid screening.
The MATCHMAKER LexA Two-Hybrid system (Clontech) was used to detect specific interaction between Gα16 and the proteins encoded by a human brain cDNA library cloned in pB42AD. The Gα16QL cDNA (27) was used as a template for PCR, and the product was cloned into the XhoI site in the polylinker of the pLexA plasmid in frame with the LexA DNA-binding domain. Both pLexA-Gα16QL and the cDNA library were cotransformed into the LacZ/LEU2 S. cerevisiae reporter strain according to the manufacturer's protocol. The S. cerevisiae cells were assayed for reporter activities, and the cDNA insert from the positive colonies was sequenced.
RNA preparation and detection by reverse transcriptase PCR.
Total RNA was isolated from the cells by using TRIZOL reagent (Life Technologies), followed by the addition of chloroform. The aqueous phase was collected, and the RNA was precipitated with isopropanol. The mRNA was reverse transcribed using an oligo(dT) primer. PCR amplification of the Gα16 cDNA resulted in a 361-bp product, using the primer pair 5′-GCCCTCATCTACCTGGCCTC-3′ and 5′-TGTGGCACATGTGTAGTGGCTG-3′. TPR1 cDNA was amplified as a 435-bp fragment, using a pair of primers with the sequence 5′-GTCGAGCCCTCGAAATGTGC-3′ and 5′-CGAAATTGATGGAGTACGAGCC-3′. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene.
Cell culture and transient transfection.
The human embryonic kidney epithelial cell line HEK293T was maintained in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum. The human cervical carcinoma cell line HeLa was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. For coimmunoprecipitation and immunoblot analyses, HEK293T cells were grown at 60 to 80% confluence in 10-cm-diameter tissue culture dishes and transfected using Lipofectamine Plus (Life Technologies) according to the manufacturer's instructions. For the functional assays such as glutathione S-transferase (GST)-RBD pull-down and ERK phosphorylation, HeLa cells were transfected at 50% confluence. In all cases, the total amount of DNA per dish was normalized with an empty vector to 4 μg. The DNA was added to the cells in serum-free medium and incubated for 4 h before an equal volume of 20% serum-containing medium was added.
Immunoprecipitation and Western blotting.
Twenty-four hours after transfection, the cells were harvested on ice and lysed in 1 ml of buffer containing 50 mM Tris-HCl (pH 7.4), 200 mM NaCl, 2.5 mM MgCl2, 10% glycerol, 1% Igepal, 1 mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor cocktail set I (Calbiochem) by agitation for 20 min at 4°C. The cell lysates were cleared of debris by centrifugation at 10,000 × g for 10 min at 4°C. For control of protein expression, 60-μl portions of the homogenates were mixed with 10 μl of 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and boiled for 5 min before loading on the gel. For immunoprecipitation studies, 800 μl of the lysate was incubated for 1 h at 4°C with 20 μl of the anti-FLAG M2 affinity gel (Sigma). The beads were washed twice with 1 ml of lysis buffer and twice with phosphate-buffered saline. The beads were resuspended in 50 μl of 2× SDS-PAGE loading buffer and boiled for 5 min to release bound proteins. The samples were resolved by Western blotting.
The proteins were usually electrophoresed on an SDS-10% polyacrylamide gel and then transferred to a nitrocellulose membrane (Schleicher & Schuell). The blots were blocked with 5% nonfat dry milk in TBS/T buffer (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 0.1% Tween 20) for 2 h at room temperature. After the membranes were washed three times with TBS/T for 5 min each time, they were incubated with primary antibodies (1 μg/ml for monoclonal antibody [MAb] and 1:1,000 for antiserum) overnight at 4°C. The peroxidase-conjugated anti-rabbit (Bio-Rad) or anti-mouse (Calbiochem) secondary antibodies were added to the membranes at a 1:3,000 dilution for 1 h at room temperature. The bands on the blots were visualized by chemiluminescence (Pierce).
Preparation of the GST fusion proteins and pull-down assay.
pGEX-2T and pGEX-TPR1, encoding GST and GST-TPR1 fusion protein, respectively, were made and introduced into Escherichia coli strain DH10B. Similarly, the pGEX-Raf(1-140) RBD construct (kindly provided by L. A. Quilliam) encoding amino acids 1 to 140 of c-Raf-1 fused to GST was also transformed in E. coli. Bacteria were grown in 500 ml of Luria-Bertani medium, and protein expression was induced with 0.2 mM isopropyl-1-thio-β-d-galactopyranoside for 2 h at 37°C. The bacterial pellet was resuspended in 10 ml of buffer containing 20 mM Tris-HCl (pH 8.0), 500 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor cocktail set II (Calbiochem) and sonicated four times on ice. After centrifugation at 10,000 rpm in a Sorvall HB-6 rotor for 10 min at 4°C, the supernatant was snap-frozen for storage in 10% glycerol. When needed, 5 ml of bacterial lysate was incubated with 750 μl of 50% (vol/vol) slurry of glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) for 2 h at 4°C. The beads were washed three times in 10 ml of washing buffer containing 20 mM Tris-HCl (pH 8.0), 50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 20% glycerol, and 1 mM phenylmethylsulfonyl fluoride and resuspended in 375 μl of washing buffer. For pull-down assays, the cells were transfected and lysed essentially as described above, and 500 μl of clear cell lysate was incubated with 15 μl of GST-, GST-TPR1-, or GST-RBD-coupled beads and agitated for 1 h at 4°C. The beads were subsequently washed three times in cell lysis buffer and twice in phosphate-buffered saline before being resuspended in 50 μl of 2× SDS-PAGE loading buffer and boiled for 5 min prior to electrophoretic analysis.
In vitro binding assay.
The cDNAs coding for wild-type Gα16 (Gα16wt) and Gα16QL were cloned into the pBluescript SK(+) vector (Stratagene) and used for in vitro translation. Briefly, labeled Gα16 was made with the T7 RNA polymerase-coupled transcription/rabbit reticulocyte translation system (Promega) in the presence of [35S]methionine (NEN Life Science Products) following the manufacturer's instructions. Equal amounts of [35S]methionine-labeled Gα16wt and Gα16QL were incubated with equivalent amounts of either GST or GST-TPR1 fusion protein precoupled to the glutathione-Sepharose 4B beads. The binding reaction was performed in cell lysis buffer (described above) overnight at 4°C. The beads were centrifuged to recover the unbound proteins and washed several times, and the bound proteins were eluted in 50 μl of 5× SDS-PAGE loading buffer. The samples were analyzed by SDS-PAGE and autoradiography.
RESULTS
Selective interaction between TPR1 and Gα proteins.
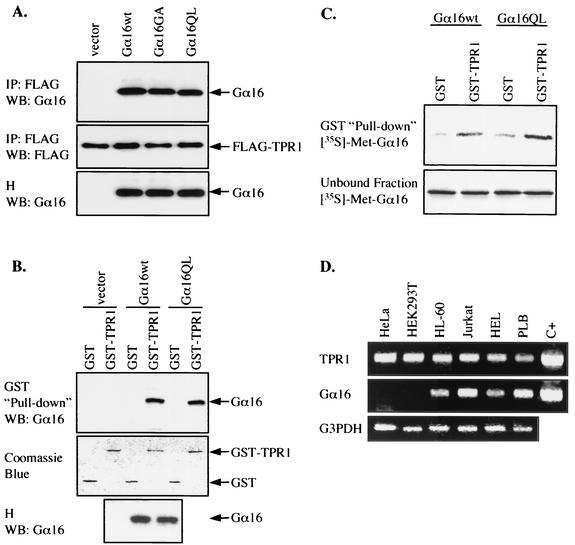
To identify proteins that interact with G proteins of the Gq family, a GTPase-deficient (constitutively active) mutant, Gα16 (Q212L), was fused to the LexA DNA-binding domain and used as bait for an S. cerevisiae two-hybrid screening. Several positive clones were isolated from a human brain cDNA library fused to the B42 activation domain, which activates transcription when brought in proximity to the LexA DNA-binding domain. Upon DNA sequencing, one clone was found to encode TPR1, a 292-residue protein of unknown function that contains three TPR motifs (24). To confirm that TPR1 interacts with Gα16 in mammalian cells, an N-terminal FLAG-tagged TPR1 (FLAG-TPR1) expression construct was prepared. This vector was cotransfected with a Gα16 expression construct into HEK293T cells devoid of endogenous Gα16 (Fig. 1D). We also examined Gα16QL and Gα16GA, the active and inactive mutants of Gα16, respectively. Gα16QL is known for its ability to mediate NF-κB luciferase reporter expression through accumulation of inositol phosphates (34). Twenty-four hours after transfection, the tagged TPR1 was immunoprecipitated from the cell lysates with an anti-FLAG MAb. The precipitates were then analyzed by Western blotting using an anti-Gα16 Ab. As shown in Fig. 1A, Gα16 was detected in the immunoprecipitates from cells transfected with Gα16 but was not found in mock (vector)-transfected cells. TPR1 interacted equally well with the GTPase-deficient Gα16QL with the Gln212-to-Leu mutation and with Gα16GA with the Gly211-to-Ala mutation, a mutation in the contact site with the βγ complex that abolishes GTP binding to Gα and subsequent dissociation of βγ (Fig. 1A). Taken together, these observations suggest that TPR1 bind to Gα16 regardless of its activation state.
FIG. 1.
Interaction between Gα16 and TPR1. (A) HEK293T cells were transiently transfected with the FLAG-TPR1 construct either alone or with expression vectors for Gα16wt, Gα16GA (inactive mutant), and Gα16QL (active mutant). Whole-cell lysates were immunoprecipitated with an anti-FLAG agarose affinity gel. The immunoprecipitates (IP) and cell homogenates (H) were resolved by SDS-PAGE (10% polyacrylamide). Coimmunoprecipitation of Gα16 with FLAG-TPR1 was examined by Western blotting (WB) with anti-Gα16 serum (top panel). FLAG-TPR1 in the IP was detected with an anti-FLAG MAb and serves as a loading control (middle panel). The blot was probed for the expression of Gα16 in the cell homogenates (bottom panel). In the absence of FLAG-TPR1, no Gα16 is detectable in the IP fraction (not shown). (B) Cells were transfected with empty vector or with expression vectors for Gα16wt or Gα16QL. Whole-cell lysates were incubated with GST (negative control) or GST-TPR1 fusion protein immobilized on glutathione-Sepharose beads. Bound proteins were eluted and analyzed by Western blotting. The ability of Gα16 to interact with GST or GST-TPR1 was determined by blotting the eluate with anti-Gα16 (top panel). The expression of Gα16 in the homogenates was assessed as in panel A (bottom panel). GST and GST-TPR1 were detected by staining with Coomassie blue (middle panel) and served as loading controls. (C) Equivalent amounts of GST or GST-TPR1, immobilized on glutathione-Sepharose beads, were incubated with in vitro-translated [35S]methionine-labeled Gα16wt or Gα16QL. After the beads were pelleted, both the unbound proteins recovered from the supernatants (bottom panel) and the bound proteins (top panel) were subjected to SDS-PAGE and autoradiography. (D) Total RNA was isolated from the human cervical carcinoma cell line HeLa, kidney epithelial cell line HEK293T, promyelocytic cell line HL-60, leukemic T-cell line Jurkat, erythroleukemic cell line HEL, and myelomonoblastic cell line PLB 985. The expression of TPR1 (top panel) and Gα16 (middle panel) mRNAs was detected by reverse transcriptase PCR using specific primers. In parallel, 1 ng of DNA encoding TPR1 or Gα16 was used as a positive control (C+) for the PCR. Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) expression serves as PCR and loading controls (bottom panel). All results presented are representative of at least three independent experiments.
The interaction between TPR1 and Gα16 was verified using a pull-down assay in which TPR1 was fused to the C terminus of GST. As shown in Fig. 1B, the GST-TPR1 fusion protein, but not the GST control protein, successfully pulled down Gα16 from the transfected HEK293T cell lysates. Both Gα16wt and Gα16QL interacted with GST-TPR1 equally well. Furthermore, Gα16wt and Gα16QL prepared by in vitro translation in the presence of [35S]methionine were able to associate with GST-TPR1 (Fig. 1C). These results suggest direct interaction between Gα16 and TPR1.
We next investigated whether the cells used in our study contain endogenous TPR1. The transcript for TPR1 was present in all six cell lines tested (Fig. 1D). In comparison, the Gα16 transcript was detected only in the four hematopoietic cell types, not in the epithelial cell lines HeLa and HEK293T. These observations are consistent with previous studies reporting that TPR1 is ubiquitously expressed (24), whereas the distribution of Gα16 is restricted to hematopoietic cells (1). The coexpression of these two proteins in the same cell implies a potential role of TPR1 in Gα16 signaling.
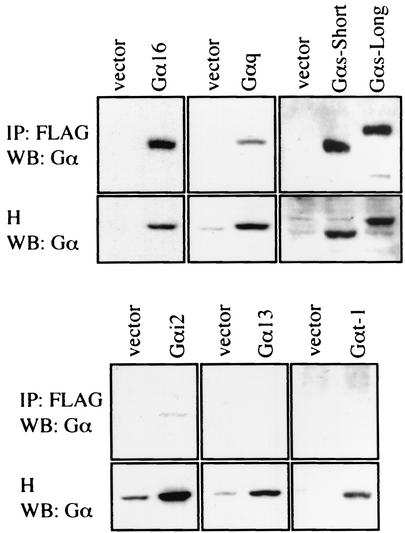
There are four classes of Gα proteins, namely, Gs, Gi, Gq, and G12 (32). We examined whether TPR1 interacts with Gα proteins other than Gα16. The expression constructs for Gαq, Gαi2, Gα13 (a member of the G12 family), Gαt-1 (a member of the Gi family), and both the long and short forms of Gαs were individually transfected into HEK293T cells together with FLAG-TPR1. After immunoprecipitation with an anti-FLAG MAb, Western blotting was conducted with antibodies against these Gα subunits. It was found that TPR1 also interacts with Gαq, with both the short and long isoforms of Gαs, and to a much lesser extent with Gαi2. However, TPR1 did not interact with either Gα13 or Gαt-1 (Fig. 2), indicating that its association with G proteins is selective.
FIG. 2.
Interactions between TPR1 and selected Gα proteins. HEK293T cells were transiently transfected with the FLAG-TPR1 construct either alone or with individual expression vectors encoding Gα16wt, Gαqwt, the short and long forms of Gαswt, Gαi2wt, Gα13wt, and Gαt-1wt. The cell homogenates were subjected to immunoprecipitation with an anti-FLAG agarose affinity gel and analyzed by Western blotting. The Western blot (WB) was probed for the respective Gα protein contents in the immunoprecipitates (IP) (top panels) and homogenates (H) (bottom panels). An equal amount of FLAG-TPR1 protein was precipitated in each sample (not shown). Data shown are representative of at least three independent experiments.
TPR1 interacts with Ha-Ras.
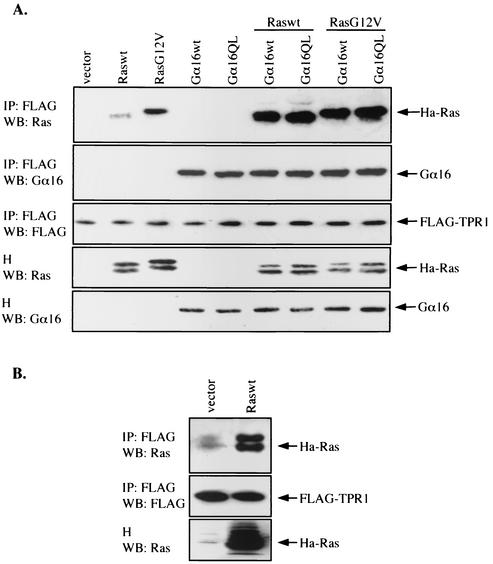
The N-terminal region of the NADPH oxidase component p67phox contains four TPR motifs that interact with the small GTPases Rac1 and Rac2 (17, 20). We therefore tested whether TPR1, with three TPR motifs, can also interact with Rac. However, results from immunoprecipitation and Western blotting experiments indicate that Rac1 and its GTPase-deficient mutant (Q61L) do not bind to TPR1. Similarly, neither Cdc42 nor RhoA were found to interact with TPR1 (data not shown). In contrast, the constitutively active mutant of Ha-Ras (G12V), and to a lesser extent, the wild-type Ha-Ras, coimmunoprecipitated with the FLAG-tagged TPR1 (Fig. 3A). The same result was obtained with a hemagglutinin-tagged TPR1 and in pull-down assays with GST-TPR1 (data not shown), thus excluding the possibility that RasG12V interacts with the FLAG tag attached to the N terminus of TPR1. As expected, the endogenous Ras was confirmed to bind to the TPR1 construct using coimmunoprecipitation and Western blotting analysis (Fig. 3B).
FIG. 3.
Interaction of Ha-Ras with TPR1 and facilitation of the interaction by Gα16. (A) HEK293T cells were transiently transfected with the FLAG-TPR1 construct either alone or in combination with expression vectors for Ras and/or Gα16 as indicated. The cell homogenates were subjected to immunoprecipitation with an anti-FLAG agarose affinity gel and analyzed by Western blotting. Total FLAG-TPR1 in the immunoprecipitates (IP) was shown by probing the Western blot (WB) with an anti-FLAG MAb (middle panel). The IP (top two panels) and cell homogenates (H) (bottom two panels) were probed with anti-Ras and anti-Gα16 sera to assay for the abilities of Ras and Gα16 to coimmunoprecipitate with FLAG-TPR1 and for their expression in the cells. Results are representative of at least three independent experiments. (B) FLAG-TPR1 was transiently expressed in HEK293T cells and used for coimmunoprecipitation (IP) of Ras with or without exogenous Raswt. Samples were analyzed by Western blotting.
Since Gα16 binds to TPR1, we tested whether Gα16 interferes with TPR1 binding to Ras. The two molecules were coexpressed in HEK293T cells together with FLAG-TPR1. Surprisingly, either the wild-type Gα16 or the GTPase-deficient mutant of Gα16 could facilitate binding of RasG12V to TPR1 (Fig. 3A, top panel). Moreover, the wild-type Ras (Raswt), which binds poorly to TPR1, could interact with TPR1 to the same degree as RasG12V in the presence of Gα16. In contrast, the interaction between Gα16 and TPR1 was not affected by expression of either Raswt or RasG12V (Fig. 3A, second panel).
Structural requirements for TPR1 interaction with Gα16 and Ha-Ras.
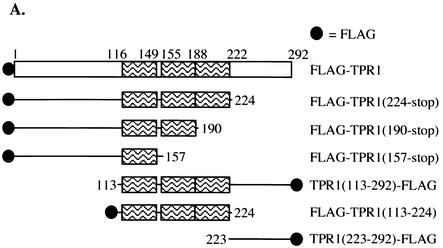
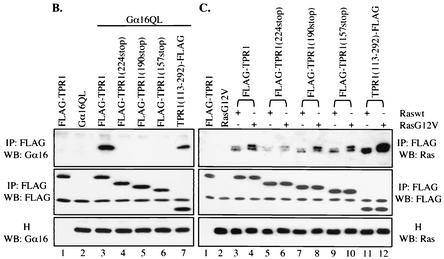
TPR1 contains three segments: an N-terminal segment (amino acids 1 to 115), a central portion with three TPR motifs (amino acids 116 to 222), and a C-terminal segment (amino acids 223 to 292). To identify structures required for the interaction with Gα16 and Ras, three C-terminal progressive deletion mutants were generated (Fig. 4A). In addition, an N-terminal deletion mutant was also prepared. These mutants were tagged with FLAG and individually transfected into HEK293T cells along with either Gα16QL (Fig. 4B) or Ha-Ras (Fig. 4C). In immunoprecipitation and Western blotting experiments, none of the three C-terminal deletion mutants retained interaction with Gα16 (Fig. 4B), suggesting that the C-terminal segment is essential for Gα16 binding. This notion was supported by the observed interaction between Gα16QL and the N-terminal deletion mutant of TPR1, although the interaction was not as strong as with the wild-type TPR1 (Fig. 4B, lane 7 versus lane 3).
FIG. 4.
Identification of TPR1 structures required for Gα16 and Ha-Ras binding. (A) Schematic representation of the human TPR1 with the three TPR motifs (amino acids 116 to 149, 155 to 188, and 189 to 222) shown as boxes. Three C-terminal progressive deletion mutants of TPR1 and an N-terminal deletion mutant were generated. Two additional mutants, comprising either the TPR-containing domain alone or the C-terminal 70 residues of TPR1 were also prepared. All the deletion mutants were tagged with FLAG. The full-length TPR1 and the mutants were overexpressed in HEK293T cells with Gα16QL (B) or Raswt or RasG12V (C). In both cases, the cell lysates were precipitated with anti-FLAG agarose affinity gels. Western blotting (WB) analysis of the immunoprecipitates (IP) with the anti-FLAG Ab identified the expression of the various TPR1 constructs, which serve as loading controls (middle panel). The abilities of the various mutants to retain Gα16 or Ras binding were assessed by blotting the immunoprecipitates with anti-Gα16 or anti-Ras (top panel). Equal levels of Gα16QL, Raswt, and RasG12V expression were demonstrated by probing the homogenates (H) with the appropriate Ab (bottom panel). The results shown are representative of at least three independent experiments.
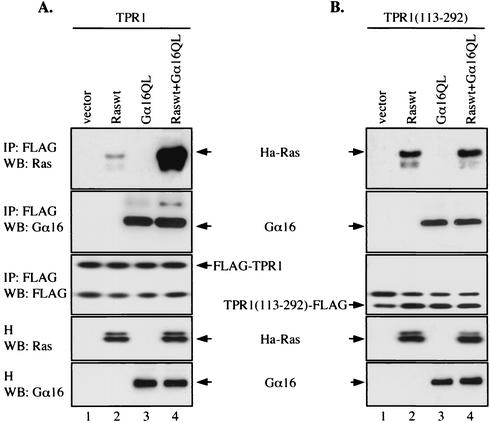
A different pattern of TPR1 interaction was observed with Ras (Fig. 4C). The deletion of the C-terminal fragment alone (224-stop) markedly reduced its interaction with Ras. However, further deletion into the TPR motifs partially restored Ras binding. More interestingly, removal of the N-terminal segment noticeably enhanced the interaction between the resultant protein, TPR1 with amino acids 113 to 292 TPR1(113-292) and Ras, but the characteristic difference in binding Raswt versus RasG12V remained (Fig. 4C, lanes 11 and 12 versus lanes 3 and 4). This latter finding suggests that the N-terminal domain of TPR1 might be folded in a manner that impedes Ras binding, raising the possibility that promotion of Ras binding by Gα16 involves this domain. Indeed, even though TPR1(113-292) binds better to Raswt than the full-length TPR1 does, this interaction cannot be further enhanced by Gα16QL in the absence of the N-terminal segment (Fig. 5).
FIG. 5.
Implication of the TPR1 N terminus in Gα16-dependent enhancement of TPR1-Ras interaction. The full-length TPR1 (A) or the N-terminal deletion mutant (residues 113 to 292 deleted) (B) were transiently transfected in HEK293T cells, together with expression vectors coding for either Raswt or Gα16QL or both, as indicated. Immunoprecipitation (IP) was performed with anti-FLAG agarose affinity gels before Western blotting (WB) with anti-Ras (top panels), anti-Gα16 (second panels from top), and anti-FLAG (third panels from top) antibodies. Expression of Ras and Gα16 constructs was verified in the homogenates (H) (two bottom panels).
Our results indicate that the C-terminal portion of TPR1 is required for Gα16 binding. To determine whether this fragment (70 residues) is also sufficient for Gα16 interaction, we prepared a FLAG-tagged TPR1(223-292) and a GST fusion protein of TPR1(223-292). The expression of FLAG-tagged TPR1(223-292) in cells was unsuccessful, probably due to its small size. We detected no binding between the purified GST-TPR1(223-292) protein and Gα16 (data not shown). Likewise, the GST-TPR1(223-292) protein did not bind to Ras. TPR1(113-224), which contains the three TPR motifs, did not retain any binding to Ras either (data not shown). Taken together, our data suggest that the last 70 residues of TPR1 are essential for the maintenance of a structure necessary for the interaction with Gα16 and Ras, but this fragment alone might be insufficient for binding of Gα16 or Ras.
Potential role of TPR1 in Ras activation.
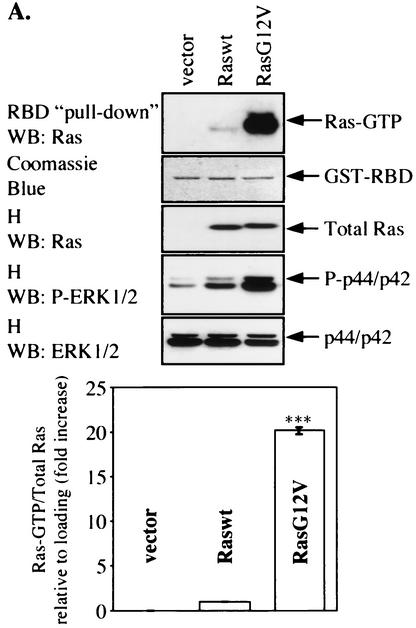
We investigated whether the expression of TPR1 affects Ras activation. The assay used to detect GTP-bound Ras is based on a previous finding that active Ras binds efficiently to Raf-1 (6). A pull-down assay with the RBD of Raf-1 fused to GST was performed to confirm that GST-RBD binds strongly to RasG12V (Fig. 6A) but weakly to Raswt (Fig. 6A) in both HeLa cells (Fig. 6A) and HEK293T cells (data not shown). The results of densitometry and statistical analyses of data obtained from HeLa cells transiently expressing either RasG12V or Raswt indicate a 20-fold (±0.36-fold) difference in their binding to GST-RBD.
FIG. 6.
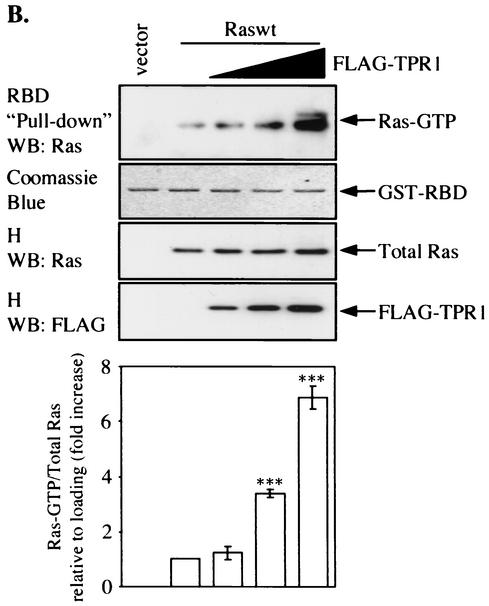
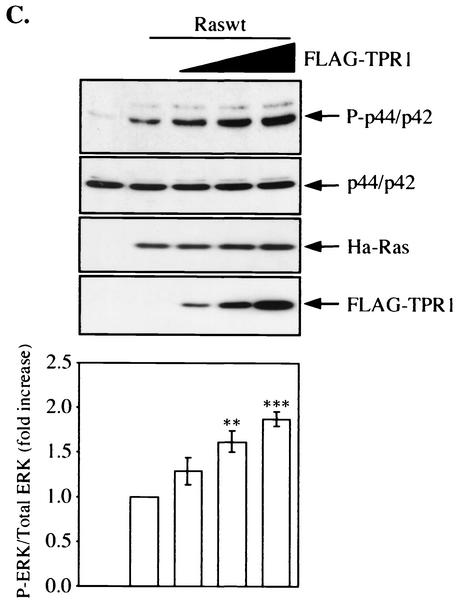
Involvement of TPR1 in accumulation of active Ras and phosphorylation of ERKs. (A) HeLa cells were transiently transfected with empty vector or with the Raswt or RasG12V expression construct. The cells were serum starved for 24 h, and Ras activity was measured using the RBD of Raf-1. The cell lysates were precipitated with the GST-RBD fusion protein coupled to glutathione-Sepharose beads. The bound Ras-GTP was detected in the precipitates by Western blotting (WB) using an anti-Ras Ab (top panel). The GST-RBD fusion protein was detected by Coomassie blue staining (second panel from top) and serves as a loading control. The total amount of Ras protein in each sample before GST-RBD binding was determined by probing the cell homogenates (H) with an anti-Ras Ab (middle panel). The homogenates were also assayed for any changes in the phosphorylation of the intrinsic ERK1/2 (P-ERK1/2) (second panel from bottom) and compared to the nonphosphorylated form of the two kinase isoforms (bottom panel). (B) Raswt was expressed alone (second lane from the left) or together with increasing amounts of FLAG-TPR1 in HeLa cells. As in panel A, after a 24-h serum starvation period, active Ras (top panel) was precipitated from the cell homogenates (H) using the GST-RBD affinity reagent. (C) The TPR1-dependent ERK1/2 phosphorylation was analyzed in cells transfected under the same conditions. Results shown are representative of at least three independent experiments. Quantitative analyses of all blots were performed using the ImageQuant program (Molecular Dynamics). For each individual experiment, values for the relative amounts of Ras-GTP were calculated as fold increase compared to the basal level Raswt condition, which was assigned a value of 1. The bars in the histograms are the means ± standard errors of the means for three experiments (n = 3). Values that were significantly different from the basal level are indicated (**, P < 0.01; ***, P < 0.001).
Expression of FLAG-TPR1 enhanced association of Ras with GST-RBD in a dose-dependent manner (Fig. 6B). The ratio of activated Ras to total Ras increased from 1.23- to 3.38- and 6.87-fold (with FLAG-TPR1 DNA input of 0.5, 1, and 3 μg per transfection, respectively) over the basal level (with no FLAG-TPR1). One of the downstream effectors of the Ras-Raf-1 pathway is the MAP kinase ERK1/2 (p44/p42), and Ras activation is known to stimulate the phosphorylation of ERK1/2. Using an anti-phospho-ERK antibody, we observed that the expression of FLAG-TPR1 also stimulated the phosphorylation of ERKs (Fig. 6C).
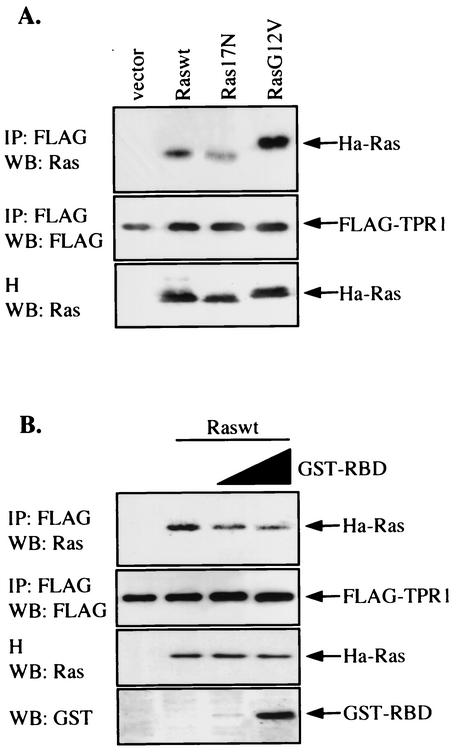
The above findings suggest that TPR1 can induce the accumulation of active Ras. This may result from stimulation of Ras activation or stabilization of Ras in an active conformation by TPR1. To test these possibilities, we first conducted an in vitro Ras activation assay with the nonhydrolyzable GTP analogue, guanosine 5′-O-(3-thiotriphosphate) (GTP-γ-S). GST-Ras fusion protein was purified from E. coli lysate, dialyzed, and loaded with cold GDP. The GDP-bound Ras was then incubated with either purified GST-TPR1 or concentrated lysates from TPR1-transfected HEK293T cells in the presence of GTP-γ-[35S]. We observed no difference in GTP-γ-S binding between assays with recombinant TPR1 and assays without TPR1 (data not shown), evidence against TPR1 acting as a GEF for Ras. Moreover, we tested TPR1 binding to a dominant-negative Ras, Ras17N (Ras with T17N mutation). This mutant functions by forming stable and inactive complexes with Ras GEFs, thus preventing activation of endogenous Ras. Ras17N has been described to bind more tightly to Ras GEFs than does wild-type Ras (8). The Ras17N construct was coexpressed with FLAG-TPR1. After selective immunoprecipitation of TPR1 from the cell lysate, we observed a much lower level of binding with Ras17N than with Raswt (Fig. 7A). These results do not support the role of TPR1 as a Ras GEF.
FIG. 7.
Interactions between Ras-TPR1 and Ras-Raf-1 RBD. (A) HeLa cells were transiently transfected with the FLAG-TPR1 construct either alone or in combination with expression vectors for the three forms of Ras. The FLAG-TPR1 protein was then immunoprecipitated (IP) from the cell lysates with an anti-FLAG agarose affinity gel. The Ras that was associated with FLAG-TPR1 was detected by Western blotting (WB) with an anti-Ras Ab (top panel). Ras17N exhibited reduced ability to bind TPR1. H, cell homogenates. (B) HeLa cells were transfected similarly as in panel A but with Raswt only. Cell lysates were prepared and preincubated for 30 min at 4°C with two different concentrations of purified GST-RBD derived from Raf-1, which were also present during immunoprecipitation of TPR1. The final reaction mixture contains 1.5 μg of cell lysates per μl and 6 and 27 ng of GST-RBD per μl. The Ras associated with FLAG-TPR1 was detected by Western blotting (top panel) and found to decrease with increasing concentrations of GST-RBD (bottom panel). Data shown are representative of at least three independent experiments.
We next examined whether TPR1 helps to stabilize active Ras by interacting with regions (switch I and II) that also contain binding sites for the Ras effectors (such as Raf-1) and regulators (such as Ras-GAPs). Indeed, the Ras-binding domain of Raf-1 (GST-RBD) could reduce the interaction between FLAG-TPR1 and Ras (Fig. 7B), suggesting that the Ras-binding site for TPR1 overlaps at least in part with its binding site for Raf-1. Taken together, these results suggest that stabilization by TPR1 contributes to the accumulation of active Ras.
Effect of Gα16 on TPR1-dependent accumulation of Ras-GTP.
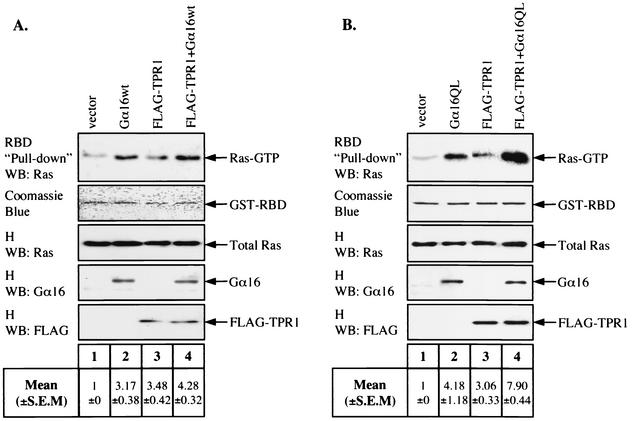
Since expression of Gα16 can increase the recruitment of Ras to TPR1 (Fig. 3A), we examined whether Gα16 can further enhance accumulation of active Ras. Using GST-RBD pull-down assay, we first observed that Gα16 could enhance Ras-GTP accumulation in the absence of FLAG-TPR1 (Fig. 8A, lane 2 versus lane 1). Interestingly, in the presence of FLAG-TPR1, the active Gα16QL was clearly more efficient in potentiating Ras-GTP accumulation than Gα16wt (a 2.58-fold increase for Gα16QL versus a 1.23-fold increase for Gα16wt; Fig. 8A and B, lanes 4 versus lanes 3).
FIG. 8.
Gα16 expression increases accumulation of active Ras. (A) HeLa cells were transfected with plasmids expressing Raswt and either empty vector, Gα16wt, FLAG-TPR1, or both FLAG-TPR1 and Gα16wt combined. After 24 h of serum starvation, active Ras was detected from cell lysates using GST-RBD coupled to beads. The samples were Western blotted (WB) with an anti-Ras Ab (top panel), and the GST-RBD protein was shown by staining with Coomassie blue (second panel from top) and serves as a loading control. Total cell lysates (homogenate [H]) were probed with the appropriate Ab to detect the relative levels of the expressed Ras, Gα16, and FLAG-TPR1 (three bottom panels). (B) The same experiment was performed with Gα16QL instead of Gα16wt. Blots shown are representative of a typical experiment. Quantitative analyses of blots were performed using the ImageQuant program. For each individual experiment, values for the relative amounts of Ras-GTP were calculated as fold increase compared to the basal level. The mean values ± standard errors of the means (S.E.M.) from three independent experiments (n = 3) are shown below the blots.
We have shown that deletion of the N-terminal portion of TPR1 allows for better interaction with Ras (Fig. 4C) but abolishes regulation by Gα16 (Fig. 5). When examined for its functional effect on Ras, the TPR1(113-292) truncation mutant could indeed promote the accumulation of active Ras. Consistent with the Ras binding data shown in Fig. 4C, the N-terminal deletion mutant slightly increased accumulation of active Ras (by approximately 25%) compared to full-length TPR1 (data not shown).
A TPR-containing protein, LGN, interacts with Ras.
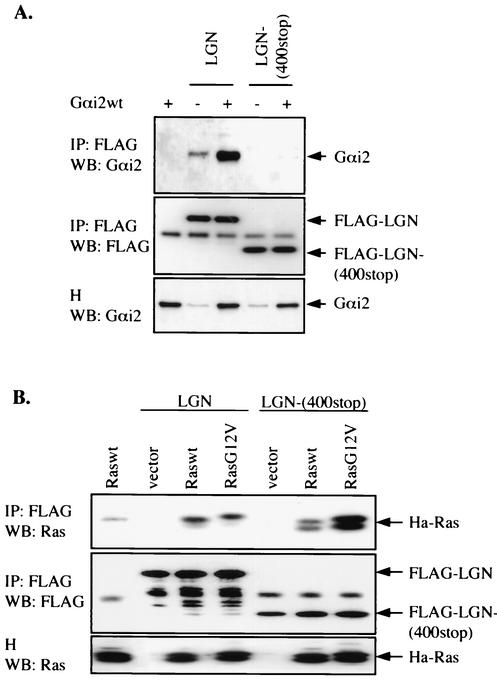
To determine whether the ability to interact with Ras is unique to TPR1, we examined another protein that contains TPR motifs. The human mosaic protein LGN was originally isolated by S. cerevisiae two-hybrid screening using Gαi2 as bait (23). LGN protein and its rat homologue AGS3 contain an N-terminal domain with clustered TPR motifs and a C-terminal domain with G-protein regulatory motifs (also called GoLoco motifs). LGN protein has been characterized for the regulation of Gαi signaling. It is believed that this function is mediated through the G-protein regulatory motifs (25, 26), whereas the role of the seven TPR motifs that are enriched with the sequence of leucine-glycine-asparagine (LGN) remains unknown. We transiently transfected a FLAG-tagged construct of the LGN protein with either Ras or Gαi2 into HEK293T cells. After immunoprecipitation with an anti-FLAG MAb, the presence of Ras and Gαi2 in the immunoprecipitates was determined using the appropriate antibody. Gαi2 was found to interact with the full-length LGN protein. As expected, it did not interact with a truncated LGN protein with only the N-terminal 400 amino acids (Fig. 9A). In comparison, both the full-length LGN protein and the truncated LGN protein bound to Raswt and RasG12V, suggesting that the N-terminal fragment, which contains the TPR motifs, is essential for interaction with Ras (Fig. 9B). It was also noted that the truncated LGN protein interacts better with RasG12V than with Raswt, a property similar to that of TPR1.
FIG. 9.
Interaction of Ha-Ras with the TPR-containing LGN protein. (A) Interaction between LGN and Gαi2 was confirmed in HEK293T cells that transiently expressed a FLAG-tagged full-length LGN protein or a C-terminal truncation mutant of LGN protein (400-stop). Cell lysates were subjected to immunoprecipitation using an anti-FLAG agarose affinity gel, and the immunoprecipitates (IP) were analyzed for Gαi2 by Western blotting (WB). (B) Same experiment as in panel A but with Raswt and RasG12V instead of Gαi2. The LGN-associated Ras was determined by Western blotting using an anti-Ras Ab (top panel). The FLAG-tagged proteins in the IP were shown with an anti-FLAG Ab and serve as loading controls (middle panels). Blots shown are representative of three experiments.
DISCUSSION
In this study, we have identified TPR1 as a direct Gα16-binding partner and reported in addition that it can bind with specificity to other Gα proteins. TPR1 was previously isolated in an S. cerevisiae two-hybrid screen using a truncated mutant of the GAP-related domain of neurofibromin as bait (24). The structure of TPR1 consists of unique amino- and carboxy-terminal domains of approximately 100 amino acids and three clustered TPR motifs (24). The TPR motif was originally identified in the S. cerevisiae cell division cycle protein Cdc23p (31) and in the Schizosaccharomyces pombe nuclear protein nuc2+ (16). It has since been found in organisms ranging from cyanobacteria to humans (2, 19). TPR motifs are usually present in tandem arrays of 3 to 16 units, and the proteins harboring TPR motifs are important for basic cellular functions, including DNA replication, transcriptional control, cell division, protein chaperoning, and mitochondrial and peroxisomal protein transport (reviewed in reference 2). TPR motifs generally mediate protein-protein interactions. The finding that TPR motif-containing proteins can selectively interact with Gα proteins suggests a potential function of these proteins in mediating G-protein signaling.
The detailed structural determinants for the direct interaction of TPR1 with various G proteins have not been identified. We have consistently observed the interaction of TPR1 with Gα16 using several approaches including S. cerevisiae two-hybrid screening, GST-TPR1 binding, coimmunoprecipitation, and in vitro binding assay with purified components. Our data support a direct association between TPR1 and Gα16. Analysis of the deletion mutants of TPR1 has revealed that the C-terminal 70 amino acids are required for TPR1 interaction with Gα16. This fragment, however, is not sufficient for Gα16 association when expressed as a GST fusion protein. Since TPR1 is a relatively small protein (292 amino acids), deleting the C-terminal 70 residues could possibly affect the overall structure of the protein, which may contain other sites or determinants for Gα16 binding. While this manuscript was being revised, Yamaguchi et al. reported that Gα12 and Gα13 can interact with the serine/threonine protein phosphatase type 5 (PP5), which contains a stretch of TPR motifs in its amino terminus (33). Their study provides evidence that the expression of the TPR motifs is sufficient for Gα12 or Gα13 binding. However, it was not determined whether the interaction between PP5 and Gα12 or Gα13 is direct. Both Gα12wt and Gα12QL interact with PP5, although Gα12wt does not stimulate PP5 activity as Gα12QL does. Likewise, we have shown TPR1 interaction with both Gα16wt and Gα16QL, indicating that the activation status of these G proteins is not a critical determinant for their interactions with TPR1. Interestingly, Gα13, a member of the Gα12 class of G proteins, does not bind TPR1 in our study. Although Gαq and Gαi2 interact with TPR1, they do not bind to PP5 (33). Information extracted from these two studies (this study and that of Yamaguchi et al. 33) suggest that TPR motif-containing proteins can discriminate between different G proteins.
Using coimmunoprecipitation and Western blotting analysis, we observed an interaction between TPR1 and Ha-Ras. Furthermore, we have found that TPR1 binds better to the active Ras than Raswt. This is the second reported case of interactions between a TPR motif-containing protein and a small GTPase. It was previously reported that the NADPH oxidase component p67phox can bind the small GTPases Rac1 and Rac2 through its TPR motifs (17). Notably, the TPR motifs in p67phox recognize Rac in its GTP-bound form but not in the GDP-bound form. This feature is similar to that of TPR1. Taken together, these observations suggest that the TPR motif-containing proteins may interact differently with small GTPases than with Gα subunits of heterotrimeric G proteins. We have further expanded our study to another TPR-containing protein, the human mosaic protein LGN protein (23). LGN protein interacts with Gαi through the GoLoco motifs located in its carboxyl-terminal domain and stabilizes Gαi in the GDP-bound state. We show that Ras can interact with LGN protein and that a carboxyl-terminal truncation mutant lacking the GoLoco motifs but containing the integral TPR domain retains the ability to bind to Ras. We investigated the possible presence of a common stretch of amino acids between the three TPR-containing proteins (TPR1, LGN, and p67phox) that might define their binding properties to small GTPases. However, as expected from p67phox in which a complex structure serves as the determinant for Rac binding (5, 12, 20), the alignment performed with these three proteins and more precisely with the isolated TPR domains was inconclusive and yielded no significant similarity at the primary structure level. We speculate that TPR motif may be the determinant for binding small GTPases, but optimal interaction requires structural determinants at higher levels. We further speculate that the actual sequence of TPR motif and/or its flanking regions define the specificity for interaction with different small GTPases.
The expression of Gα16 is restricted to hematopoietic cells. Our data show that these cells also contain TPR1 (Fig. 1D), raising the possibility that Gα16 functionally interacts with TPR1. In the transfected cells, expression of Gα16 markedly increases TPR1 binding to Ras and abolishes the ability of TPR1 to discriminate between Raswt and active Ras (Fig. 3A). There are two major possibilities. First, Gα16 may trigger structural alterations in TPR1 favoring the recruitment of Ras. Second, Gα16 alone may induce Ras activation, thereby increasing the amount of GTP-bound Ras that preferably interacts with TPR1. We have examined both possibilities and concluded that, even though Gα16 can stimulate small to moderate levels of Ras activation, presumably through endogenous TPR1 or another mechanism, this does not account for the potent effect of Gα16 on Ras recruitment to TPR1 (Fig. 5A). Moreover, the TPR1(113-292) mutant is capable of binding Ras and discriminating between active Ras and Raswt (Fig. 4C), but expression of Gα16QL does not lead to increased binding of Ras to TPR1(113-292) (Fig. 5B). Therefore, it is most likely that binding of Gα16 to TPR1 induces a conformational change that involves the N terminus of TPR1, resulting in significantly stronger interaction with Ras.
The ability of TPR1 to interact better with active Ras in a Gα16-independent manner (Fig. 3A and 4C) suggests that TPR1 can recognize the conformational change associated with Ras activation (Ras-GTP versus Ras-GDP). However, our results also show that overexpression of TPR1 can induce accumulation of active Ras as measured by binding to the Raf-1 RBD. Thus, TPR1 may activate Ras by stimulating guanine nucleotide exchange or may stabilize Ras in its active conformation and cause accumulation of the active Ras. To determine whether TPR1 can serve the function of a GEF for Ras, we conducted a GTP-γ-[35S] binding assay but found no difference between the test sample (with TPR1) and control (without TPR1). Also, a careful analysis of our data argues against TPR1 being a GEF for Ras. As a Ras GEF, TPR1 would be expected to bind relatively strongly to Ras17N (8). However, experimental data indicate that the interaction of TPR1 with Ras17N is much weaker than its interaction with RasG12V and Raswt (Fig. 7A). These observations suggest that TPR1 binds to the active Ras and stabilizes it in this conformation. A possible region for TPR1 interaction with Ras is one mostly influenced by the active and inactive states, namely, switch I (loop L2/N-terminal β2) and switch II (loop L4/helix α2). Since this region also contains binding sites for the Ras effectors (such as Raf-1) and regulators (e.g., GAPs), we predicted that TPR1 and GST-RBD compete for binding of the active Ras. Our finding that increasing the amount of GST-RBD reduces association of TPR1 and Ras (Fig. 7B) supports the notion that TPR1 binds to the region that is also essential for Ras interaction with Raf-RBD, thereby offering a possible mechanism for the accumulation of active Ras in the presence of TPR1.
In summary, we have characterized TPR1 as a novel adaptor that can bind to specific Gα proteins and to Ras. Data shown here support Gα16 playing a role in activating Ras, in part through its interaction with TPR1. However, the precise role of TPR1 in mediating this function remains unclear. The ability of Gα16 to interact with TPR1 is not affected by its activation status, whereas a constitutively active Gα16 is more effective than Gα16wt in causing the accumulation of active Ras. Therefore, there may also be a TPR1-independent mechanism for Gα16 to activate Ras. Although the present study was initiated with identification of TPR1 using Gα16 as bait, we are aware that this G protein differs from most other Gα subunits in that it can interact with GPCRs less specifically. The detailed mechanism underlying Gα16-induced cellular functions requires further investigation. With TPR motif-containing proteins becoming an emerging group of molecules that interact with heterotrimeric G proteins and small GTPases, it will be important to examine how TPR1 associates with other Gα proteins, such as Gαs and Gαq, and what the functional consequences are. TPR1 was originally identified as a protein that interacts in S. cerevisiae with a truncated form of the GAP-related domain of neurofibromin (24). Based on results shown above, TPR1 may compete with the GAP-related domain and thereby increase the levels of Ras-GTP. This possibility and its implication in the pathogenesis of neurofibromatosis type I will be investigated in future studies.
Acknowledgments
We thank Lawrence Quilliam and Isabel Lopez for helpful discussions.
This work was made possible in part by National Institutes of Health grants AI33503 and AI40176 (to R.D.Y.). Caroline Marty is a recipient of a predoctoral fellowship from the American Heart Association Midwest Affiliate.
REFERENCES
- 1.Amatruda, T. T., D. A. Steel, V. Z. Slepak, and M. I. Simon. 1991. G alpha 16, a G protein alpha subunit specifically expressed in hematopoietic cells. Proc. Natl. Acad. Sci. USA 88:5587-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blatch, G. L., and M. Lassle. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21:932-939. [DOI] [PubMed] [Google Scholar]
- 3.Cismowski, M. J., A. Takesono, M. L. Bernard, E. Duzic, and S. M. Lanier. 2001. Receptor-independent activators of heterotrimeric G-proteins. Life Sci. 68:2301-2308. [DOI] [PubMed] [Google Scholar]
- 4.Crespo, P., N. Xu, W. F. Simonds, and J. S. Gutkind. 1994. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature 369:418-420. [DOI] [PubMed] [Google Scholar]
- 5.Das, A. K., P. W. Cohen, and D. Barford. 1998. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 17:1192-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Rooij, J., and J. L. Bos. 1997. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene 14:623-625. [DOI] [PubMed] [Google Scholar]
- 7.De Vries, L., B. Zheng, T. Fischer, E. Elenko, and M. G. Farquhar. 2000. The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40:235-271. [DOI] [PubMed] [Google Scholar]
- 8.Feig, L. A. 1999. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1:E25-E27. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara, S., C. Murga, M. Zohar, T. Igishi, and J. S. Gutkind. 1999. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 274:5868-5879. [DOI] [PubMed] [Google Scholar]
- 10.Gilman, A. G. 1987. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56:615-649. [DOI] [PubMed] [Google Scholar]
- 11.Goebl, M., and M. Yanagida. 1991. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem. Sci. 16:173-177. [DOI] [PubMed] [Google Scholar]
- 12.Grizot, S., F. Fieschi, M. C. Dagher, and E. Pebay-Peyroula. 2001. The active N-terminal region of p67phox. Structure at 1.8 A resolution and biochemical characterizations of the A128V mutant implicated in chronic granulomatous disease. J. Biol. Chem. 276:21627-21631. [DOI] [PubMed] [Google Scholar]
- 13.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 14.Hart, M. J., X. Jiang, T. Kozasa, W. Roscoe, W. D. Singer, A. G. Gilman, P. C. Sternweis, and G. Bollag. 1998. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280:2112-2114. [DOI] [PubMed] [Google Scholar]
- 15.Hawes, B. E., T. van Biesen, W. J. Koch, L. M. Luttrell, and R. J. Lefkowitz. 1995. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J. Biol. Chem. 270:17148-17153. [DOI] [PubMed] [Google Scholar]
- 16.Hirano, T., N. Kinoshita, K. Morikawa, and M. Yanagida. 1990. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell 60:319-328. [DOI] [PubMed] [Google Scholar]
- 17.Koga, H., H. Terasawa, H. Nunoi, K. Takeshige, F. Inagaki, and H. Sumimoto. 1999. Tetratricopeptide repeat (TPR) motifs of p67phox participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J. Biol. Chem. 274:25051-25060. [DOI] [PubMed] [Google Scholar]
- 18.Kozasa, T., X. Jiang, M. J. Hart, P. M. Sternweis, W. D. Singer, A. G. Gilman, G. Bollag, and P. C. Sternweis. 1998. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280:2109-2111. [DOI] [PubMed] [Google Scholar]
- 19.Lamb, J. R., S. Tugendreich, and P. Hieter. 1995. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem. Sci. 20:257-259. [DOI] [PubMed] [Google Scholar]
- 20.Lapouge, K., S. J. Smith, P. A. Walker, S. J. Gamblin, S. J. Smerdon, and K. Rittinger. 2000. Structure of the TPR domain of p67phox in complex with Rac.GTP. Mol. Cell 6:899-907. [DOI] [PubMed] [Google Scholar]
- 21.Macara, I. G., K. M. Lounsbury, S. A. Richards, C. McKiernan, and D. Bar-Sagi. 1996. The Ras superfamily of GTPases. FASEB J. 10:625-630. [DOI] [PubMed] [Google Scholar]
- 22.McCormick, F., G. A. Martin, R. Clark, G. Bollag, and P. Polakis. 1991. Regulation of ras p21 by GTPase activating proteins. Cold Spring Harb. Symp. Quant. Biol. 56:237-241. [DOI] [PubMed] [Google Scholar]
- 23.Mochizuki, N., G. Cho, B. Wen, and P. A. Insel. 1996. Identification and cDNA cloning of a novel human mosaic protein, LGN, based on interaction with G alpha i2. Gene 181:39-43. [DOI] [PubMed] [Google Scholar]
- 24.Murthy, A. E., A. Bernards, D. Church, J. Wasmuth, and J. F. Gusella. 1996. Identification and characterization of two novel tetratricopeptide repeat-containing genes. DNA Cell Biol. 15:727-735. [DOI] [PubMed] [Google Scholar]
- 25.Natochin, M., K. G. Gasimov, and N. O. Artemyev. 2001. Inhibition of GDP/GTP exchange on G alpha subunits by proteins containing G-protein regulatory motifs. Biochemistry 40:5322-5328. [DOI] [PubMed] [Google Scholar]
- 26.Peterson, Y. K., M. L. Bernard, H. Ma, S. Hazard III, S. G. Graber, and S. M. Lanier. 2000. Stabilization of the GDP-bound conformation of Gialpha by a peptide derived from the G-protein regulatory motif of AGS3. J. Biol. Chem. 275:33193-33196. [DOI] [PubMed] [Google Scholar]
- 27.Qian, N. X., M. Russell, A. M. Buhl, and G. L. Johnson. 1994. Expression of GTPase-deficient G alpha 16 inhibits Swiss 3T3 cell growth. J. Biol. Chem. 269:17417-17423. [PubMed] [Google Scholar]
- 28.Quilliam, L. A., R. Khosravi-Far, S. Y. Huff, and C. J. Der. 1995. Guanine nucleotide exchange factors: activators of the Ras superfamily of proteins. Bioessays 17:395-404. [DOI] [PubMed] [Google Scholar]
- 29.Ridley, A. J. 1997. The GTP-binding protein Rho. Int. J. Biochem. Cell Biol. 29:1225-1229. [DOI] [PubMed] [Google Scholar]
- 30.Ross, E. M., and T. M. Wilkie. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69:795-827. [DOI] [PubMed] [Google Scholar]
- 31.Sikorski, R. S., M. S. Boguski, M. Goebl, and P. Hieter. 1990. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell 60:307-317. [DOI] [PubMed] [Google Scholar]
- 32.Simon, M. I., M. P. Strathmann, and N. Gautam. 1991. Diversity of G proteins in signal transduction. Science 252:802-808. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi, Y., H. Katoh, K. Mori, and M. Negishi. 2002. Galpha(12) and galpha(13) interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr. Biol. 12:1353-1358. [DOI] [PubMed] [Google Scholar]
- 34.Yang, M., H. Sang, A. Rahman, D. Wu, A. B. Malik, and R. D. Ye. 2001. Galpha(16) couples chemoattractant receptors to NF-κB activation. J. Immunol. 166:6885-6892. [DOI] [PubMed] [Google Scholar]