Abstract
Usefulness of adenoviral vectors derived from human adenovirus (HAd) type 5 (HAd5) is mainly limited by wide prevalence of preexisting anti-HAd5 immunity as well as nonspecific tissue tropism of these vectors. As an alternative, nonhuman adenoviral vectors including bovine adenovirus type 3 (BAd3), are currently being investigated. Non-prevalence of BAd3 in humans and its ability to evade preexisting HAd immunity are some of the features that make BAd3 a promising vector for human gene delivery. BAd3 appears to have a tissue tropism distinct from that of HAd5 and also the repertoire of cells efficiently transduced by BAd3 is different. We performed antibody-mediated receptor blocking experiments to show that BAd3 internalization was independent of coxsackievirus adenovirus receptor (CAR), the primary determinant of HAd5 tropism, or integrin αvβ3, a secondary molecule involved in HAd5 entry. Using homologous and heterologous knob-mediated competition assays with recombinant knobs of HAd5, porcine adenovirus type 3 (PAd3), or BAd3, we observed that BAd3 internalization was independent of the primary receptors of HAd5 and PAd3. These results provide support for further exploration of BAd3 vectors for designing targeted vectors for human gene therapy.
INTRODUCTION
Nonhuman adenoviruses have been proposed as alternative gene delivery vectors in order to extend the range of adenoviral vectors and overcome the limitations associated with human adenoviral (HAd) vectors based on HAd type 5 (HAd5) [1-6]. In general, nonhuman adenoviruses are species specific, can enter human cells, and are naturally not prevalent in the human population [4; 7]. Bovine adenovirus type 3 (BAd3) is a nonhuman adenovirus belonging to the genus Mastadenovirus and was originally isolated from a normal healthy cow [8] and is not known to cause any serious disease in its natural host. BAd3 genome organization and transcription map are similar to those of subtype C HAd [9]. BAd3 effectively evades HAd5-specific immune response [10] and is not cross-neutralized by HAd-specific preexisting neutralizing antibodies in humans [4]. Based on these observations, BAd3 vectors have been proposed as a promising gene delivery vehicle for human and veterinary use [11; 12].
Therapeutic application of any gene delivery vector is dependent on its ability to specifically transduce the target cell types. Adenoviral transduction is mediated by high affinity interaction of the fiber knob domain with the cell surface receptor(s) followed by secondary interactions of the penton base with the cell surface integrins [13]. HAd5 entry involves cosackievirus-adenovirus receptor (CAR) as a primary receptor [14], and integrin αvβ3 and αvβ5 as secondary receptors [13]. Although BAd3 possesses a unique fiber protein consisting of an N-terminal tail, a long shaft region containing 46.5 hydrophobic repeats, and a globular carboxy-terminal knob [15], the nature of primary receptor(s) for BAd3 is unknown. Identification of the cellular receptors involved in BAd3 entry will be important in determining the potential cell or tissue types that are susceptible or refractory to BAd3 vectors.
Transduction efficiency of BAd3 vectors has been tested in various cell lines [11; 16; 17]. In a recent study, we observed significant differences in transduction efficiencies of BAd3, HAd5 and porcine adenovirus type 3 (PAd3) vectors in a panel of human, murine, bovine and porcine cell lines [11], suggesting that these vectors may use distinct primary receptors for entry into the susceptible cells. Furthermore, the exchange of the fiber knob domain of BAd3 with that of HAd5 resulted in a significant alteration in cell tropism of the chimeric BAd3 vector [18]. Using homologous and heterologous knob-mediated competition assays, here we provide the evidence that the primary receptors of BAd3 is/are distinct from that of HAd5 or PAd3. Further experiments are needed to define the exact nature of the BAd3 primary receptor/s, and to design BAd3-based targeted vectors for human gene delivery.
MATERIALS AND METHODS
Adenoviral genome sequence alignment
The complete genomic sequences of HAd5 (accession #NC_001405), PAd3 (accession #AF083132) and BAd3 (NC_001876) were obtained from GenBank to compare amino acid sequences of their receptor-binding fiber knob domains using CLUSALW alignment software [19].
Cells and adenoviral vectors
Madin Darby bovine kidney (MDBK) cells, human embryonic kidney cells expressing adenoviral E1 (293), fetal bovine retina cells expressing adenoviral E1 (FBRT HE1)(van Olphen et al., 2002), and fetal porcine retina cells expressing adenoviral E1 (FPRT HE1-5)[4] were propagated as described previously [4]. The generation and propagation of replication-defective HAd5 (HAd-GFP), PAd3 (PAd-GFP) and BAd3 (BAd-GFP) vectors carrying the green fluorescent protein (GFP) gene driven by the cytomegalovirus promoter is described elsewhere [11]. BAd-GFP, HAd-GFP, and PAdGFP were grown and titrated in FBRT HE1, 293, or FPRT HE1-5, respectively. These vectors replicated similar to their respective wild-type counterparts in these E1-complementing cell lines. The physical particle count of purified vector stocks was estimated by spectrophotometry [20]. Briefly, each vector stock was diluted to 10- and 100-fold in 10 mM Tris (pH 8.0), and the absorbance was measured at 260 nm using a spectrophotometer. The optical density value of 1 at 260nm was considered equivalent to 4 × 1011 particles. The ratio of particle number to the plaque forming unit (p.f.u.) for purified preparations of BAd-GFP, HAd-GFP, and PAd-GFP vectors were 120, 80 and 138, respectively.
Antibodies
The mouse hybridoma RmcB that expresses anti-hCAR antibody [21] was purchased from American Type Culture Collection (ATCC), Rockville, MD; and purified as previously described [22]. The mouse anti-human αvβ3 integrin (clone LM609) monoclonal antibody was obtained from Chemicon International, Inc., Temecula, CA.
Expression, purification and characterization of recombinant BAd3, HAd5 and PAd3 fiber knobs
BAd3 fiber sequences encoding the knob domain and the last shaft motif present between nucleotides 30278 and 30898 (corresponding to the BAd3 genome sequence in GenBank accession #NC_001876) were amplified by PCR using the forward 5′-GCAGACGGATCCCAGGTTAGGGTTA-3′ and the reverse 5′ GCTAAGCTTCCAACTTATTTAAGTCCAG-3′ primers, and the genomic BAd3 DNA as a template. The PCR product was ligated into the BamHI-HindIII site of pQE30 vector (Qiagen, Inc., Valencia, CA) to obtain pQE30.B3knob, and expressed in E. coli M5 (pREP4) (Qiagen) following the supplier's recommendations. Briefly, the bacterial culture was grown to an OD600 of 0.5-0.6 followed by induction of protein expression by 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) and the incubation was continued for 3-4 h at 37°C. Due to the presence of the 6xHis-tag in-frame with the knob, the recombinant protein was purified from the soluble fraction by nickel-nitrilotriacetic acid (Ni-NTA) metal chelation chromatography using His.Bind protein purification kit (Novagen, Madison, WI) as per the supplier's instructions. Purified knobs were dialyzed against PBS (137 mM NaCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, and 2.7 mM KCl, pH 7.4) and protein concentrations were determined by Coomassie Protein Assay Reagent (Pierce Biotechnology, Inc., Rockford, IL) using bovine serum albumin (BSA) as a standard. Purification of HAd5 and PAd3 trimeric knob domains was carried out as described previously [22]. The ability of the recombinant adenoviral knobs to form trimers was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using both boiled and unboiled samples followed by Coomassie staining or Western blot using a rabbit anti-BAd3, anti-HAd5, or anti-PAd3 hyperimmune serum (data not shown).
Transduction assay in MDBK cells
Approximately 1×105 MDBK cells were seeded in 12-well tissue culture plates. Twenty-four h later, cells were infected with BAd-GFP, HAd-GFP, or PAd-GFP at a multiplicity of infection (m.o.i.) of 4, 20, or 100 p.f.u. per cell. At 24 h post-infection, cells were visualized under Olympus IX 70 fluorescence microscope (Olympus America Inc., Melville, NY) and photographed using Olympus DP70 CCD digital camera (Olympus). Subsequently, cells were harvested and analyzed by flow cytometry as described below.
Antibody-mediated blocking experiments
Anti-CAR antibody or anti-αvβ3 antibody-mediated blocking assays for BAd-GFP, HAd-GFP, or PAd-GFP in MDBK cells were conducted essentially as previously described [22].
Fiber knob-mediated virus inhibition assays
Experiments involving BAd3 (B3), HAd5 (H5), or PAd3 (P3) knob-mediated inhibition in transduction efficiencies of homologous and heterologous adenoviral vectors in MDBK cells were performed as described previously [22].
Flow cytometric analysis of GFP-expressing cells
Flow cytometry analyses were carried out as described [4]. Briefly, vector-infected MDBK cells were harvested by trypsinization, washed once with PBS and resuspended at a density of approximately 106cell per ml in PBS containing 2% formaldehyde and immediately used for flow cytometry at Purdue University Flow Cytometry Laboratories using Cytomics™ FC-500 Flow Cytometer (Beckman Coulter, Inc., Fullerton, CA) with Ar 488 nm excitation filter and FL-1 525 nm BP emission filter. At least 5000 cells were enumerated for each sample.
Statistical analysis
Data were compared by Student's ‘t’ test for statistical significance. P<0.05 was considered significant.
RESULTS AND DISCUSSION
Comparison of BAd3, HAd5 and PAd3 fiber knob amino acid sequences
The initial attachment of adenovirus to the cell surface receptor/s is mediated by the adenoviral fiber knob, hence, it was important to compare the amino acid sequences of BAd3, HAd5 and PAd3 fiber knobs. For a number of HAd types including HAd5, CAR serves as the primary receptor [14]. Mutagenesis studies of fiber knob domains of a number of CAR-binding HAd types have led to identification of amino acid residues that are critical for or peripherally effect CAR binding [23; 24]. Comparison of BAd3, HAd5 and PAd3 fiber knob amino acid sequences showed that the residues critical in CAR-binding (Ser408, Pro409, Tyr477 and Leu485) or those that peripherally effect CAR-binding (Ala406, Arg412 and Arg481) were not conserved in the BAd3 or PAd3 fiber knob (Fig.1). The consensus Thr-Leu-Trp-Thr (TLWT) motif that marks the beginning of the knob domain in Mastadenovirus species [25] was also conserved in the BAd3 and PAd3 fiber knobs. The similarity between BAd3 and HAd5, PAd3 and HAd5, or BAd3 and PAd3 fibers was 29.3, 29.1, and 26.2%, respectively. When compared at the fiber knob domains, these values were 25.3%, 27.3, and 33.6%, respectively. In addition, the BAd3 fiber is very long (976 residues with 46.5 repeats in the shaft region) [15], the PAd3 fiber is short (448 residues with 15 repeats in the shaft region) [9; 26], compared to the HAd5 fiber (581 residues with 22 repeats in the shaft region) [25]. Furthermore, the integrin-binding motif such as the Arg-Gly-Asp (RGD) or Leu-Asp-Val (LDV) is absent in the BAd3 or PAd3 penton base [9]. On the basis of above-mentioned dissimilarities in fiber knob domains of BAd3, HAd5 and PAd3, it was anticipated that BAd3 internalization into susceptible cells might be CAR-as well as integrin-independent. Recently we have demonstrated that PAd3 internalization was independent of CAR and αvβ3 or αvβ5 integrin [22].
Fig. 1.
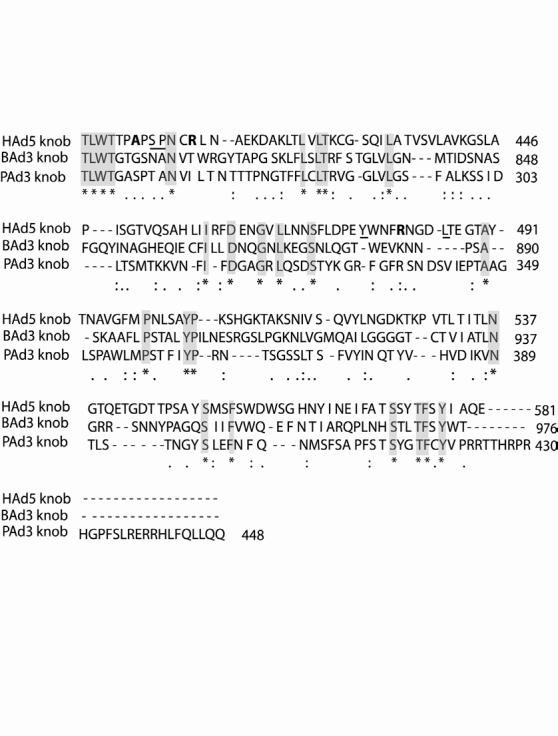
Homology comparison of the BAd3 fiber knob with the corresponding region of PAd3 and HAd5 fibers using CLUSALW alignment software. Identical, similar, and related residues are depicted by asterisks (and highlighted), colons, and dots, respectively. The HAd5 fiber knob residues that are known to be important for CAR-binding or that peripherally involved in CAR-binding are underlined or shown in bold, respectively.
Transduction by BAd-GFP, HAd-GFP, or PAd-GFP in MDBK cells
We used bovine MDBK cell line for performing all competition experiments in this study. None of these vectors replicate in MDBK cells due to a lethal deletion in the E1 region of all three vectors. We have previously shown that transduction efficiencies of BAd-GFP, HAd-GFP, and PAd-GFP in MDBK cells at an m.o.i. of 25 p.f.u. per cell were 85, 58 and 48%, respectively [11]. Similar differences were also observed in the transduction experiments conducted in this study. As indicated by the number of green fluorescent cells, BAd-GFP showed significant transduction at an m.o.i. of 4 p.f.u. per cells, whereas both HAd-GFP- and PAd-GFP-mediated transduction was clearly visible at an m.o.i. of 20 p.f.u. per cell (Fig. 2). These results clearly demonstrated a dose-dependent increase in transduction with each vector. The number of green fluorescent cells was determined by flow cytometry at 36 h post infection. Transduction efficiencies of BAd-GFP, HAd-GFP, and PAd-GFP were approximately 55, 40 and 32%, respectively. The differences in transduction efficiencies of BAd-GFP, HAd-GFP, and PAd-GFP in MDBK cells will not significantly impact interpretation of our results because the relative transduction of various treatment groups was calculated taken into consideration the transduction efficiency of each vector.
Fig. 2.
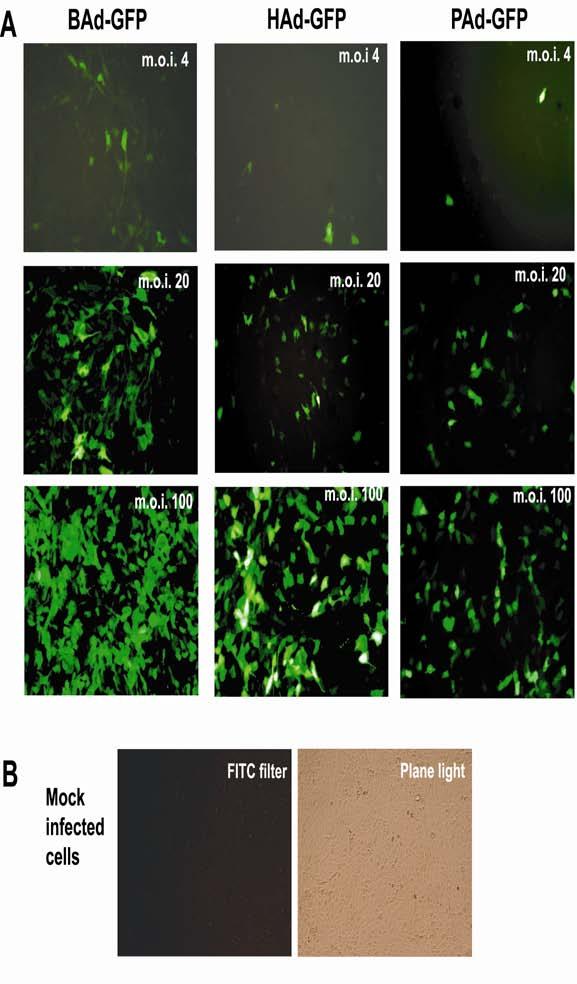
Comparison of green fluorescent protein (GFP) expression in MDBK cells infected either with BAd-GFP, HAd-GFP or PAd-GFP. A) MDBK cells were infected either with BAd-GFP, HAd-GFP or PAd-GFP at an m.o.i. of 4, 20, or 100 p.f.u. per cell. At 36 h post-infection, cells were observed under a fluorescence microscope. GFP expressing cells appear green. B) Mock-infected MDBK cells under a fluorescence microscope (left) or a light microscope (right) are shown as a negative control. ×100
CAR-and integrin-independent transduction by BAd-GFP
Our choice of conducting various transduction experiments in MDBK cells was based on the fact that BAd3, HAd5 and PAd3 efficiently transduce these cells [11], MDBK cells express detectable levels of CAR as demonstrated by Western blot analysis [11], and they express very high levels of αvβ3 integrin as detected by flow cytometry (data not shown).
The CAR-specific mouse monoclonal antibody, RmcB, is proven to occupy CAR receptors on the cell surface and thereby inhibiting infection of CAR-binding adenoviruses [14; 27]. We noticed a dose-dependent inhibition of HAd-GFP transduction of MDBK cells treated with CAR-specific antibody, but antibody-mediated blocking of CAR molecules did not significantly (P>0.05) affect the transduction efficiency of BAd-GFP or PAd-GFP (Fig. 2) suggesting a CAR-independent internalization of BAd3 or PAd3. In a previous study, we also observed a drastic reduction in HAd-GFP transduction of MCF-10A cells (non-transformed human breast epithelial cells) preincubated with CAR-specific antibody [22]. These results corroborated the pre-established model of CAR-mediated entry of HAd5 [14; 27].
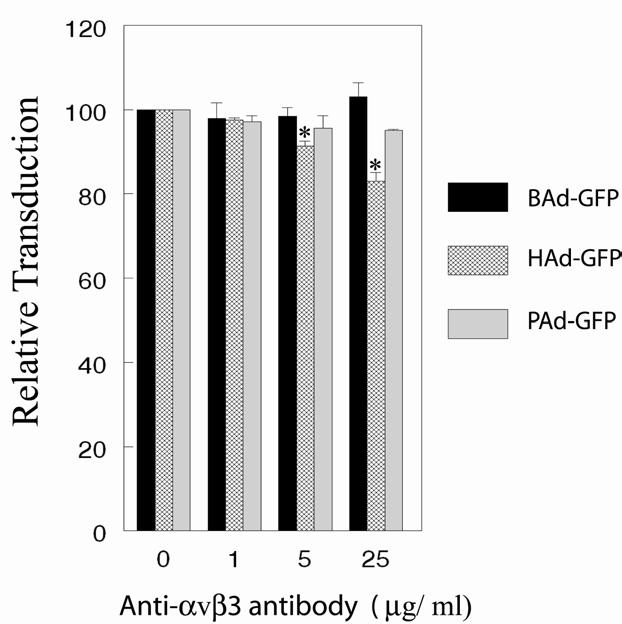
In addition to CAR, integrins have been implicated as secondary receptors in HAd5 internalization [13]. We have previously demonstrated that preincubation of MCF-10A cells with function-blocking antibody against αvβ3 or αvβ5 integrin resulted in significant inhibition of HAd5 transduction [22]. Similarly, here we observed that the treatment of MDBK cells with an anti-αvβ3 integrin antibody diminished HAd5 infectivity and the effect was dose-dependent (Fig. 3). Whereas, preincubation of MDBK cells with an anti-αvβ3 integrin antibody did not significantly (P>0.05) inhibited transduction efficiencies of BAd3 or PAd3 (Fig. 3). We did not attempt blocking assays using an anti-αvβ5 integrin antibody mainly because MDBK seems to express very low level of αvβ5 integrin (data not shown). These observations and the absence of the RGD or LDV motif in the BAd3 penton base indicate that CAR and αvβ3 integrin may not play a major role in BAd3 internalization into MDBK cells.
Fig. 3.
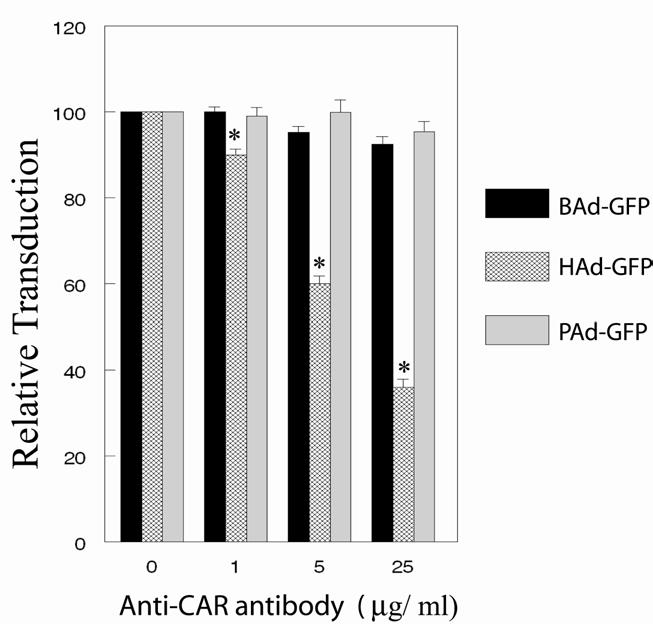
Inhibition of CAR-mediated internalization using a CAR-specific antibody. MDBK cells in monolayer cultures were treated with 1, 5, or 25 μg/ml of CAR-specific antibody followed by infection either with BAd-GFP, HAd-GFP, or PAd-GFP at an m.o.i. of 20 p.f.u. per cell. At 36 h post-infection, cells were harvested by trypsinization and the cells expressing GFP were sorted by flow cytometry. For positive controls, cell monolayers without the CAR-specific antibody treatment were infected similarly with BAd-GFP, HAd-GFP, or PAd-GFP. The transduction efficiencies of positive controls were taken as 100% for calculating the relative transduction of various treatment groups. The results are presented as the mean ± standard deviation of the percent relative transduction of minimum of three independent observations. *, P <0.05.
Inhibition in adenoviral vector internalization in the presence of vector-specific fiber knob
Since the primary receptor-binding domain of adenovirus is located in the fiber knob [28], the purified adenoviral fiber or knob domain has been effectively used in competitive inhibition assays to investigate distinct receptor usage by various HAd serotypes [14; 24; 29; 30]. For our homologous and heterologous competitive inhibition assays, we needed purified BAd3, HAd5, and PAd3 trimeric fiber knobs. Purification of HAd5 and PAd3 trimeric fiber knobs is described elsewhere [22] and the successful expression and purification of BAd3 trimeric knob was conducted for this study (data not shown).
MDBK cells were preincubated with various concentrations of recombinant B3, H5, or P3 knobs prior to infection with BAd-GFP, HAd-GFP, or PAd-GFP. The B3 knob significantly (P<0.05) inhibited BAd-GFP transduction in a dose-dependent manner, but there was no significant (P>0.05) inhibition in HAd-GFP or PAd-GFP transduction even at the highest concentration of the B3 knob (Fig. 4A). Similarly, the H5 knob significantly (P<0.05) reduced HAd-GFP transduction in a dose-dependent manner, whereas there was no significant (P>0.05) inhibition in BAd-GFP or PAd-GFP transduction even at the highest concentration of the H5 knob (Fig. 4B). The P3 knob significantly (P<0.05) diminished PAd-GFP transduction in a dose-dependent fashion, whereas there was no significant (P>0.05) reduction in BAd-GFP or HAd-GFP transduction even at the highest concentration of the P3 knob (Fig. 4C). Most striking inhibition in transduction of homologous vector was observed in case of HAd-GFP. We observed low levels of inhibition of homologous vector transduction in case of BAd-GFP as well as PAd-GFP. This may be due to the variability in quality of these three recombinant knob preparations and/or the variability in the number of primary receptor/s for BAd3, HAd5, or PAd3 on the surface of MDBK cells. Since knob domain is the primary receptor-seeking moiety of most Mastadenovirus species [28], we attempted blocking studies with recombinant knob domain only and not with the entire fiber of BAd3. BAd3 has a uniquely long fiber with unusual kinks in the shaft region [15]. Therefore, it is quite possible that fiber domain/s other than the knob play more important role in BAd3 internalization. Knob domain of BAd3 does, however, play some role in virus attachment and entry as suggested by a recent report describing altered tropism of BAd3 when its fiber knob was exchanged with the HAd5 knob [18].
Fig. 4.
Transduction efficiencies of BAd-GFP, HAd-GFP, or PAd-GFP in the presence of αvβ3 integrin-specific antibody. MDBK cells in monolayer cultures were treated with 1, 5, or 25 μg/ml of αvβ3 integrin-specific antibody followed by infection either with BAd-GFP, HAd-GFP, or PAd-GFP at an m.o.i. of 20 p.f.u. per cell. At 36 h post-infection, cells were harvested by trypsinization and the cells expressing GFP were sorted by flow cytometry. For positive controls, cell monolayers without the αvβ3 integrin-specific antibody treatment were infected similarly with BAd-GFP, HAd-GFP, or PAd-GFP. The transduction efficiencies of positive controls were taken as 100% for calculating the relative transduction of various treatment groups. The results are presented as the mean ± standard deviation of the percent relative transduction of minimum of three independent observations. *, P <0.05.
Putting together the results of homologous and heterologous competitive inhibition assays, it is clearly evident that significant inhibition in vector transduction was only observed in the homologous system suggesting that the primary receptor/s for BAd3, HAd5 and PAd3 are distinct. These findings are a further extension of our earlier work where we have shown that the primary receptor/s for PAd3 was/were distinct from that of HAd5 [22]. Furthermore, in vivo tropism of BAd3 in a cotton rat model appear to be distinct from that of HAd5 [31]. Therefore, it will be important to investigate whether usage of distinct primary receptor/s by BAd3 vectors will also affect vector biodistribution or vector toxicity in experimental animal models. In addition to CAR, several other cell surface molecules have been shown to play a role in adenovirus binding and internalization. Heparan sulfate glycosaminoglycans (HSG) are involved in fiber-mediated entry of HAd type 2 and 5 [32; 33]; sialic acid has been shown to mediate attachment and infection of subgroup D adenoviruses [30]; membrane cofactor protein (CD46) has been implicated as a receptor for subgroup B [34] as well as subgroup D [18] adenoviruses. Similarly, CD80 and CD86 have been shown to be the attachment receptors for HAd3, a subgroup B adenovirus [35]. The role of any of these cell surface molecules as the primary BAd3 receptor/s is yet to be explored.
We have previously shown that BAd3-, PAd3-, or HAd5-specific neutralizing antibody response was not cross-neutralizing [10]. In addition, we have also shown that human serum samples having HAd-neutralizing antibodies did not cross-neutralize BAd3 or PAd3 [4; 11]. The study of comparative transduction efficiencies of BAd3, HAd5 and PAd3 vectors in a number of human, murine, bovine and porcine cell lines clearly indicated that the repertoire of cell types transduced by these vectors was quite different [11]. These observations and the results of the present study clearly demonstrate the usage of distinct receptor/s by BAd3. Additional experiments are needed to further understand the nature of BAd3 primary receptor/s.
Fig. 5.
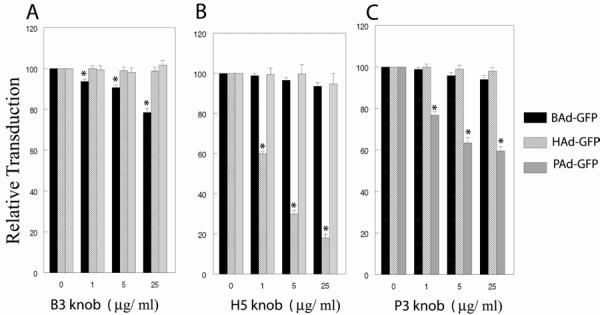
Inhibition in adenoviral vector internalization in the presence of vector-specific fiber knob. MDBK cells in monolayer cultures were treated with 1, 5, or 25 μg/ml of the recombinant A) BAd3 (B3), B) HAd5 (H5), or C) PAd3 (P3) fiber knob followed by infection either with BAd-GFP, HAd-GFP, or PAd-GFP at an m.o.i. of 20 p.f.u. per cell. At 36 h post-infection, cells were harvested by trypsinization and the cells expressing GFP were sorted by flow cytometry. For positive controls, cell monolayers without any fiber knob treatment were infected similarly with BAd-GFP, HAd-GFP, or PAd-GFP. The transduction efficiencies of positive controls were taken as 100% for calculating the relative transduction of various treatment groups. The results are presented as the mean ± standard deviation of the percent relative transduction of minimum of three independent observations. *, P <0.05.
ACKNOWLEDGEMENTS
We thank David Curiel, University of Alabama at Birmingham, Birmingham, AL for providing the clone of the HAd5 fiber knob, and Jane Kovach for excellent secretarial assistance. This work was supported by NIH, Purdue Research Foundation, and Hatch grants.
REFERENCES
- 1.Klonjkowski B, Gilardi-Hebenstreit P, Hadchouel J, Randrianarison V, Boutin S, Yeh P, Perricaudet M, Kremer EJ. A recombinant E1-deleted canine adenoviral vector capable of transduction and expression of a transgene in human-derived cells and in vivo. Hum.Gene Ther. 1997;8:2103–2115. doi: 10.1089/hum.1997.8.17-2103. [DOI] [PubMed] [Google Scholar]
- 2.Zakhartchouk A, Zhou Y, Tikoo SK. A recombinant E1-deleted porcine adenovirus-3 as an expression vector. Virology. 2003;313:377–386. doi: 10.1016/s0042-6822(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 3.Reddy PS, Idamakanti N, Hyun BH, Tikoo SK, Babiuk LA. Development of porcine adenovirus-3 as an expression vector. J.Gen.Virol. 1999;80:563–570. doi: 10.1099/0022-1317-80-3-563. [DOI] [PubMed] [Google Scholar]
- 4.Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Mittal SK, Prevec L, Graham FL, Babiuk LA. Development of a bovine adenovirus type 3-based expression vector. J.Gen.Virol. 1995;76:93–102. doi: 10.1099/0022-1317-76-1-93. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann C, Loser P, Cichon G, Arnold W, Both GW, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J.Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, Marsh J, Ertl HC, Wilson JM. Replication-defective vector based on a chimpanzee adenovirus. J.Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darbyshire JH, Dawson PS, Lamont PH, Ostler DC, Pereira HG. A new adenovirus serotype of bovine origin. J.Comp Pathol. 1965;75:327–330. doi: 10.1016/0021-9975(65)90038-1. [DOI] [PubMed] [Google Scholar]
- 9.Reddy PS, Idamakanti N, Song JY, Lee JB, Hyun BH, Park JH, Cha SH, Bae YT, Tikoo SK, Babiuk LA. Nucleotide sequence and transcription map of porcine adenovirus type 3. Virology. 1998;251:414–426. doi: 10.1006/viro.1998.9418. [DOI] [PubMed] [Google Scholar]
- 10.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- 11.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem.Biophys.Res.Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 12.Reddy PS, Idamakanti N, Pyne C, Zakhartchouk AN, Godson DL, Papp Z, Baca-Estrada ME, Babiuk LA, Mutwiri GK, Tikoo SK. The immunogenicity and efficacy of replication-defective and replication-competent bovine adenovirus-3 expressing bovine herpesvirus-1 glycoprotein gD in cattle. Vet.Immunol.Immunopathol. 2000;76:257–268. doi: 10.1016/s0165-2427(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 13.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 14.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 15.Ruigrok RW, Barge A, Mittal SK, Jacrot B. The fibre of bovine adenovirus type 3 is very long but bent. J.Gen.Virol. 1994;75(Pt 8):2069–2073. doi: 10.1099/0022-1317-75-8-2069. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen UB, Benchaibi M, Meyer V, Schlesinger Y, Schughart K. Novel human gene transfer vectors: evaluation of wild-type and recombinant animal adenoviruses in human-derived cells. Hum.Gene Ther. 1999;10:2587–2599. doi: 10.1089/10430349950016636. [DOI] [PubMed] [Google Scholar]
- 17.Reddy PS, Idamakanti N, Chen Y, Whale T, Babiuk LA, Mehtali M, Tikoo SK. Replication-defective bovine adenovirus type 3 as an expression vector. J.Virol. 1999;73:9137–9144. doi: 10.1128/jvi.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Tikoo SK. Altered tropism of recombinant bovine adenovirus type-3 expressing chimeric fiber. Virus Res. 2004;99:9–15. doi: 10.1016/j.virusres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol.Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 21.Hsu KH, Lonberg-Holm K, Alstein B, Crowell RL. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J.Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangari DS, Mittal SK. Porcine adenovirus serotype 3 internalization is independent of CAR and alpha(v)beta(3) or alpha(v)beta(5) integrin. Virology. 2005;332:157–166. doi: 10.1016/j.virol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Kirby I, Davison E, Beavil AJ, Soh CP, Wickham TJ, Roelvink PW, Kovesdi I, Sutton BJ, Santis G. Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J.Virol. 2000;74:2804–2813. doi: 10.1128/jvi.74.6.2804-2813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW, Brough DE, Kovesdi I, Wickham TJ. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J.Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chroboczek J, Ruigrok RW, Cusack S. Adenovirus fiber. Curr.Top.Microbiol.Immunol. 1995;199(Pt 1):163–200. doi: 10.1007/978-3-642-79496-4_10. [DOI] [PubMed] [Google Scholar]
- 26.Reddy PS, Nagy E, Derbyshire JB. Sequence analysis of putative pVIII, E3 and fibre regions of porcine adenovirus type 3. Virus Res. 1995;36:97–106. doi: 10.1016/0168-1702(94)00105-l. [DOI] [PubMed] [Google Scholar]
- 27.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc.Natl.Acad.Sci.U.S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry LJ, Xia D, Wilke ME, Deisenhofer J, Gerard RD. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J.Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J.Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J.Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 31.Mittal SK, Middleton DM, Tikoo SK, Babiuk LA. Pathogenesis and immunogenicity of bovine adenovirus type 3 in cotton rats (Sigmodon hispidus) Virology. 1995;213:131–139. doi: 10.1006/viro.1995.1553. [DOI] [PubMed] [Google Scholar]
- 32.Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- 33.Smith TA, Idamakanti N, Rollence ML, Marshall-Neff J, Kim J, Mulgrew K, Nemerow GR, Kaleko M, Stevenson SC. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum.Gene Ther. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- 34.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat.Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 35.Short JJ, Pereboev AV, Kawakami Y, Vasu C, Holterman MJ, Curiel DT. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology. 2004;322:349–359. doi: 10.1016/j.virol.2004.02.016. [DOI] [PubMed] [Google Scholar]


