Abstract
There are three mechanisms of transcriptional repression in eukaryotes. The first is quenching, whereby repressors and activators co-occupy closely linked sites and then the repressor inhibits adjacent activators. The second is direct repression, in which repressors block the function of the core transcription complex. The third is competition, in which repressors compete with activators for a common DNA-binding site. Previous studies have shown that the Drosophila melanogaster CtBP corepressor (dCtBP) is essential for the quenching activity of three short-range sequence-specific repressors in the early Drosophila embryo: Krüppel, Knirps, and Snail. Here we demonstrate that dCtBP is dispensable for target enhancers that contain overlapping activator and repressor binding sites. However, it is essential when Krüppel and Knirps repressor sites do not overlap activator sites but are instead located adjacent to either activators or the core promoter. These findings provide evidence that competition is distinct from quenching and direct repression. Quenching and direct repression depend on dCtBP, whereas competition does not.
Competition is the premiere mechanism of transcriptional repression in prokaryotes. Both prototypic transcriptional repressors, the lambda and lac repressors, inhibit gene expression by binding to specific recognition elements located near the core promoter (24). Once bound, these repressors block access of RNA polymerase. It has been proposed that a variation in this mechanism is essential for establishing localized patterns of gene expression in the early Drosophila melanogaster embryo (for reviews see references 7, 9, and 19). For example, the even-skipped (eve) stripe 2 enhancer is located ∼1 kb 5′ of the core promoter (32, 33, 37). It contains overlapping binding sites for sequence-specific transcriptional activators and repressors, including the Bicoid activator and the Krüppel repressor. The Krüppel repressor is not thought to interfere with the binding of the RNA polymerase II transcription complex at the core promoter due to the large distance separating the eve enhancer and core promoter. Rather, it was proposed that Krüppel mediates repression by blocking the binding of the Bicoid activator to overlapping sites within the enhancer.
Although the organization of Bicoid and Krüppel binding sites within the eve stripe 2 enhancer strongly suggested repression by competition, subsequent studies demonstrated that the repressor binding sites need not overlap the activator sites (6, 8). Krüppel can inhibit adjacent activators even when the Krüppel binding sites are positioned 100 bp away from the activator sites. Presumably, the Krüppel repressor does not impede the binding of activators over this distance. Instead, it was suggested that Krüppel mediates repression via quenching, whereby activators and repressors bind to linked sites and the repressors somehow inhibit the function of the activators.
Repression by quenching appears to depend on a corepressor protein called CtBP, which was originally identified as a protein that binds to the carboxyl terminus of the adenovirus E1A protein in cultured cells (2, 31). CtBP-E1A interactions attenuate E1A-mediated transcriptional activation and tumorigenesis (36). CtBP binds to a conserved peptide motif in the E1A protein: PLDLS (2, 31). A variant of this motif is conserved in the Krüppel repressor (PxDLSxH) (20, 21). Alanine substitutions in this motif severely disrupt the repression activity of an otherwise normal Krüppel protein in transgenic Drosophila embryos (21, 22). The Drosophila CtBP (dCtBP) protein is maternally expressed and uniformly distributed throughout the cytoplasm of the unfertilized egg and early embryo (20, 23). Embryos derived from mutant eggs containing reduced levels of dCtBP exhibit a variety of patterning defects due to a reduction in the activities of three short-range sequence-specific transcriptional repressors in the early embryo: Krüppel, Knirps, and Snail (21). All three zinc finger repressors contain at least one copy of the conserved dCtBP interaction motif, PxDLSxBasic. These motifs mediate the binding of dCtBP in vitro and are essential for transcriptional repression in vivo.
Recent studies suggest that all three repressors retain the ability to repress a subset of target genes in the absence of dCtBP. Krüppel continues to repress the hairy stripe 7 enhancer (16), Knirps represses the eve stripe 3 enhancer (13), and there is genetic evidence that Snail represses at least one neurogenic gene in the absence of dCtBP (J. Cowden and M. Levine, unpublished results). These findings suggest that Krüppel, Knirps, and Snail may interact with additional, unknown corepressor proteins. Alternatively, it is conceivable that some of these target enhancers may be repressed by competition, whereby activator and repressor sites overlap and the DNA-binding domains of the repressors are sufficient to exclude activators from DNA. To address these issues, we have examined the dCtBP-independent repression activities of Krüppel.
We investigate the role of dCtBP for each of the three modes of repression: quenching, direct repression, and competition. First, an in vivo repression assay demonstrates that only the dCtBP interaction motif within the carboxyl-terminal Krüppel repression domain is required for quenching and direct repression. Second, it is shown that the quenching activity of Krüppel and the direct-repression activities of Krüppel and Knirps are abolished in dCtBP mutant embryos. Third, an artificial enhancer that contains a 14-bp sequence with optimal Bicoid, Dorsal, and Krüppel binding sites was created. These recognition sequences directly overlap one another so that the binding of Krüppel interferes with the binding of the Bicoid and Dorsal activators. The synthetic enhancer thereby permits the visualization of repression by competition in transgenic embryos. No repression activity was observed in mutant embryos that lack Krüppel. However, Krüppel continues to repress the synthetic enhancer in dCtBP mutant embryos. These studies demonstrate that Krüppel can mediate repression by simple competition without the dCtBP corepressor. We conclude that competition and quenching plus direct repression represent two distinct mechanisms of short-range transcriptional repression.
MATERIALS AND METHODS
Protein expression and gel shift assays.
Escherichia coli BL21(DE3)pLysS bacteria transformed with His-tagged Krüppel or the His-tagged Dorsal Rel domain were grown to an optical density at 500 nm of 0.5 to 0.8 at 37°C and then induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h. Expressed proteins were denatured and purified by affinity chromatography using Ni-nitrilotriacetic acid columns (Qiagen) according to the manufacturer's instructions, with the addition of 10 μM ZnSO4 in the binding and washing steps. Gel shift assays with recombinant proteins were performed as follows. The nucleotide sequence of the probe is 5′-gaagatctGGGATTAACCCGTTggatccag. Lowercase letters represent linker sequences. The DNA probe was end labeled with [γ-32P]ATP and T4 polynucleotide kinase, and an amount of the probe corresponding to approximately 50,000 cpm was incubated for 15 min at room temperature after the addition of each protein in 10 mM Tris (pH 7.9)-50 mM NaCl-1 mM EDTA-10 μM ZnSO4-8% glycerol-20 mg of bovine serum albumin. Protein-DNA complexes were resolved by electrophoresis through an 8% acrylamide-bisacrylamide (29:1) gel in 0.5× Tris-boric acid-EDTA (40 mM Tris, 45 mM boric acid, 1 mM EDTA) at 200 V for 1 h. The gels were dried and autoradiographed with an intensifying screen.
Plasmid constructions.
Gal4-Krüppel fusion genes Gal4-Kr 402-502 and Gal4-Kr 402-502ΔPED were placed under the control of either the twist or Krüppel 2.5 enhancer. The details of these fusion genes are described in reference 21.
The Gal4-Kr 62-92 fusion gene was constructed as follows. The putative N-terminal repression domain (amino acids [aa] 62 to 92) of Krüppel (16) was amplified by PCR from cDNA using PCR primers containing KpnlI and XbaI sites, digested, and cloned into KpnlI and XbaI sites of either a TWIG vector, which contains the twist enhancer and Gal4 DNA-binding domain, or a KREG vector, which contains the Krüppel enhancer and the Gal4 DNA-binding domain. The following primers were used: 5′-TAAGGTACCGCCTCAGCTTTTGGAATGCTA and 5′-ATTTCTAGACTACAATGTGCTCATGGGCAGTTGG.
All of the transgenic lacZ reporter genes except NEE-5xUAS-lacZ (see Fig. 1 and 2) were described previously (21). The NEE-5xUAS-lacZ reporter gene containing five copies of the upstream activation sequence (UAS) was constructed as follows. A CaSpeR-AUG-βgal transformation vector (38) containing the eve basal promoter, starting at −42 bp and continuing through codon 22 fused in frame with lacZ (33), was modified by adding multiple restriction enzyme sites (BglII, AscI, PacI, NotI, PmeI, and EcoRI sites; GAATTAGATCTATGGCGCGCCTCTTAATTAACTGCGGCCGCTAGTTTAAACTAGCATGCTTGAATTC) at the original unique EcoRI site (this new version is called the Casp-GANE vector). A 0.7-kb BamHI NEE region lacking a Snail site fragment was inserted into the unique BglII site of the Casp-GANE vector. A NotI-BglII fragment containing five copies of the UAS and the hsp70 promoter region of pUAST was created by PCR amplification using primers 5′-TAAGCGGCCGCTTGCATGCCTGCAGGTCGG and 5′-TAGAGACATCCATCAAACAGG (a NotI site is underlined) and subsequent digestion with NotI and BglII. The fragment was cloned into NotI and BamHI sites of the Casp-GANE vector containing the NEE enhancer by removing the eve promoter.
FIG. 1.
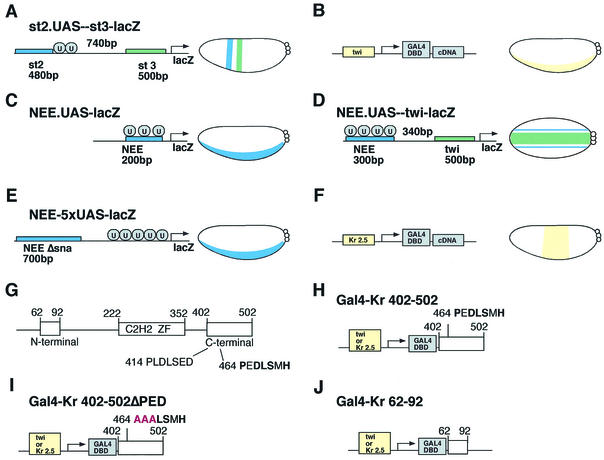
Transgenic-embryo assay for transcriptional repression. Four different lacZ reporter genes were used to analyze the repression activities of different Gal4-Krüppel fusion proteins. Embryo diagrams are oriented with the anterior side to the left. Small circles at the right of each embryo represent pole cells. (A) The st2.UAS-st3-lacZ reporter gene contains Gal4 UAS (U) binding sites near the distal eve stripe 2 enhancer (st2; blue box). The 480-bp stripe 2 enhancer and the 500-bp eve stripe 3 enhancer (st3; green box) are separated by 740 bp. This reporter gene normally exhibits stripes of lacZ expression in both dorsal and ventral regions (as depicted in the embryo diagram). (B) Gal4-Krüppel fusion proteins were expressed in ventral regions of transgenic embryos by using the twist enhancer. The expression profile produced by this expression vector is indicated in the embryo diagram. (C) NEE.UAS-lacZ reporter gene driven by a modified 200-bp rhomboid NEE (blue box) that contains three Gal4 binding sites (U) and three Dorsal activator sites. This reporter gene is normally activated in the ventral mesoderm (as indicated in the embryo diagram). (D) The NEE.UAS-twi-lacZ reporter gene possesses a modified 300-bp rhomboid NEE (blue box) containing four Gal4 binding sites (U) placed upstream of the 500-bp twist proximal enhancer (twi; green box). The two enhancers are separated by a 340-bp spacer sequence. This reporter gene normally exhibits both lateral lines of lacZ expression from the modified NEE (blue) and a broad band of staining in the ventral mesoderm from the twist enhancer (green) (as indicated in the embryo diagram). (E) The NEE-5xUAS-lacZ reporter gene contains five Gal4 (U) binding sites immediately upstream of the TATA box in the minimal hsp70 promoter. A modified 700-bp NEE enhancer lacking Snail binding sites (blue box) was placed upstream of the five UAS sites. This reporter gene is normally activated in the ventral mesoderm (as indicated in the embryo diagram). (F) Gal4-Krüppel fusion proteins were expressed in central regions of transgenic embryos by using the 2.5-kb Krüppel enhancer (Kr 2.5). (G) Structure of the Krüppel protein. It is composed of 502 aa and contains five C2H2 zinc fingers (aa 222 to 352; C2H2 ZF). Repression domains are located near the N terminus (aa 62 to 92) and C terminus (aa 402 to 502). The C-terminal repression domain contains a strong dCtBP interaction motif located at residues 464 to 470 and a weak interaction motif at residues 414 to 420. (H) The C-terminal repression domain (aa 402 to 502) of Krüppel was linked to the Gal4 DNA-binding domain (Gal4-Kr 402-502). (I) The strong dCtBP interaction motif located at residues 464 to 470 in the C-terminal repression domain was mutagenized by alanine substitutions (red; Gal4-Kr 402-502ΔPED). (J) The N-terminal repression domain (aa 62 to 92) was linked to the Gal4 DNA-binding domain (Gal4-Kr 62-92). The Gal4-Kr fusion genes were placed downstream of either the twist or Kr 2.5 enhancer.
FIG. 2.
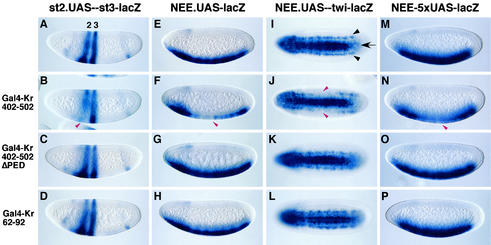
The C-terminal repression domain of Krüppel is essential for quenching and direct repression. Shown are transgenic embryos expressing different lacZ reporter genes and Gal4-Krüppel fusion proteins. In all cases, lacZ expression was visualized by in situ hybridization with a lacZ antisense RNA probe. Embryos are oriented with the anterior side to the left and the dorsal side up (except panels I to L, which display ventral views). (A to D) The st2.UAS-st3-lacZ reporter gene directs two stripes of lacZ expression, one from the eve stripe 2 enhancer (2 in panel A) and the other from the eve stripe 3 enhancer (3 in panel A). In the absence of Gal4-Krüppel fusion proteins, the two stripes exhibit symmetric expression in both dorsal and ventral regions (A). The expression of the Gal4-Kr 402-502 fusion protein in ventral regions causes diminished expression of stripe 2 in ventral regions (B, red arrowhead). The stripe 3 enhancer is located 740 bp downstream of the UAS sites and is not affected by the fusion protein. However, neither the Gal4-Kr 402-502ΔPED (C) nor the Gal4-Kr 62-92 (D) fusion protein altered stripe 2 staining. (E to H) The NEE.UAS-lacZ transgene exhibits uniform staining in the ventral mesoderm in the absence of Gal4-Krüppel fusion proteins (E). However, expression of the Gal4-Kr 402-502 fusion gene under control of the Krüppel 2.5-kb (Kr 2.5) enhancer represses staining in central regions (F, arrowhead). Neither Gal4-Kr 402-502ΔPED (G) nor Gal4-Kr 62-92 (H) altered the staining pattern. (I to L) The NEE.UAS-twi-lacZ gene directs staining in the ventral mesoderm (I, arrow) and in two lateral lines in the neurogenic ectoderm (I, arrowheads). The Gal4-Kr 402-502 fusion protein represses the lateral lines produced by the modified NEE enhancer when expressed in central regions with the Kr 2.5 enhancer (J, arrowheads). There is no repression from the neighboring twist enhancer, so staining in the ventral mesoderm is uniform. Neither the Gal4-Kr 402-502ΔPED fusion protein (K) nor the Gal4-Kr 62-92 fusion protein (L) alters the normal staining pattern. (M to P) The NEE-5xUAS-lacZ reporter gene directs uniform staining in the ventral mesoderm in the absence of Gal4-Krüppel fusion proteins (M). This reporter gene differs from the one used in panels E to H by virtue of the positions of the Gal4 (UAS) binding sites (Fig. 1E). In this case, the five UAS sites were placed near the core promoter but far from the distal NEE enhancer. The Gal4-Kr 402-502 fusion protein diminishes staining in central regions when expressed under the control of the Kr 2.5 enhancer (N). Neither the Gal4-Kr 402-502ΔPED (O) nor the Gal4-Kr 62-92 fusion protein alters the expression pattern.
The coding sequence for the entire Rel domain of Dorsal (aa 1 to 403), Dl DBD, was amplified by PCR using appropriate primers containing NcoI and HindIII sites, digested, and cloned into the pRSET C vector. Likewise, the coding sequence for a full-length Krüppel (aa 1 to 502) was amplified by PCR using appropriate PCR primers containing BglII and KpnI sites, digested, and cloned into the pRSET B vector.
The following oligonucleotides were used to create a synthetic enhancer which contains optimal Bicoid, Dorsal, and Krüppel binding sites: 5′-gatctGGGATTAACCCGTTgtacGGGATTAACCCGTTg-3′ and 5′-gatccAACGGGTTAATCCCgtacAACGGGTTAATCCCa-3′. Lowercase letters represent the linker or spacer sequences. These were annealed, kinased, ligated, and digested by both BamHI and BglII. Three or seven (corresponding to 6 or 14 copies of the binding sites) head-to-tail tandem repeats of the oligonucleotides were inserted within the BamHI-BglII sites of modified pBluescript II KS(+) plasmid pBlueG, which contains a unique BglII site in place of SmaI. An additional EcoRI site was then created in pBlueG between the BamHI and XbaI sites. Six or 14 copies of the binding sites were isolated as EcoRI fragments and were inserted into the unique EcoRI site of the CaSpeR-AUG-βgal transformation vector containing the eve basal promoter, starting at −42 bp and continuing through codon 22 fused in frame with lacZ.
For transfection assays, the six head-to-tail tandem copies of overlapping Bicoid, Dorsal, and Krüppel sites plus the adjacent eve promoter were amplified by PCR from the CaSpeR-AUG-βgal vector, which is described above, with the appropriate primers containing BglII and NcoI sites and then inserted into pGL3-Basic. To construct the Krüppel DNA binding domain, expression vectors pPac Kr and pPac Kr9, which both correspond to aa 217 to 401, were amplified by PCR from plasmids previously described (32). The primers contained BamHI sites, and the upstream and downstream primers introduced start and stop codons, respectively. The products were cut with BamHI and ligated into the pPac vectors pPac Kr DBD and pPac Kr9 DBD, respectively. pPac Dorsal was a gift from Stephen Small.
In situ hybridization assays and transgenic embryos.
Embryos were collected from wild-type (yw) and mutant adults carrying different lacZ transgenes and then fixed and hybridized with digoxigenin-labeled antisense lacZ RNA probes as described previously (12). Transgenic strains were obtained by injecting yw embryos with various P element transformation vectors as described previously (27).
Germ line mosaics and fly stocks.
dCtBP germ line clones were generated as described previously (21) by the FLP-DFS technique (3). Briefly, hsFLP; FRT82B ovoD1/TM3 Sb males were mated with FRT82B P1590/TM3 Sb females. Embryos were collected for 24 h, aged another 48 h, heat shocked for 3 successive days at 37°C for 3 h, and then grown to adults. Virgin females lacking the Sb marker were selected and mated with yw males carrying lacZ reporter genes. Embryos were collected from this final mating and then fixed and hybridized with digoxigenin-labeled lacZ RNA probes.
The mutant alleles used in this study were bicoid (bcdE1), Krüppel (Kr1), and gastrulation defective (gd7). For the maternal mutation, males carrying the transgene were mated with virgin females homozygous for bcd or gd. For the Krüppel mutant, male flies containing both a single copy of the Kr1 allele and a single copy of the transgene were crossed to virgin females heterozygous for Kr1.
The following transgenic reporter strains were used in these studies: 2UG3-1 and 2UG3-2 (st2.UAS-st3-lacZ; see Fig. 2A to D) (21), G18.2 and G18.3 (NEE.UAS-lacZ; see Fig. 2E to H) (21), and RUCPT-3 and RUCPT-5 (NEE.UAS-twi-lacZ; see Fig. 2I to L) (21). The following Gal4-Kr driver lines were used in these studies: YN20-2, -3, and -4 (twi-Gal4-Kr 402-502; see Fig. 2B) (21); YN18-2, -3, and -4 (Kr-Gal4-Kr 402-502; see Fig. 2F, J, and N) (21); YN21-1, -2, and -3 (twi-Gal4-Kr 402-502ΔPED; see Fig. 2C) (21); and YN19-1, -2, and -3 (Kr-Gal4-Kr 402-502ΔPED; see Fig. 2G, K, and O). The laboratory fly stock G5.5 was used for the modified 700-bp NEE enhancer containing two synthetic Krüppel binding sites (see Fig. 3A to C) (8). G27.2 was used for the twist-NEE fusion gene containing a single Krüppel binding site proximal to the promoter (see Fig. 3D to F) (8). Strain A58 was used for the NEE-twist enhancer containing two Knirps binding sites proximal to the promoter (see Fig. 3G to I) (1).
FIG. 3.
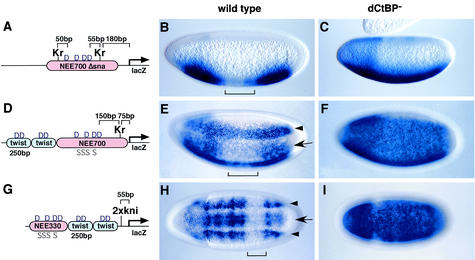
dCtBP is required for quenching and direct repression. Transgenic embryos express lacZ reporter genes containing synthetic Krüppel or Knirps binding sites (A, D, and G). Reporter gene expression was visualized by in situ hybridization using a digoxigenin-labeled lacZ antisense RNA probe. Embryos are oriented with the anterior side to the left. (A to C) Modified rhomboid NEE enhancer containing two synthetic Krüppel binding sites (Kr in panel A). These sites are located 50 bp from the nearest Dorsal activator sites (D in panel A). In normal embryos, there is a gap in the staining pattern (bracket) in central regions, where there are high levels of the Krüppel repressor (B). This repression is not observed in dCtBP mutants (C). (D to F) Reporter gene containing two copies of the 250-bp twist enhancer and 700-bp NEE enhancer (D). A single synthetic Krüppel binding site was placed 75 bp 5′ of the transcription start site. D and S, Dorsal and Snail binding sites, respectively (D). Both the lateral stripe of lacZ staining produced by the NEE enhancer (arrowhead) and the mesoderm staining (arrow) directed by the twist enhancer are diminished in central regions, where there are high levels of the Krüppel repressor (E, bracket). This repression is not observed in dCtBP mutants (F). (G to I) Reporter gene containing a 330-bp NEE enhancer and two copies of the 250-bp twist enhancer (G). Two tandem Knirps binding sites (2xkni) were placed 55 bp 5′ of the transcription start site. There is a gap in both lacZ staining patterns (H, arrow and arrowhead) in normal embryos. This gap occurs in the presumptive abdomen, where there are high levels of the Knirps repressor (H, bracket). This repression is lost in dCtBP mutants (I).
Transient-transfection assays.
Drosophila mbn-2 cells (4) were grown in Schneider's medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum. For each transfection 106 cells were plated per well of a 12-well plate. Transfections were performed by the calcium phosphate method (26). The following amounts of DNA were used: 0.5 μg of the 6×dl/Kr-eve-Luc reporter, 100 ng of pPac Dorsal, 500 ng of pPac Kr DBD, 500 ng of pPac Kr9 DBD, and 1 μg of CMVZ, which served as an internal control for transfection efficiency. The total amount of DNA in each sample was adjusted to 2.1 μg with an empty pPac vector. Two days after transfection cell extracts were made with lysis buffer (Promega) and luciferase activity was measured on a TD-20/20 luminometer (Turner Designs, Sunnyvale, Calif.). The results shown in Fig. 4D represent the averages of two separate experiments. Dorsal mediated nearly a 10-fold induction in the first experiment and just a 3- to 4-fold induction in the second. In both cases, the Krüppel DNA binding domain repressed luciferase activity, whereas the mutant form of the peptide, Kr9 DBD, had no effect on expression.
FIG. 4.
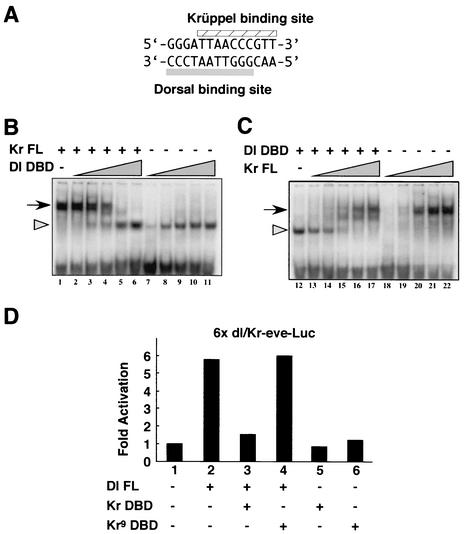
Krüppel and Dorsal proteins compete for overlapping binding sites. (A) Sequence of the synthetic 14-bp sequence containing overlapping Bicoid, Dorsal, and Krüppel binding sites. Bicoid recognizes the core sequence GGATTA. The Dorsal and Krüppel sites are indicated. (B and C) Gel shift assays. A 30-bp DNA fragment, containing the core 14-bp sequence shown in panel A plus 8 bp of linker sequence on either side was labeled with [γ-32P]ATP by T4 polynucleotide kinase. A His-tagged Krüppel protein (Kr FL) and a His-tagged Rel domain from Dorsal (Dl DBD) were used for the binding assays. Increasing amounts of Dl DBD protein with or without constant amounts of Kr FL protein (B) and the reciprocal condition (C) were used. After the binding, the resulting complexes were resolved by electrophoresis in nondenaturing 8% polyacrylamide gels. Arrows and arrowheads, Krüppel-probe and the Dorsal-probe complexes, respectively. Supershifted complexes were not observed, indicating that the two proteins did not bind to the probe at the same time. (D) Transient-transfection assays. Six tandem copies of the 14-bp synthetic sequence were attached to an eve-luciferase reporter gene containing the minimal eve promoter. Addition of a Dorsal expression vector caused more than a fivefold induction in expression above background levels (compare lanes 1 and 2). However, this induction was lost upon coexpression of the Krüppel DNA-binding domain (Kr DBD; lane 3). The Kr DBD lacks both the N-terminal and C-terminal repression domains, including the optimal dCtBP interaction motif at positions 464 to 470 of the wild-type protein. Expression of a mutant form of the Kr DBD that contains a single amino acid substitution in one of the C2H2 zinc fingers fails to repress activation by Dorsal (Kr9 DBD; lane 4). The mutant peptide is unable to bind DNA (25).
RESULTS
In vivo analysis of Krüppel repression domains.
Krüppel is a zinc finger DNA-binding protein that is composed of 502 aa residues (Fig. 1G) (16, 17). The quenching activity of the C-terminal repression domain (aa 402 to 502) requires a dCtBP interaction motif located at amino acids (aa) 464 to 470 (21). Another repression domain has been identified in cultured cells. It is located between aa 62 and 92 and does not contain a dCtBP interaction motif (Fig. 1G) (17). A transgenic embryo assay was used to determine whether this N-terminal repression domain might be a source for CtBP-independent repression in early embryos (16) (see below). The Gal4-Krüppel fusion proteins and lacZ reporter genes that were tested in these assays are summarized in Fig. 1.
A Gal4-Krüppel fusion protein containing aa 402 to 502 (Fig. 1H) created gaps in the staining patterns directed by st2.UAS-st3-lacZ, NEE.UAS-lacZ, and NEE.UAS-twi-lacZ (Fig. 2B, F, and J). For the st2.UAS-st3-lacZ and NEE.UAS-twi-lacZ reporter genes, repression was observed only for the staining pattern produced by the enhancer containing UAS binding sites. For example, the binding of the Gal4-Krüppel fusion protein to the stripe 2 enhancer did not alter expression from the neighboring stripe 3 enhancer (Fig. 2B) (34). Similarly, the binding of the fusion protein to the rhomboid NEE enhancer did not alter expression from the twist enhancer (Fig. 2J). Substitutions in three of the amino acid residues within the dCtBP interaction motif (PEDLSMH to AAALSMH) (Fig. 1I) eliminated the repression activity of an otherwise normal Gal4-Krüppel fusion protein (Fig. 2C, G, and K).
There is a second potential dCtBP interaction motif, located between aa 414 and 420 (PLDLSED), that weakly binds dCtBP in vitro (data not shown; Fig. 1G). However, this second motif was not sufficient to support discernible repression activity in vivo (Fig. 2C, G, and K). These results suggest that most or all of the repression activity of the Gal4-Krüppel 402-502 fusion protein resides within the major dCtBP interaction motif between amino acid residues 464 and 470. Moreover, repression was not observed for a Gal4-Krüppel fusion protein that contains the N-terminal repression domain (aa 62 to 92). These results suggest that the C-terminal dCtBP motif mediates most or all of the quenching activity in the early embryo.
The proximal UAS site within the NEE.UAS-lacZ reporter gene (Fig. 1C and 2E) is located 120 bp 5′ of the core promoter, slightly beyond the range of Krüppel-mediated repression (8, 21). In contrast, the UAS sites map within 50 bp of critical Dorsal sites within the NEE. Thus, repression of the reporter gene is most likely due to quenching rather than the direct repression of the core promoter. Another lacZ reporter was created to investigate this issue, NEE-5xUAS-lacZ (Fig. 1E and 2M). The most distal UAS site is located 250 bp 5′ of the most proximal Dorsal binding site within the modified 700-bp NEE enhancer, while the most proximal UAS site is located just 57 bp 5′ of the transcription start site of the hsp70 promoter. The Gal4-Krüppel 402-502 fusion protein attenuated lacZ expression (compare Fig. 2N with O and P). This direct repression was not obtained with the mutagenized fusion protein lacking the dCtBP interaction motif or with a fusion protein containing the N-terminal repression domain (Fig. 2O and P). These results suggest that the C-terminal dCtBP interaction motif is essential for both quenching and direct repression.
dCtBP is essential for both quenching and direct repression.
Previous studies suggest that Krüppel mediates quenching by recruiting dCtBP to distal enhancers, such as the eve stripe 2 enhancer (21). An NEE-lacZ reporter gene that contains two synthetic Krüppel recognition sequences located 50 bp 5′ of the most distal Dorsal binding site and 50 bp 3′ of the most proximal site was created (Fig. 3A) (8, 21). This enhancer lacks the native Snail repressor sites and therefore directs lacZ expression in both lateral and ventral regions of early embryos. lacZ staining was diminished in central regions due to the localized expression of the Krüppel repressor (Fig. 3B). This gap in the pattern was eliminated in Kr1/Kr1 mutant embryos (data not shown; see below). Krüppel also failed to repress the reporter gene in mutant embryos derived from dCtBP germ line clones (Fig. 3C). These results indicate that dCtBP+ gene activity is required for the quenching activity of the Krüppel repressor.
Subsequent experiments were done to determine whether dCtBP is required for the direct-repression activity of Krüppel and another short-range repressor, Knirps. lacZ transgenes with either Krüppel or Knirps binding sites located near the core promoter were examined (Fig. 3D and G). Both transgenes contain two tandem copies of the 250-bp twist proximal enhancer placed either upstream or downstream of rhomboid lateral stripe enhancers (NEE). In wild-type embryos, the enhancers direct additive patterns of expression in the lateral neurogenic ectoderm and ventral mesoderm (1, 8). A single Krüppel binding site located 75 bp 5′ of the transcription start site (Fig. 3D) was sufficient to create a central gap in both staining patterns (Fig. 3E). Staining directed by the tandem twist enhancers was nearly eliminated, whereas the lateral stripe produced by the rhomboid NEE was diminished (Fig. 3E). Repression of the twist pattern is almost certainly due to direct repression, since the solo Krüppel site mapped more than 800 bp from the nearest Dorsal activator site in the twist enhancer (Fig. 3D). Krüppel-mediated repression was lost when the transgene was introduced into embryos obtained from dCtBP germ line clones (Fig. 3F). There was no longer a central gap in the staining pattern. Moreover, there was a fusion of the expression patterns directed by the twist and NEE enhancers due to a loss in the activity of the Snail repressor. Normally, Snail binds to the NEE enhancer and represses expression in the ventral mesoderm, thereby restricting the staining pattern to lateral stripes in the neurogenic ectoderm (Fig. 3D and G). The broad uniform staining pattern obtained in dCtBP mutants suggests that the dCtBP corepressor is required for the direct repression of the core promoter.
Similar results were obtained with the Knirps repressor. In this case, two tandem Knirps binding sites were placed 55 bp 5′ of the transcription start site (Fig. 3G). In wild-type embryos, there was a clean gap in both the NEE-mediated lateral stripes and the twist-mediated staining pattern in the ventral mesoderm (Fig. 3H). This gap coincided with the site of Knirps expression in the presumptive abdomen. As seen for Krüppel, the gap in the staining patterns disappeared in dCtBP mutant embryos (Fig. 3I). These results suggest that dCtBP is required for the direct repression activities of both Krüppel and Knirps.
Competitive binding of the Krüppel repressor and Dorsal activator.
The preceding experiments suggest that dCtBP is required for both quenching and the direct repression of the core promoter. A synthetic lacZ reporter gene was prepared to determine whether Krüppel can mediate repression by competition and, if so, whether dCtBP is required for this repression. A 14-bp oligonucleotide that contains overlapping Dorsal and Krüppel binding sites was synthesized (Fig. 4A). Each subunit of the Dorsal homodimer binds to an inverted half-site: GGG…CCC (10). Krüppel binds DNA as a monomer, and the core recognition sequence includes the CCC Dorsal half-site. This short sequence also contains an optimal Bicoid binding site (GGATTA) (see, e.g., reference 33). This motif is located between the two half-sites of the Dorsal recognition sequence and overlaps the Krüppel consensus sequence.
Gel shift assays were done to determine whether Dorsal and Krüppel bind the synthetic 14-bp sequence in a mutually exclusive manner (Fig. 4B and C). A 30-bp fragment that contains the 14-bp sequence along with 8 bp of flanking sequence at each end was synthesized. In the first set of experiments, a full-length Krüppel protein produced in E. coli was mixed with the 30-bp fragment and fractionated on an agarose gel (Fig. 4B, lane 1). A shifted Krüppel-DNA complex was observed. The addition of increasing amounts of the Dorsal DNA-binding domain (Dl DBD; aa 1 to 403) resulted in the gradual loss of this complex (Fig. 4B, lanes 2 to 6). A new complex that is identical in size to those obtained with the Dorsal protein alone was observed (Fig. 4B, lanes 7 to 11). These results suggest that high concentrations of the Dorsal DNA-binding domain can displace Krüppel.
Similar results were obtained in reciprocal DNA-binding assays (Fig. 4C). In this case, the shifted Dorsal-DNA complex was formed in the absence of Krüppel (Fig. 4C, lane 12). The addition of increasing amounts of the Krüppel protein resulted in the gradual loss of the Dorsal-DNA complex (Fig. 4C, lanes 13 to 17). A new complex was obtained that has the same size as the one observed with increasing amounts of Krüppel in the absence of the Dorsal protein (Fig. 4C, lanes 18 to 22). These results suggest that increasing amounts of Krüppel can displace Dorsal-DNA complexes. Thus, the gel shift assays indicate mutually exclusive binding of Dorsal and Krüppel to the overlapping binding sites contained within the 14-bp fragment.
Transient-transfection assays were used to determine whether the Krüppel DNA-binding domain is sufficient to mediate transcriptional repression. Six tandem copies of the synthetic oligonucleotide used in the preceding DNA-binding assays were attached to an eve-luciferase reporter gene containing the minimal eve promoter. This reporter gene was introduced into mbn-2 cultured cells (a Drosophila blood cell line) along with various expression vectors containing Dorsal or Krüppel coding sequences (Fig. 4D). An expression vector containing the full-length Dorsal coding sequence (Dl FL) produced a 6× induction in luciferase activity (Fig. 4D, compare lane 2 with lane 1). However, an expression vector containing the Krüppel DNA-binding domain (Kr DBD; aa 217 to 401) reduced luciferase activity to background levels (Fig. 4D, lane 3). This reduction in reporter gene expression was not obtained with a Krüppel expression vector that contains a single amino acid substitution in the zinc finger DNA-binding domain (Kr9 DBD; Fig. 4D, lane 4). These results suggest that Krüppel can repress the synthetic enhancer by simply binding DNA and excluding the Dorsal activator. Repression does not depend on Krüppel protein sequences that map outside the DNA-binding domain. Subsequent experiments were done to determine whether Krüppel can mediate repression by competition in transgenic embryos.
dCtBP is dispensable for the competition activity of Krüppel.
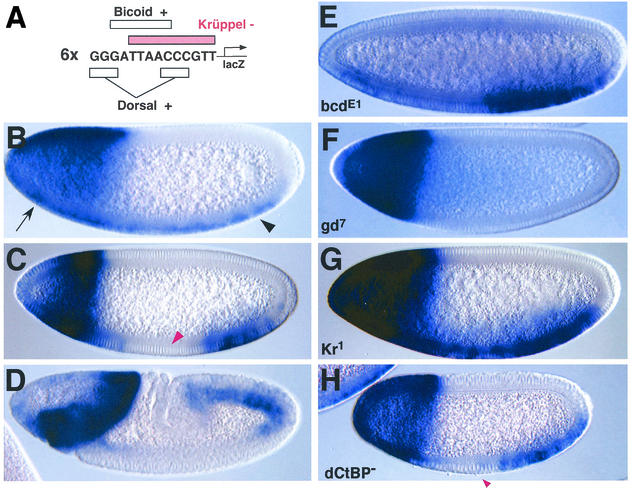
Either 6 or 14 tandem copies of the 14-bp synthetic enhancer sequence were attached to a lacZ reporter gene containing the minimal, 42-bp eve promoter region (Fig. 5A). Similar results were obtained with both fusion genes, and most of the following results were obtained with individual strains carrying the transgene with six copies attached. The transgene exhibits a combinatorial pattern of lacZ staining in wild-type (yw) embryos (Fig. 5B to D). Staining was first detected in the anterior 40% of 120-min embryos, presumably in response to the broad Bicoid activator gradient (Fig. 5B) and was also detected in both anterior regions and along the entire length of the ventral mesoderm (Fig. 5B). Mesoderm expression was first seen at the time when the maternal Dorsal protein was released from the cytoplasm and entered nuclei (see, e.g., reference 10). During cellularization, staining was lost in central regions, presumably due to the onset of Krüppel expression (Fig. 5C). In addition, there was a refinement in the anterior staining pattern, so that it became restricted to the anterior one-fourth of the embryo and exhibited a reasonably sharp posterior border. This staining pattern persisted during gastrulation and germ band elongation (Fig. 5D).
FIG. 5.
dCtBP is not required for repression by competition. (A) Sequence of the synthetic enhancer. Six tandem copies were attached to a minimal eve-lacZ reporter gene. The exact binding sites for Bicoid, Dorsal, and Krüppel are indicated. (B to D) lacZ expression in wild-type (yw) embryos at nuclear cycle 12 or 13 (B), mid-cycle 14 (C), and germ band elongation (D). Transgenic embryos were hybridized with a digoxigenin-labeled lacZ antisense RNA probe. They are oriented with the anterior side to the left and the dorsal side up. The transgene is activated by Bicoid in anterior regions (B, arrow) and by Dorsal in ventral regions (B, arrowhead). Staining is repressed in central regions by Krüppel (C, red arrowhead). (E to G) The transgene was introduced into bicoid, gd, and Krüppel mutants (panels E, F, and G, respectively). There is a loss of staining in anterior regions in bicoid mutants (compare panels C and E). The reduced staining in anterior regions of the ventral mesoderm is probably due to the anterior expansion of the Krüppel staining pattern in bicoid mutants. In gd7 mutants, lacZ staining is lost in the ventral mesoderm (compare panels C and F). The central gap formed in wild-type (yw) embryos (C) is derepressed in Kr1 mutants (G). (H) The bicoid-dorsal-Krüppel-lacZ transgene introduced into dCtBP mutants. The central gap in the staining pattern is not lost (H, arrowhead; compare panels H and C). This result suggests that dCtBP is dispensable for the Krüppel-mediated repression of the synthetic enhancer.
The transgene was introduced into different mutant backgrounds in order to confirm that the synthetic enhancer is regulated by Bicoid, Dorsal, and Krüppel (Fig. 5E to G). The anterior staining pattern was eliminated when the transgene was introduced into embryos derived from females homozygous for a null mutation in bicoid (Fig. 5E versus C). However, staining persisted in ventral regions in response to the Dorsal gradient. The loss of staining in the anterior regions correlates with an anterior expansion of the Krüppel expression pattern in bicoid mutants (5). The maternal Dorsal gradient was eliminated in embryos derived from females that are homozygous for a null mutation in gastrulation defective (gd7/gd7). lacZ staining in the ventral mesoderm of these mutants was lost (Fig. 5F versus C). However, staining persisted in anterior regions, presumably in response to the Bicoid gradient, which is unaffected in gd mutants. The transgene was also crossed into Kr1/Kr1 mutant embryos (Fig. 5G). The central gap of repression seen in wild-type embryos was essentially abolished in Kr mutants (Fig. 5G versus C). There may be a subtle attenuation in central regions due to the low levels of Krüppel protein that are retained in this mutant (Kr1 is not quite a null allele [25]). The anterior staining pattern directed by the Bicoid gradient may be a bit broader in Kr mutants than in wild-type embryos (Fig. 5G versus C), suggesting that the Krüppel repressor might help refine the pattern. These results indicate that the artificial enhancer is activated by Bicoid and Dorsal but repressed by Krüppel (Fig. 5C). Competition is the likely form of repression since the Krüppel repressor sites directly overlap the Bicoid and Dorsal activator sites.
One of the central goals of this study was to determine whether Krüppel requires dCtBP when it mediates repression by competition. This issue was investigated by crossing the transgene into mutant embryos derived from germ line clones produced in dCtBP/+ females (Fig. 5H). Krüppel continued to induce a central gap of repression in these mutants (Fig. 5H). In fact, the repression obtained in dCtBP mutants was comparable to that observed in wild-type embryos (Fig. 5H versus C). These results provide a clear example of Krüppel-mediated repression in the absence of the dCtBP corepressor. In contrast, Krüppel failed to repress transcription in dCtBP mutants when Krüppel and Dorsal sites did not overlap (Fig. 3).
DISCUSSION
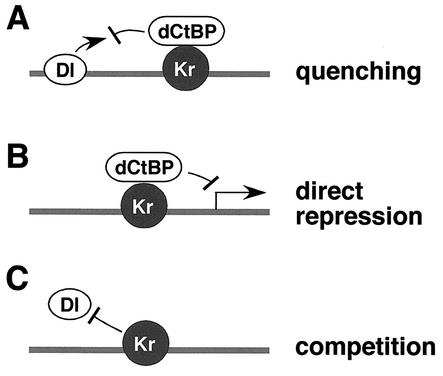
This study provides evidence for two distinct mechanisms of short-range repression, corepressor-dependent (quenching and direct repression) and corepressor-independent (competition) repression (summarized in Fig. 6). In addition, this is the first demonstration that transcriptional repression by competition does not require a corepressor in transgenic Drosophila embryos. dCtBP is dispensable when Krüppel binding sites directly overlap Dorsal activator sites. However, dCtBP is essential for repression when the Krüppel and Dorsal sites are nonoverlapping and can be coordinately occupied (21). The previous analysis of eve stripe 2 regulation led to the proposal that the Krüppel repressor establishes the posterior stripe 2 border via competition (32). Two of the Krüppel repressor sites contained within the stripe 2 enhancer overlap Bicoid activator sites. Subsequent studies led to the surprising observation that Krüppel binding sites need not overlap activator sites in order to mediate transcriptional repression (6, 8).
FIG. 6.
Role of dCtBP in the three major modes of repression. There are at least three distinct mechanisms of transcriptional repression (for a review, see reference 7): quenching, direct repression, and competition. We demonstrated that quenching and direct repression require dCtBP, while repression by competition is dCtBP independent. (A) Repression by quenching. Repressors and activators bind to adjacent sites, and then the repressor inhibits the function of the neighboring activators. dCtBP is required for the quenching activities of three short-range repressors: Krüppel, Knirps, and Snail (21). The Dorsal (Dl) activator is inhibited by a complex of the short-range Krüppel repressor (Kr) and dCtBP when Dorsal binding sites are within 100 bp of the Krüppel binding sites. (B) Direct repression of the core promoter. A repressor binds within 100 bp from the transcription start site and directly blocks promoter activity. Krüppel (Kr) and Knirps, along with dCtBP, can mediate direct repression when bound near the promoter. (C) Repression by competition. Activators and repressors can compete for a common binding site. We created an artificial enhancer containing overlapping Bicoid, Dorsal, and Krüppel binding sites. Krüppel and Dorsal proteins compete for binding to DNA in vitro and in vivo. In the absence of dCtBP, Krüppel continues to compete with Dorsal and to repress the reporter gene. Moreover the Krüppel DNA-binding domain is sufficient to mediate repression in cultured mbn-2 cells.
There are three Krüppel binding sites in the minimal, 480-bp eve stripe 2 enhancer (33). Two of the sites directly overlap Bicoid activator sites. In both cases, it is likely that the binding of the Krüppel repressor precludes the binding of Bicoid. This type of simple competition is probably not restricted to the regulation of eve stripe 2. For example, one of the mixed Bicoid/Krüppel binding sites in the stripe 2 enhancer is conserved in a newly identified ftz enhancer, which appears to be activated by Bicoid but repressed by Krüppel (V. Calhoun and M. Levine, unpublished data). The two enhancers contain the same composite recognition sequence, ACGGATTAA. Repression by competition probably governs, in part, the regulation of the rhomboid lateral stripe enhancer (NEE) since some of the Snail repressor sites directly overlap critical Dorsal and basic helix-loop-helix activator sites (11).
An implication of this study is that the residual activity of the Krüppel repressor observed in dCtBP mutants might be due to repression by competition. For example, Krüppel can repress the hairy stripe 7 enhancer when misexpressed throughout early embryos using the heat-inducible hsp70 promoter (16). This repression is retained in dCtBP mutants. Moreover, a mutant form of Krüppel that lacks the dCtBP interaction motif can repress hairy stripe 7 expression (16). hairy stripe 7 is activated, at least in part, by Caudal and repressed by Krüppel (15). Interestingly, five Krüppel binding sites directly overlap Caudal activator sites within the hairy stripe 7 enhancer. Similar arguments apply to the Knirps repressor, which helps establish the posterior border of eve stripe 3 (35). The stripe 3 pattern expands in kni−/kni− mutant embryos but is essentially unchanged in dCtBP mutants (13). Knirps repressor sites might overlap critical activator sites, such as binding sites for D-Stat (40) or an unknown activator(s) within the stripe 3 enhancer. Previous studies suggest that Brinker can also function independently of corepressors when bound to sites that directly overlap critical Smad activator sites within cis regulatory regions of Dpp target genes (14, 29, 30). Direct evidence for simple competition was obtained in transient-transfection assays. The Krüppel DNA-binding domain is sufficient to inhibit activation of the synthetic enhancer by Dorsal in cultured mbn-2 cells (Fig. 4D).
The results reported in this study exclude another possible explanation for the residual activity of the Krüppel and Knirps repressors in dCtBP mutants: direct repression of the core promoter. In principle, direct repression could involve distinct corepressor proteins (Fig. 6). If so, then target genes that contain promoter-proximal Krüppel and Knirps binding sites might be repressed in dCtBP mutants. However, the lacZ fusion genes containing either a single Krüppel site or two tandem Knirps sites located near the transcription start site are no longer repressed in dCtBP mutants. Thus, we favor the possibility that the residual Krüppel and Knirps repression activities depend on competition between overlapping activator and repressor binding sites within selected target enhancers.
The demonstration that both quenching and direct repression require dCtBP raises the possibility that these two seemingly distinct forms of repression employ similar mechanisms. At least three types of models come to mind. First, dCtBP could disrupt physical interactions between upstream activators and the RNA polymerase II transcription machinery/mediator complex at the core promoter (18, 39). Perhaps dCtBP masks or modifies the activation domains of upstream activators. However, this model can account for quenching but not direct repression. A second type of model involves local chromatin modification. dCtBP contains a well-conserved dehydrogenase catalytic center and binds NADH (41). Perhaps dCtBP modifies proteins such as histones and helps condense DNA within the limits of a nucleosome. In Saccharomyces cerevisiae, the Rpd3 histone deacetylase (HDAC) causes histone deacetylation over a distance of just two nucleosomes (28). A third model is that dCtBP “poisons” the RNA polymerase II transcription machinery and impedes its binding, assembly, or function at the core promoter. This poisoning can be accomplished by placing dCtBP-dependent repressors near the core promoter or by looping distal enhancers to the promoter. According to the latter model, the linkage requirement seen for short-range repressors (they must bind within 100 bp of adjacent activators) might reflect a reliance of the repressors on linked activators in order to loop to the core promoter.
Acknowledgments
We thank Kevin Wright for construction of the His-Dorsal vector and Stephen Small for providing the pPac Dorsal vector. We also thank Angelike Stathopoulos and Anna Di Gregorio for valuable advice.
Y.N. was supported by the Uehara Memorial Foundation. This work was funded by an NIH grant (GM 34431).
REFERENCES
- 1.Arnosti, D. N., S. Gray, S. Barolo, J. Zhou, and M. Levine. 1996. The gap protein knirps mediates both quenching and direct repression in the Drosophila embryo. EMBO J. 15:3659-3666. [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, J. M., T. Subramanian, U. Schaeper, M. La Regina, S. Bayley, and G. Chinnadurai. 1993. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 12:469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou, T. B., and N. Perrimon. 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gateff, E., L. Gissmann, R. Shrestha, N. Plus, H. Pfister, J. Schroeder, and H. Zur Hausen. 1980. Characterization of two tumorous blood cell lines of Drosophila melanogaster and the viruses they contain, p. 517-533. In E. Kurstak, K. Maramarosch, and A. Dubendorfer (ed.), Invertebrate systems in vitro. Elsevier/North-Holland Biomedical Press, Amsterdam, The Netherlands.
- 5.Gaul, U., and H. Jackle. 1989. Analysis of maternal effect mutant combinations elucidates regulation and function of the overlap of hunchback and Kruppel gene expression in the Drosophila blastoderm embryo. Development 107:651-662. [DOI] [PubMed] [Google Scholar]
- 6.Gray, S., P. Szymanski, and M. Levine. 1994. Short-range repression permits multiple enhancers to function autonomously within a complex promoter. Genes Dev. 8:1829-1838. [DOI] [PubMed] [Google Scholar]
- 7.Gray, S., H. Cai, S. Barolo, and M. Levine. 1995. Transcriptional repression in the Drosophila embryo. Phil. Trans. R. Soc. Lond. B Biol. Sci. 349:257-262. [DOI] [PubMed] [Google Scholar]
- 8.Gray, S., and M. Levine. 1996. Short-range transcriptional repressors mediate both quenching and direct repression within complex loci in Drosophila. Genes Dev. 10:700-710. [DOI] [PubMed] [Google Scholar]
- 9.Gray, S., and M. Levine. 1996. Transcriptional repression in development. Curr. Opin. Cell Biol. 8:358-364. [DOI] [PubMed] [Google Scholar]
- 10.Ip, Y. T., R. Kraut, M. Levine, and C. A. Rushlow. 1991. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell 64:439-446. [DOI] [PubMed] [Google Scholar]
- 11.Ip, Y. T., R. E. Park, D. Kosman, E. Bier, and M. Levine. 1992. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 6:1728-1739. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, J., D. Kosman, Y. T. Ip, and M. Levine. 1991. The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 5:1881-1891. [DOI] [PubMed] [Google Scholar]
- 13.Keller, S. A., Y. Mao, P. Struffi, C. Margulies, C. E. Yurk, A. R. Anderson, R. L. Amey, S. Moore, J. M. Ebels, K. Foley, M. Corado, and D. N. Arnosti. 2000. dCtBP-dependent and -independent repression activities of the Drosophila Knirps protein. Mol. Cell. Biol. 20:7247-7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick, H., K. Johnson, and A. Laughon. 2001. Repression of dpp targets by binding of brinker to Smad sites. J. Biol. Chem. 276:18216-18222. [DOI] [PubMed] [Google Scholar]
- 15.La Rosee, A., T. Häder, H. Taubert, R. Rivera-Pomar, and H. Jäckle. 1997. Mechanism and Bicoid-dependent control of hairy stripe 7 expression in the posterior region of the Drosophila embryo. EMBO J. 16:4403-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Rosee-Borggreve, A., T. Häder, D. Wainwright, F. Sauer, and H. Jäckle. 1999. hairy stripe 7 element mediates activation and repression in response to different domains and levels of Krüppel in the Drosophila embryo. Mech. Dev. 89:133-140. [DOI] [PubMed] [Google Scholar]
- 17.Licht, J. D., W. Hanna-Rose, J. C. Reddy, M. A. English, M. Ro, M. Grossel, R. Shaknovich, and U. Hansen. 1994. Mapping and mutagenesis of the amino-terminal transcriptional repression domain of the Drosophila Krüppel protein. Mol. Cell. Biol. 14:4057-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik, S., and R. G. Roeder. 2000. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25:277-283. [DOI] [PubMed] [Google Scholar]
- 19.Mannervik, M., Y. Nibu, H. Zhang, and M. Levine. 1999. Transcriptional coregulators in development. Science 284:606-609. [DOI] [PubMed] [Google Scholar]
- 20.Nibu, Y., H. Zhang, and M. Levine. 1998. Interaction of short-range repressors with Drosophila CtBP in the embryo. Science 280:101-104. [DOI] [PubMed] [Google Scholar]
- 21.Nibu, Y., H. Zhang, E. Bajor, S. Barolo, S. Small, and M. Levine. 1998. dCtBP mediates transcriptional repression by Knirps, Krüppel, and Snail in the Drosophila embryo. EMBO J. 17:7009-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nibu, Y., H. Zhang, and M. Levine. 2001. Local action of long-range repressors in the Drosophila embryo. EMBO J. 20:2246-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poortinga, G., M. Watanabe, and S. M. Parkhurst. 1998. Drosophila CtBP: a Hairy-interacting protein required for embryonic segmentation and hairy-mediated transcriptional repression. EMBO J. 17:2067-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptashne, M., and A. Gann. 2001. Genes and signals. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Redemann, N., U. Gaul, and H. Jackle. 1988. Disruption of a putative Cys-zinc interaction eliminates the biological activity of the Kruppel finger protein. Nature 332:90-92. [DOI] [PubMed] [Google Scholar]
- 26.Rio, D. C., and G. M. Rubin. 1985. Transformation of cultured Drosophila melanogaster cells with a dominant selectable marker. Mol. Cell. Biol. 5:1833-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin, G. M., and A. C. Spradling. 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218:348-353. [DOI] [PubMed] [Google Scholar]
- 28.Rundlett, S. E., A. A. Carmen, N. Suka, B. M. Turner, and M. Grunstein. 1998. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature 392:831-835. [DOI] [PubMed] [Google Scholar]
- 29.Rushlow, C., P. F. Colosimo, M. C. Lin, M. Xu, and N. Kirov. 2001. Transcriptional regulation of the Drosophila gene zen by competing Smad and Brinker inputs. Genes Dev. 15:340-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saller, E., and M. Bienz. 2001. Direct competition between Brinker and Drosophila Mad in Dpp target gene transcription. EMBO Rep. 2:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeper, U., J. M. Boyd, S. Verma, E. Uhlmann, T. Subramanian, and G. Chinnadurai. 1995. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl. Acad. Sci. USA 92:10467-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Small, S., R. Kraut, T. Hoey, R. Warrior, and M. Levine. 1991. Transcriptional regulation of a pair-rule stripe in Drosophila. Genes Dev. 5:827-839. [DOI] [PubMed] [Google Scholar]
- 33.Small, S., A. Blair, and M. Levine. 1992. Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J. 11:4047-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small, S., D. N. Arnosti, and M. Levine. 1993. Spacing ensures autonomous expression of different stripe enhancers in the even-skipped promoter. Development 119:767-772. [PubMed] [Google Scholar]
- 35.Small, S., A. Blair, and M. Levine. 1996. Regulation of two pair-rule stripes by a single enhancer in the Drosophila embryo. Dev. Biol. 175:314-324. [DOI] [PubMed] [Google Scholar]
- 36.Sollerbrant, K., G. Chinnadurai, and C. Svensson. 1996. The CtBP binding domain in the adenovirus E1A protein controls CR1-dependent transactivation. Nucleic Acids Res. 24:2578-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanojevic, D., T. Hoey, and M. Levine. 1989. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature 341:331-335. [DOI] [PubMed] [Google Scholar]
- 38.Thummel, C. S., A. M. Boulet, and H. D. Lipshitz. 1988. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene 74:445-456. [DOI] [PubMed] [Google Scholar]
- 39.Woychik, N. A., and M. Hampsey. 2002. The RNA polymerase II machinery: structure illuminates function. Cell 108:453-463. [DOI] [PubMed] [Google Scholar]
- 40.Yan, R., S. Small, C. Desplan, C. R. Dearolf, and J. E. Darnell, Jr. 1996. Identification of a Stat gene that functions in Drosophila development. Cell 84:421-430. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, Q. D., W. Piston, and R. H. Goodman. 2002. Regulation of corepressor function by nuclear NADH. Science 295:895-897. [DOI] [PubMed] [Google Scholar]