Abstract
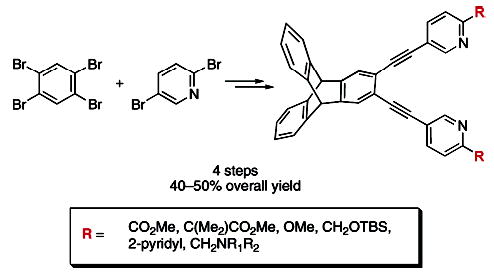
An efficient route to a new family of dinucleating ligands has been developed. A convergent strategy to these ligands involved dual Sonogashira cross-coupling of 2,3-diethynyltriptycene with a variety of functionally diverse 5-bromopyridines. The resultant ligands were accessed in four steps and 40–50% overall yield from 1,2,4,5-tetrabromobenzene. Synthesis of an imidazole and a quinoline derivative by this method is also described.
Enzymes that utilize diiron active sites catalyze a variety of key functions in Nature. These include the selective hydroxylation of methane to methanol (MMOH),1 conversion of deoxyribonucleotides to ribonucleotides (RNR-R2),2 and dehydrogenation of fatty acid side chains (Δ9D).3 The active sites of this family of enymes have several common structural features, including a carboxylate-rich coordination environment and syn histidine N-donor substituents. To illustrate, the diiron(II) active site of reduced MMOH is shown in Figure 1.
Figure 1.
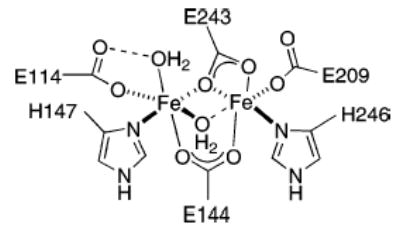
Representation of methane monooxygenase hydroxylase (MMOH) active site in its reduced, diiron(II) state.
Much progress has been made over the last two decades in creating synthetic ligands to model the active sites of these diiron enzymes, although many challenges remain.4 One goal that has not been realized is to prepare the diiron(IV) oxo intermediate of MMOH in a synthetic complex.5 This achievement would be valuable because this high-valent intermediate can insert an oxygen atom into the strong C–H bond (104 kcal/mol) of methane. DFT calculations have suggested that enforcing a syn coordination geometry of the N-donors with respect to the Fe–Fe vector could have an important stereoelectronic consequence in reproducing the hydrocarbon oxidation activity of MMOH.6
Recently, a ligand capable of inducing syn coordination of two N-donors was described.7 This molecule, termed Et2-BCQEB (Figure 2), was used to synthesize the diiron compound [Fe2(Et2BCQEB)(μ-O2CArTol)3](OTf)], where −O2CArTol is 2,6-di(p-tolyl)benzoate. Although Et2BCQEB produced a syn N-donor complex, the ligand was not readily available. The synthesis involved seven steps and furnished Et2BCQEB in only 2% overall yield from anthranilic acid. Therefore, efforts were made to develop a more efficient route to a second generation of syn N-donor ligands to facilitate further studies in this area.
Figure 2.
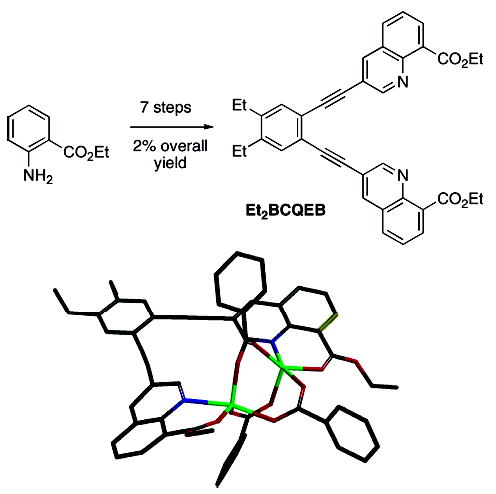
Synthetic route to Et2BCQEB and structure of [Fe2(Et2-BCQEB)(μ-O2CArTol)3](OTf)]. The triflate counterion and tolyl groups of O2CArTol are omitted for clarity.
To expedite the synthesis of the syn N-donor ligands, a more convergent strategy was sought. The new plan incorporates a late-stage coupling between the heteroaryl N-donors and a diethynylarene.8 The linker 2,3-diethynyltriptycene 2 (Scheme 1) was chosen as a modification of the original diethynylbenzene scaffold of Et2BCQEB, in anticipation that the extra aromatic rings would enhance the crystallinity of its metal complexes. Dual Sonigashira coupling of 2,3-dibromotriptycene (1)9 and 2.5 equiv of trimethylsilylacetylene using a catalyst combination of 2 mol % Pd(PPh3)4 and 2 mol % CuI in piperidine at 100 °C furnished the coupled product, which was desilylated with K2CO3 in MeOH, giving 2 in 81% yield over two steps.
Scheme 1 .
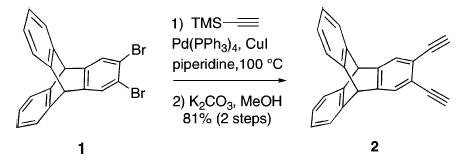
Synthesis of 2,3-Diethynyltriptycene (2)
To provide greater access to a variety of heteroaryl coupling partners, the syn N-donor substituents were changed from quinoline to pyridine. Several bromopyridines (4a–d) that could serve as components in ligands similar to Et2-BCQEB were readily available from 2,5-dibromopyridine 3 (Scheme 2),10 a versatile and commercially available starting material that can be selectively functionalized in either the two or the five position.11
Scheme 2 .
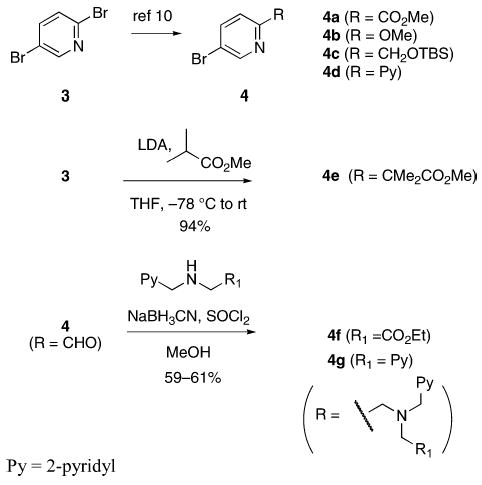
Heteroaryl Coupling Partners from 2,5-Dibromopyridine
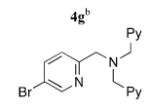
To add to the collection of available coupling partners, routes to three other 5-bromopyridines were developed (Scheme 2). Reaction between the lithium enolate of methyl isobutyrate and 3 proceeded smoothly,12 providing the pyridine acetic acid derivative 4e in one step and 94% yield from 3.13 Reductive aminations between 2-formyl-5-bromopyridine (4, R = CHO)10c and two aminomethyl pyridine derivatives14 were mediated by thionyl chloride and NaBH3-CN in MeOH, which afforded 4f and 4g in 61 and 63% yield, respectively.
Next, Sonogashira coupling reactions between 2,3-diethynyltriptycene (2) and the bromopyridines 4a–g were investigated (entries 1–7, Table 1). Optimal conditions for coupling of 2 with 4a–g incorporated 2.1–2.5 equiv of the 5-bromopyridine, 10 mol % of Pd(PPh3)4 as the catalyst, and a combination of Et3N and THF at 55 °C. A number of functional moieties that ligate metal ions were tolerated in the coupling reaction, including bipyridyl (4d) and tripyridylamine (4g). Typical coupling reaction times ranged from 18 to 48 h. Efforts to incorporate a Cu(I) cocatalyst, such as CuI, resulted in only trace amounts of product being formed. Nevertheless, the syn N-donor ligands 5a–g were obtained in 79–99% yield under the optimized conditions.
Table 1.
Synthesis of Syn N-Donor Ligands 5a–i
| entry | ArXb-d | product | yield (%) (overall yield)e |
|---|---|---|---|
| 1 |
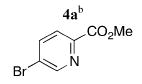
|
5a | 93 (47)f |
| 2 |
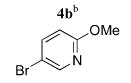
|
5b | 79 (40)f |
| 3 |
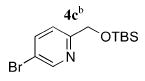
|
5c | 97 (40)f |
| 4 |
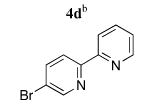
|
5d | 93 (49)f |
| 5 |
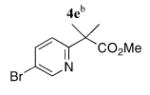
|
5e | 80 (47)d |
| 6 |
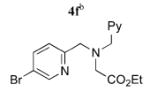
|
5f | 99 (50)f,g |
| 7 |
|
5g | 87 (44)f,g |
| 8 |
|
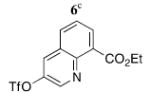
5h | 67 (9)h |
| 9 |
|
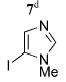
5i | 63 (32)f |
2.2–2.5 equiv of ArX was used.
10 mol % of Pd(PPh3)4, Et3N, THF, 55 °C.
10 mol % of PdCl2(PPh3)2, 5 mol % of CuI, Et3N, THF, rt.
10 mol % of PdCl2(PPh3)2, piperidine, 65 °C.
The overall yield is calculated for the longest linear sequence from commercially available materials.
Overall yield from 1,2,4,5-tetrabromobenzene.
The yield was measured by 1H NMR spectroscopy using an internal standard.
Overall yield from anthranilic acid.
Cross-coupling of 2 with two other heteroaryls was also examined. To provide a point of comparison to the original synthesis of Et2BCQEB, the reaction of 2 with quinoline triflate 67 (entry 8) was performed. Coupling of 2 with 6 gave a mixture of mono- and dicoupled product under the same conditions used to assemble 5a–g but proceeded smoothly with 10 mol % of PdCl2(PPh3)2 and 50 mol % of CuI in a mixture of Et3N and THF at rt, providing the diquinoline product 5h in 67% yield. To access an imidazole derviative, reaction of 2 with 4-iodo-3-methylimidazole15 (7, entry 9) was conducted with 10 mol % of PdCl2(PPh3)2 in piperidine at 65 °C, affording the diimidazole 5i in 65% yield.
By using a more convergent synthetic strategy and switching from quinoline to pyridine N-donor substituents, the overall efficiency of preparing the syn N-donor ligands described in this account was considerably enhanced. The pyridine-based ligands 5a–g were obtained in four steps and 40–50% overall yield from 1,2,4,5-tetrabromobenzene, a substantial improvement over the efficiency of assembling Et2BCQEB (2% over seven steps). The higher convergence of this strategy also allowed the quinoline congener 5h to be accessed in two fewer steps and nearly five times (9% overall yield from anthranilic acid16) more efficiently than Et2BCQEB. Access to the imidazole derviative 5i was also possible by using this strategy, proceeding in four steps and 33% overall yield.
In conclusion, an efficient synthesis of a new family of syn N-donor ligands is described. With ready access to these ligands, their iron coordination chemistry can now be investigated. Preliminary work indicates that the pyridine-based ligands support dimetallic structures. The mixed iron–sodium complex of ligand 5a, [FeNa(5a)(μ-O2CTrp)3] (Figure 3), was recently isolated and characterized by X-ray crystallography.17 Replacement of sodium by iron in this complex was possible, providing a rare opportunity to study metal substitution chemistry in a dinuclear structure. Further experiments involving the iron coordination chemistry of these ligands, as well as the synthesis of other derviatives by the strategy disclosed herein, are ongoing.
Figure 3.
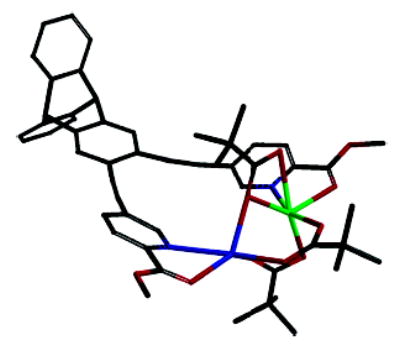
Synthesis and structure of [FeNa(5a)(μ-O2CTrp).17 The iron atom is shown in blue. The triptycene carboxylates (Trp = 9-triptycenyl) are abbreviated as pivolate groups for clarity.
Acknowledgments
This work was supported by Grant No. GM32134 from the National Institute of General Medicine Sciences. J.J.K. thanks the National Institutes of Health for a postdoctoral fellowship (F32 GM069236-01), and A.J.M. thanks the MIT UROP program for funding.
Footnotes
Supporting Information Available: Experimental procedures for preparation of for 2, 4e–g, and 5a–i including characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Angew Chem, Int Ed. 2001;40:2782–2807. doi: 10.1002/1521-3773(20010803)40:15<2782::AID-ANIE2782>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Stubbe J, van der Donk WA. Chem Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 3.Fox BG, Lyle KS, Rogge CE. Acc Chem Res. 2004;37:421–429. doi: 10.1021/ar030186h. [DOI] [PubMed] [Google Scholar]
- 4.a Tshuva EY, Lippard SJ. Chem Rev. 2004;104:987–1012. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]; b Tolman WB, Que L., Jr J Chem Soc, Dalton Trans. 2002;5:653–660. [Google Scholar]; c Du Bois J, Mizoguchi TJ, Lippard SJ. Coord Chem Rev. 2000;200–202:443–485. [Google Scholar]
- 5.A di(μ-oxo)diiron(IV) species similar to Q was thought to be generated with the ligand BPMCN, which has an all-N-donor ligand set. A recent study reveals this compound to be mononuclear, however, illustrating the difficulty of the task. See: Jensen MP, Costas M, Ho RYN, Kaizer J, Mairata i Payeras A, Münck E, Que L, Jr, Rohde JU, Stubna A.J Am Chem Soc 200512710512–10525.and ref. cited therein. [DOI] [PubMed] [Google Scholar]
- 6.Density functional theory (DFT) calculations implicate the oxygen atom trans to the two histidines in the di(μ-oxo) intermediate Q to be the reactant that is inserted into the C–H bond of methane. Baik MH, Gherman BF, Friesner RA, Lippard SJ.J Am Chem Soc 200212414608–14615. [DOI] [PubMed] [Google Scholar]
- 7.Kuzelka J, Farrell JR, Lippard SJ. Inorg Chem. 2003;42:8652–8662. doi: 10.1021/ic034928e. [DOI] [PubMed] [Google Scholar]
- 8.For an example of this type of strategy, see: Kawano T, Kuwana J, Shinomaru T, Du CX, Ueda I.Chem Lett 20011230–1231. [Google Scholar]
- 9.,3-Dibromotriptycene is available in one step and 62% yield from 1,2,4,5-tetrabromobenzene. See: Hart H, Bashir-Hashemi A, Luo J, Meador MA.Tetrahedron 1986421641–1654. [Google Scholar]
- 10.a Song JJ, Yee NK. J Org Chem. 2001;66:605–608. doi: 10.1021/jo0013554. [DOI] [PubMed] [Google Scholar]; b Comins DL, Killpack MO. J Org Chem. 1990;55:69–73. [Google Scholar]; c Wang X, Rabbat P, O’Shea P, Tillyer R, Grabowski EJJ, Reider PJ. Tetrahedron Lett. 2000;41:4335–4338. [Google Scholar]; d Fang YQ, Hanan GS. Synlett. 2003;6:852–854. [Google Scholar]
- 11.Substitution and palladium-catalyzed cross-coupling reactions occur selectively at the 2 position of 3, due to that site’s higher electrophilictiy, whereas lithium halogen exchange occurs selectively at the 5 position. For early examples, see: (a) Testaferri L, Tiecco M, Tingoli M, Bartoli D, Massoli A.Tetrahedron 1985411373–1384. [Google Scholar]; b Tilley JW, Zawoiski S. J Org Chem. 1988;53:386–390. [Google Scholar]; c Bolm C, Ewald M, Felder M, Schlingloff G. Chem Ber. 1992;125:1169–1190. [Google Scholar]
- 12.For a related example with 6,6′-dibromo-2,2′-bipyridyl, see: Zhu YZ, Li ZP, Ma JA, Tang FY, Kang L, Zhou QL, Chan AS.Tetrahedron: Asymmetry 200213161–165. [Google Scholar]; For a palladium-catalyzed example, see: Jorgensen M, Lee S, Liu X, Wolkowski JP, Hartwig JF.J Am Chem Soc 200212412557–12565. [DOI] [PubMed] [Google Scholar]
- 13.This is a major improvement from the known route to PACs of this type; 5-bromopyridine acetic acid methyl ester is available in four steps and <20% overall yield from 3-bromopyridine. Jones G, Pitman MA, Lunt E, Lythgoe DJ, Abarca B, Ballesteros R, Elmasnaouy M.Tetrahedron 1997538257–8268. [Google Scholar]
- 14.Policar C, Lambert F, Cesario M, Morgenstern-Badarau I. Eur J Inorg Chem. 1999:2201–2207. [Google Scholar]
- 15.Holden KG, Mattson MN, Cha KH, Rapoport H. J Org Chem. 2002;67:5913–5918. doi: 10.1021/jo0111210. [DOI] [PubMed] [Google Scholar]
- 16.The lower overall yield in the preparation of 6f is largely due to inefficient steps in the preparation of the quinoline triflate 7, which is available in four steps and 13% yield from anthranilic acid.7
- 17.Kodanko, J. J.; Xu, D.; Lippard, S. J. Submitted for publication.


