Abstract
Interleukin-13 (IL-13) is a cytokine secreted by Th2 lymphocytes that is capable of inducing expression of 15-lipoxygenase (15-LO) in primary human monocytes. We recently demonstrated that induction of 15-LO requires the activation of Jak2 and Tyk2 kinases and Stats 1, 3, 5, and 6. Since IL-13-induced 15-LO expression was inhibited by H7 (a serine-threonine kinase inhibitor), we predicted that Stat serine phosphorylation may also be crucial for 15-LO expression. In this study, we present evidence indicating that IL-13-induced 15-LO mRNA expression was detectable as early as 1 h by real-time reverse transcription-PCR. We found that IL-13 induced a time-dependent serine phosphorylation of both Stat1 and Stat3, detectable at 15 min after IL-13 treatment. In addition, the activation of p38 mitogen-activated protein kinase (MAPK) was detected in a time-dependent fashion, with peak phosphorylation at 15 min after IL-13 treatment. SB202190, a p38 MAPK-specific inhibitor, markedly inhibited IL-13-induced Stat1 and Stat3 serine phosphorylation as well as DNA binding. Furthermore, treatment of cells with Stat1 or Stat3 decoys significantly impaired IL-13-induced 15-LO expression. Taken together, our results provide the first evidence that IL-13 induces p38 MAPK phosphorylation/activation, which regulates Stat1 and Stat3 serine 727 phosphorylation. Both of these events are important steps in IL-13-induced 15-LO expression in human monocytes.
Monocytes are one of the unique cell types that can respond to the T lymphocyte-derived cytokines interleukin (IL)-4 and IL-13 (14). One of the novel proteins induced upon monocyte exposure to IL-4 and IL-13 is the lipid-oxidizing enzyme called 15-lipoxygenase (15-LO) (11, 35, 44). 15-LO is expressed and enzymatically active in human atherosclerotic lesions (8). Through specific lipid oxidation, it generates a series of pro- and anti-inflammatory molecules, termed HPODEs/HODEs, which have been extracted from atherosclerotic lesions and are potent mediators of inflammatory responses (4, 16, 22). This enzyme is believed to be important for the pathogenesis of atherosclerosis as well as for generating potent inflammatory mediators.
Previously, our group showed the involvement of the Jak/Stat pathway in 15-LO induction in IL-13-treated human monocytes. Our studies demonstrated that activation of Jak2 and Tyk2 kinases was required for IL-13-induced 15-LO protein expression (44). Our recent studies have defined the functional IL-13 receptor complex, association of the Jaks with the receptor constituents, and the tyrosine phosphorylation of specific Stat molecules, Stat1, Stat3, Stat5, and Stat6, in response to IL-13 (43). These studies established a novel and selective signal transduction pathway from the receptor to the nucleus in human monocytes.
Tyrosine phosphorylation of Stat proteins by specific Jak kinases facilitates the dimerization of Stat molecules by binding the SH2 domain of one Stat molecule to the phosphotyrosine of another Stat (50). The dimerized Stat complex is then translocated to the nucleus, binds DNA, and regulates the expression of the corresponding target gene (63). In addition to tyrosine phosphorylation, serine phosphorylation of the Stat molecules is necessary for optimal transcriptional activity but has no influence on either dimer formation or nuclear translocation of the Stat complex (57, 58, 62). Recent reports suggest that the serine phosphorylation of Stat molecules [e.g., Stat1α (Ser727), Stat3 (Ser727), Stat5A (Ser 725), and Stat5B (Ser730)] is mediated by different kinases (1-3, 9, 19, 20, 26, 28, 29, 34, 46, 48, 56, 60).
A group of serine-threonine kinases, including ERK1 and -2, p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinases (JNKs), all components of distinct but evolutionally conserved MAPK signaling cascade, and protein kinase Cδ have been reported to be involved in the serine phosphorylation of Stat1 and Stat3 (3, 9, 19, 20, 26, 28, 29, 34, 46, 48, 56). The best-known and best-characterized candidates of the serine-threonine kinases are the ERKs, characterized by the p42 and p44 MAP kinases ERK1 and ERK2 (10). ERK1 and -2 are activated in response to a wide variety of growth factors and mitogens (7). JNKs are related to the ERKs but are regulated differently. Usually JNKs are activated in response to stress or cytokines (13, 31, 52). The p38 MAPK family is also activated in response to osmotic stress, cytokines, or phorbol esters (21, 55, 61). Upon activation, the MAP kinases phosphorylate and activate transcription factors, including the Stats. The activation and tyrosine/threonine phosphorylation of p38 MAPK are induced in response to several hematopoietic growth factors, including IL-3 and granulocyte-macrophage colony-stimulating factor, as well as physical and chemical stresses (17).
Previous studies suggested the involvement of p38 MAPK in Stat1 serine phosphorylation and transcriptional activation induced by alpha interferon (IFN-α) and IFN-γ (19). In addition, p38 MAPK was reported to play an important role in regulating Stat1 and Stat3 serine phosphorylation in response to the combination of IL-2 and IL-12 in T cells (20). Here, we report for the first time that in primary human monocytes, IL-13 induces activation of p38 MAPK, which in turn regulates the serine 727 phosphorylation of both Stat1 and Stat3. Furthermore, our results demonstrate that activation of p38 MAPK and subsequent phosphorylation of 727 serine residues on Stat1 and Stat3 molecules are critical in IL-13-induced 15-LO expression in human monocytes.
MATERIALS AND METHODS
Reagents.
Recombinant human IL-13 was purchased from Biosource International (Camarillo, Calif.). Antibody against rabbit reticulocyte 15-LO, cross-reacting with human 15-LO, was raised in sheep and was kindly provided by Joseph Cornicelli, Parke-Davis, or purchased from Cayman Chemical (Ann Arbor, Mich.). Antibodies specific for Stat1 phosphorylated on Ser-727 (P-Ser-Stat1) and for Stat3 phosphorylated on Ser-727 (P-Ser-Stat3) were generated in rabbits and characterized previously (18). Antiphosphotyrosine-Stat antibodies raised against Stat1(Y701) and Stat3(Y705) were obtained from Upstate Biotechnology and were used to detect tyrosine-phosphorylated Stat proteins on Western blots. Each of the Stat antibodies used was non-cross-reactive with other Stat molecules and was good for detecting proteins on Western blots. Antibodies to phospho-p38 (active p38) and to total p38 MAPK were purchased from Cell Signaling Technology (Beverly, Mass.). Actinomycin D was obtained from Sigma (St. Louis, Mo.). p38 MAPK inhibitors SB202190 and SB203580 and the inactive analogue SB202474 were purchased from Calbiochem (La Jolla, Calif.). H7 and its structural analogue HA1004 were purchased from Biomol (Plymouth Meeting, Pa.). All the pharmacological inhibitors were dissolved in dimethyl sulfoxide and stored at −20°C as 10 mM stock solutions.
Isolation of human monocytes.
Human peripheral blood monocytes were isolated from heparinized whole blood as described earlier (44). Adherent cells were released from the flask with 5 mM EDTA and plated in six-well cell culture plates (Nunclon, Roskilde, Denmark). The cell preparations usually had more than 95% monocytes and were maintained in Dulbecco's modified Eagle's medium containing 10% bovine calf serum at 37°C in the presence of 10% CO2.
RNA extraction and real-time RT-PCR.
Monocytes were plated in six-well culture plates at 5 × 106 cells/well in 2 ml of medium. Two hours after plating, cells were treated with 500 pM recombinant IL-13 for different times, as indicated. In some other experiments, monocytes were either treated with inhibitors for 30 min and then with IL-13 for various times or transfected with Stat1 and Stat3 decoys for 24 h before IL-13 was added. After each treatment, cells were collected and washed with phosphate-buffered saline (PBS). Total cellular RNA was extracted with the RNeasy minikit from Qiagen (Valencia, Calif.). One microgram of total RNA from different treatments was reverse transcribed with the TaqMan reverse transcription kit (PE Applied Biosystem, Foster City, Calif.). The cDNAs were then subjected to real-time reverse transcription (RT)-PCR with Sybr Green PCR core reagents. The PCRs were carried out in an ABI Prism 7700 sequence detector (PE Applied Biosystems), with the thermal cycle conditions suggested by the manufacturer. The PCR primers for 15-LO were selected from the regions displaying minimal sequence homology to human 5- and 12-LO and spanned two introns. The sequences of the 15-LO primers were 5′-GCTGGAAGGATCTAGATGACT-3′ and 5′-TGGCTACAGAGAATGACGTTG-3′. The amplicon for 15-LO was 294 bp. Data were acquired and analyzed with the sequence detector 7700 software.
Detection of Stat1 and Stat3 serine phosphorylation and p38 MAPK phosphorylation.
Monocytes were plated at 2 × 106/ml in six-well plates and treated with inhibitors (30 min) when required and then treated with IL-13 (500 pM) for different times as indicated. All cells were treated with 100 μM sodium orthovanadate solution 15 min before lysis (43). The cells were then washed three times with PBS to remove traces of Dulbecco's modified Eagle's medium-10% bovine calf serum before lysis. Whole-cell extracts and nuclear extracts were prepared according to previously published protocols (42, 47) with the exception that 0.5 M NaCl was used in the whole-cell lysis buffer (43). After the protein concentration was determined with the Bio-Rad protein assay kit, lysates were loaded on a sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis (SDS-8% PAGE) gel (10 to 75 μg/well). The proteins were transferred to a polyvinylidene difluoride membrane, blocked with 5% nonfat milk in PBS with 0.1% Tween 20, and the membranes were probed with anti-phosphoserine-Stat1 (Ser 727), anti-phosphoserine-Stat3 (Ser 727), or anti-phospho-p38 MAPK antibody overnight (diluted 1:1,000 in 3% bovine serum albumin in PBS with 0.1% Tween 20). A horseradish peroxidase-labeled secondary antibody (diluted 1:1,000 in 3% bovine serum albumin in PBS with 0.1% Tween 20) was then added for 1 h, and the hybridization signal was detected with an ECL kit (Pierce). In several experiments, immunoblots were stripped and reprobed to assess equal loading according to the previously published protocol (43).
15-LO protein induction and detection.
IL-13 (500 pM) was added to six-well plates containing 5 × 106 cells/well treated with inhibitors when required and then washed three times with PBS to remove the traces of Dulbecco's modified Eagle's medium-10% bovine calf serum. The cells were lysed, and the 15-LO protein was detected on Western blots following the previously described protocol (44). Quantification of Western blot results was conducted with the software program NIH Image.
Nuclear protein extraction and electrophoretic mobility shift assay.
To assess the role of p38 MAPK in IL-13-induced Stat1 and Stat3 DNA binding activity, an electrophoretic mobility shift assay was performed with nuclear extracts and specific Stat1 and Stat3 probes (Santa Cruz Biotechnology Inc.). Monocytes were treated with 5 μM SB202190 for 30 min, followed by the addition of IL-13 for another 30 min. Nuclear proteins were extracted, and the electrophoretic mobility shift assay was performed following the protocol described earlier (43).
Transfection of Stat1 and Stat3 decoy and mismatched oligodeoxyribonucleotides into monocytes.
The phosphorothioated oligodeoxyribonucleotides used for Stat1 and Stat3 decoys were purchased from Sigma-Genosys Biotechnologies, Inc. (The Woodlands, Tex.). These decoys were used in previous studies and shown to provide specific inhibition of Stat1 and Stat3 activities (24). The sequences for the decoys were 5′-ATATTCCTGTAAGTG-3′ and 3′-TATAAGGACATTCAC-5′ for Stat1; 5′-ATATTGGAGTAAGTG-3′ and 3′-TATAACCTCATTCAC-5′ for the mismatched Stat1 decoy; 5′-GATCCTTCTGGGAATTCCTAGATC-3′ and 3′CTAGGAAGACCCTTAAGGATCTAG-5′ for the Stat3 decoy; and 5′-GATCCTTCTGGGCCGTCCTAGATC-3′ and 3′-CTAGGAAGACCCGGCAGGATCTAG-5′ for the mismatched Stat3 decoy. The consensus sequences are in italics and correspond to the high-affinity Ly-6E gamma interferon-activated site (GAS) (27) and the acute-phase response element in the rat α2-macroglobulin gene (6), respectively.
The single-stranded oligodeoxyribonucleotides were annealed by incubation at 65°C for 10 min and then slowly being cooled to room temperature for 2 h. Double-stranded decoys were transfected into monocytes at a final concentration of 2 μM by using Superfect (Qiagen) following the manufacturer's instruction. After 24 h of transfection, cells were treated with IL-13 for either 4 h for the real-time RT-PCR analysis or for 24 h for Western blot analysis to assess 15-LO mRNA or protein expression.
RESULTS
Induction of 15-LO expression in IL-13-treated monocytes.
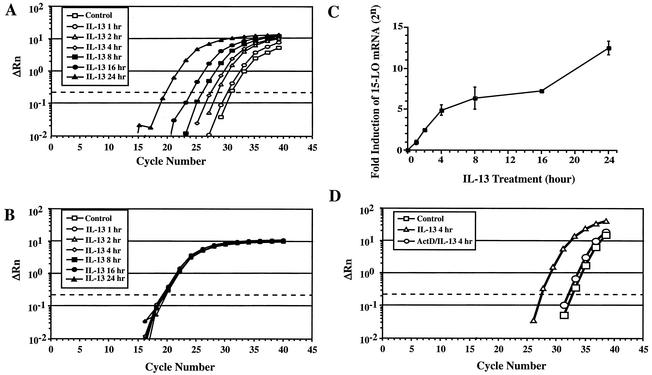
Earlier reports indicated that IL-4 and IL-13 can uniquely induce 15-LO expression and activity in human peripheral blood monocytes following 36 to 72 h of treatment (11, 35, 44). With a more quantitative approach, real-time RT-PCR, we performed a time-dependent analysis of IL-13 induction of 15-LO mRNA expression to examine this induction in greater detail. The results of representative experiments are illustrated in Fig. 1. 15-LO mRNA levels were detected in untreated monocytes and increased continuously with time of exposure to IL-13 up to 24 h (Fig. 1A and C). The induction of 15-LO mRNA was detected as early as 1 h of IL-13 treatment. The induction of the mRNA level was profound, with a remarkable >4,000-fold induction (212) by 24 h. The mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were nearly identical in all of these samples (Fig. 1B), indicating the specificity of the response. In addition, treatment of cells with 50 ng of actinomycin D per ml provided nearly complete inhibition (97.4%) of IL-13-induced 15-LO mRNA expression (Fig. 1D), suggesting the transcriptional regulation of 15-LO gene expression by IL-13.
FIG. 1.
IL-13 induces 15-LO gene expression. Amplification plots of 15-LO (A) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (B) from real-time RT-PCR. Monocytes (5 × 106) were treated with 500 pM IL-13 for different times. Total RNA was isolated, and RNA (1 μg) from each sample was used for real-time RT-PCR. The y axis shows the change in fluorescence intensity (ΔRn), and the x axis indicates the PCR cycle number. Each cycle represents a twofold difference in mRNA amounts. (C) This graph represents the induction of 15-LO mRNA expressed as induction as derived from the data presented in panel A. Data were collected from at least three independent experiments and are shown as means ± standard deviations. (D) Amplification plots showing inhibitory effects of actinomycin D on IL-13-induced 15-LO mRNA expression. The internal control glyceraldehyde-3-phosphate dehydrogenase plots (not shown) are similar to those in panel B. Data presented here are representative plots from two independent experiments.
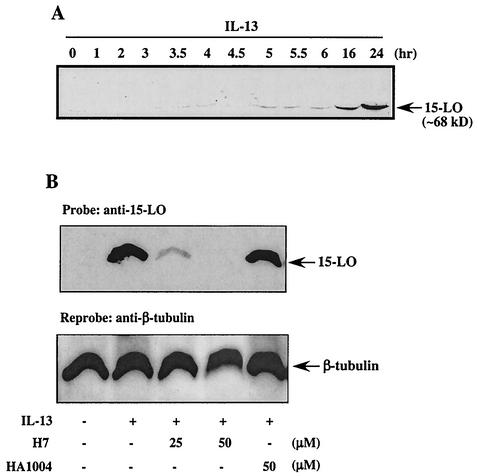
In contrast to 15-LO mRNA levels, 15-LO protein was not detectable in untreated monocytes, an observation previously reported by us and others (35, 44). A low level of 15-LO protein could be reproducibly detected as early as 5 h after IL-13 treatment. The 15-LO protein levels increased steadily and gave an easily detectable signal by 16 h (Fig. 2A). The level of protein continued to increase at 24 h. These results confirm and extend prior findings that induction of 15-LO protein by IL-13 takes as long as 16 h for a considerable level of expression. In addition, our real-time RT-PCR data provide the first careful quantification of IL-13-induced 15-LO gene expression in a time-dependent manner.
FIG. 2.
IL-13-induced 15-LO expression requires serine-threonine kinases. (A) Monocytes (5 × 106/well) were incubated with IL-13 (500 pM) for various times, and then lysates were prepared, and proteins (75 μg/lane) were separated by SDS-8% PAGE, blotted onto membranes, probed with 15-LO-specific antibody, and detected by ECL. The arrow indicates the position of 15-LO (68 kDa) as determined by migration relative to recombinant standards and molecular size markers. (B) Freshly isolated human blood monocytes (5 × 106/well) were treated for 30 min with the general serine-threonine kinase inhibitor H7 or its structural analogue HA1004 at various doses, followed by stimulation with 500 pM IL-13 for 24 h. The cells were lysed, and 50 μg of the extract was loaded in each lane on an SDS-8% PAGE gel, followed by blotting with antibody to 15-LO according to the protocols described under Materials and Methods. The blot was subsequently reprobed with β-tubulin to assess equal loading. This blot is presented in the lower panel.
Previously, we reported that IL-13-induced 15-LO expression in human monocytes is dependent on the tyrosine kinases Jak2 and Tyk2 (44). To evaluate whether serine-threonine kinases are required as well, we incubated monocytes with the general serine-threonine kinase inhibitor H7 and examined IL-13 induction of 15-LO protein expression after 24 h of treatment. As shown in Fig. 2B, treating cells with H7 provided a dose-dependent inhibition of IL-13-induced 15-LO expression, whereas HA1004, the structural analogue of H7, showed no detectable inhibitory effects, suggesting a specific role for particular serine-threonine kinases in the regulation of 15-LO expression.
IL-13 induces serine 727 phosphorylation of both Stat1 and -3.
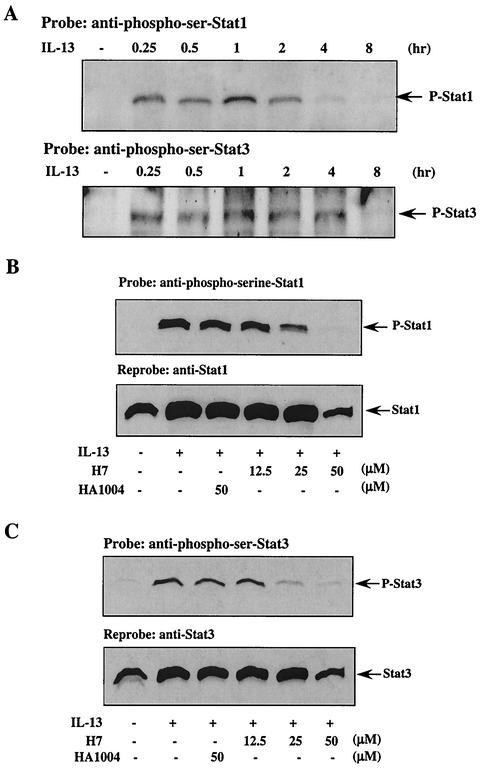
In our previous study, we showed tyrosine phosphorylation of both Stat1 and Stat3 in IL-13-treated human monocytes (43). For optimal transcriptional activity, these Stats also need to be phosphorylated on serine residues (57, 58, 62). Since serine-threonine kinase activity was required for 15-LO expression, we next evaluated whether IL-13 induced Stat serine phosphorylation. To determine whether IL-13 can induce both Stat1 and Stat3 serine phosphorylation, we used antibodies that specifically recognize phosphoserine at position 727 of Stat1 or Stat3. Monocytes were treated with IL-13 for different times from 0.25 to 8 h, and phosphoserine-Stat1 and -Stat3 were detected by Western blots. The results are presented in Fig. 3A and indicate that both Stat1 and Stat3 were phosphorylated on serine-727 in an IL-13-dependent manner and that this phosphorylation could be detected at 15 min after IL-13 treatment.
FIG. 3.
IL-13 induces Stat 1 and Stat 3 serine phosphorylation. (A) Freshly isolated human blood monocytes (10 × 106 per group) were treated with IL-13 (500 pM) for different time intervals or left untreated as indicated. The cells were lysed, and the nuclear extracts (10 μg/lane) were loaded on an SDS-8% PAGE gel and immunoblotted with anti-phosphoserine-Stat1 (p-Stat1) and anti-phosphoserine-Stat3 (p-Stat3) antibodies. The arrows indicate the positions of Stat1 (91 kDa) and Stat3 (89 kDa) based on the migration of molecular size markers in adjacent lanes. (B and C) Monocytes (5 × 106/well) were treated for 30 min with H7 or HA1004 at various doses, followed by IL-13 treatment (500 pM) for an hour. The cells were then harvested and lysed, and 50 μg of the whole-cell extract was loaded in each lane on an SDS-8% PAGE gel and immunoblotted with anti-phosphoserine-Stat1 (B) and anti-phosphoserine-Stat3 (C) antibodies. These blots were stripped and reprobed with Stat1 and Stat3 antibodies, respectively, as loading controls and are shown in the lower panels.
The time course of serine phosphorylation was comparable to that of tyrosine phosphorylation of Stat1 and Stat3 that occurred within 15 to 30 min (43). For Stat1, the serine phosphorylation peak occurred at 1 h, and after that the signal diminished. The serine phosphorylation status of Stat3 was maintained until 4 h and then declined. The general serine-threonine kinase inhibitor H7 blocked both IL-13-induced Stat1 and Stat3 Ser-727 phosphorylation in a dose-dependent manner (Fig. 3B and C). In contrast, HA1004 failed to show any inhibitory effect at similar concentrations (Fig. 3B and C).
Activation of p38 MAPK in IL-13-treated human monocytes.
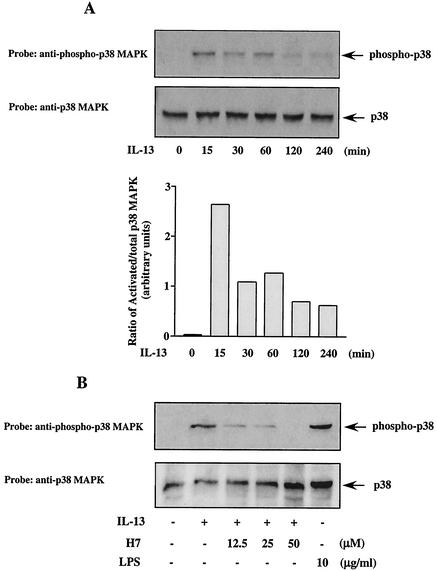
p38 MAPK has been implicated as an upstream kinase regulating Stat1 and Stat3 serine phosphorylation in several studies (19, 20, 53). To determine whether p38 MAPK was activated in response to IL-13, monocytes were stimulated with IL-13 for different lengths of time. Cell lysates were subjected to Western blot analysis with an anti-phospho-p38 MAPK antibody that specifically recognizes the activated form of this enzyme. The results revealed that p38 MAPK was activated by IL-13 from 15 min to later stages of treatments (up to 4 h) (Fig. 4A). The peak of activation was at 15 min, and the signal was gradually weaker thereafter. These results indicate that activation of p38 MAPK in response to IL-13 is an early event in the IL-13 signaling cascades. IL-13 activation of p38 MAPK was comparable to that induced by lipopolysaccharide, which is an established activator of p38 MAPK (38) (Fig. 4B). The general serine-threonine kinase inhibitor H7 also inhibited IL-13-induced p38 MAPK phosphorylation in a dose-dependent manner (Fig. 4B).
FIG. 4.
IL-13 induces phosphorylation of p38 MAPK. (A) Monocytes were left untreated or treated with IL-13 (500 pM) for different times as indicated. The cell lysates (75 μg/lane) were separated by SDS-PAGE and immunoblotted with anti-phospho-p38 antibody. The blot was subsequently stripped and reprobed with anti-p38 antibody to assess equal loading of protein in each lane. The arrow indicates the position of phospho-p38 MAPK and total p38 MAPK. The results are representative of at least three similar experiments. The ratio of activated p38 to total p38 MAPK from one of the representative data, shown in arbitrary units, was obtained with NIH Image software and is presented in the lower panel. (B) Monocytes (5 × 106/well) were treated for 30 min with H7 or lipopolysaccharide at various doses, followed by IL-13 stimulation (500 pM) for 15 min. Whole-cell lysates (50 μg/lane) were separated by SDS-8% PAGE and immunoblotted with anti-phospho-p38 MAPK antibody. The blot was stripped and reprobed with anti-p38 MAPK antibody as a loading control and is shown in the lower panel.
p38 MAPK is involved in Stat1 and Stat3 serine phosphorylation and DNA binding.
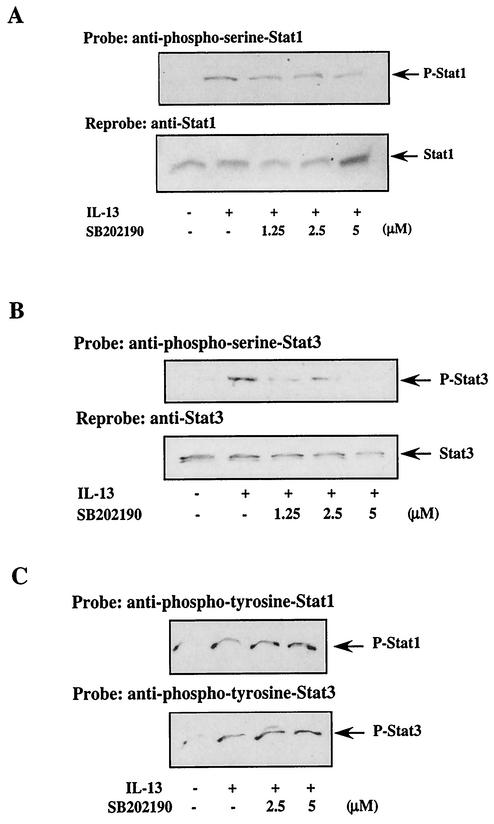
To determine whether p38 MAPK was involved in the serine phosphorylation of Stats 1 and 3, we used the reagent SB202190, which inhibits p38 and p38β activity but has no effect on p38γ and p38δ activities (15). An earlier report indicated that SB202190 was a more effective inhibitor of p38 activity than SB203580 (51), and thus we used SB202190. The results of a representative experiment are shown in Fig. 5. Monocytes were treated with SB202190 for 30 min prior to the addition of IL-13, either treated or not with IL-13 for an hour, and harvested. The serine phosphorylation status of both Stat1 and Stat3 was determined by Western blots with phosphoserine-Stat-specific antibodies. The results, presented in Fig. 5A, indicate that SB202190 inhibited Stat1 serine phosphorylation in a dose-dependent manner. The degree of inhibition was nearly complete (≈85%) at a 5 μM concentration of SB202190.
FIG. 5.
p38 MAPK activity is required for IL-13-induced Stat serine 727 phosphorylation. Freshly isolated human blood monocytes (5 × 106/well) were treated with IL-13 (500 pM) for an hour or left untreated and harvested. SB202190 (an inhibitor of p38 MAPK activity) was added to all the samples 30 min before the addition of IL-13, and the serine phosphorylation status of both Stat1 and Stat3 was studied on Western blots with anti-phosphoserine-Stat1 (A) and anti-phosphoserine-Stat3 (B) antibodies after running 50 μg/lane of the whole-cell extracts (preparation described under Materials and Methods) on an SDS-8% PAGE gel. Arrows indicate the positions of Stat1 (91 kDa) and Stat3 (89 kDa) calculated from the migration of molecular size markers in adjacent lanes. To ensure equal loading, the blots were stripped and reprobed with Stat1 and Stat3, respectively. These blots are shown in the lower panels. SB202190-treated lysates were also checked for Stat1 and Stat3 tyrosine phosphorylation on Western blots with anti-phosphotyrosine-Stat1 and anti-phosphotyrosine-Stat3 antibodies (C) after running the same amount of protein on SDS-8% PAGE gels as was loaded in panels A and B.
We conducted similar experiments in which we studied the effect of SB202190 on Stat3 serine phosphorylation. The cells were treated with SB202190 (30 min before IL-13 addition), then similarly treated with IL-13 for an hour, and harvested. Subsequently, phosphoserine-Stat3 was detected on Western blots, as shown on Fig. 5B. The results demonstrate that SB202190 (5 μM) had an effect on Stat3 serine phosphorylation (≈90% inhibition) similar to that exhibited in the case of Stat1 serine phosphorylation in Fig. 5A.
To evaluate whether SB202190 had any effects on Stat1 and Stat3 tyrosine phosphorylation, the same cell lysates were used for Western blot analysis. The phosphorylation status of Stat proteins was assessed with antiphosphotyrosine antibodies specific for either Stat1 or Stat3. As presented in Fig. 5C, treatment with SB202190 at up to 5 μM had no inhibitory effects on IL-13-induced Stat1 or Stat3 tyrosine phosphorylation.
To prove that the p38 inhibitors were not having general, nonspecific inhibitory effects on monocyte function, we examined the phosphorylation of p38 MAPK in the presence of the p38 inhibitors. Monocytes were incubated in the presence or absence of IL-13 (500 pM) and harvested at 1 h. The p38 MAPK inhibitors SB202190 (5 μM) and SB203580 (5 μM) were added into the wells 30 min before lysis. Whole-cell lysates (25 or 50 μg/lane) were loaded on an SDS-8% PAGE gel and immunoblotted with anti-phospho-p38 antibody. At these doses, neither SB202190 nor SB203580 had any effect on p38 phosphorylation (data not shown). Since these inhibitors interfere with p38 activity and not phosphorylation, the observation that p38 phosphorylation is not perturbed by these treatments was reassuring. In our hands, higher doses of these drugs can cause inhibition of p38 phosphorylation and therefore are mediating nonspecific effects on monocyte function (data not shown).
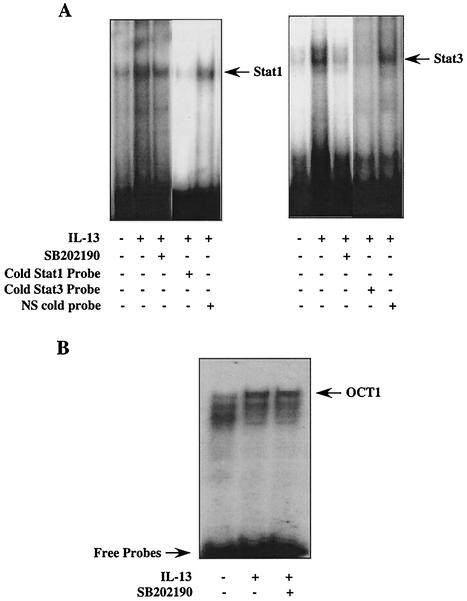
To examine whether inhibition of Stat1 and Stat3 serine phosphorylation by the p38 MAPK inhibitor affected their DNA binding activities, an electrophoretic mobility shift assay was employed with nuclear extracts from treated monocytes and 32P-labeled probes specific for Stat1 or Stat3. As demonstrated in Fig. 6, treatment of cells with the p38 MAPK inhibitor SB202190 markedly attenuated IL-13-induced Stat1 and Stat3 DNA binding activities (Fig. 6A) and had no detectable effect on DNA binding of an unrelated transcription factor, OCT1 (Fig. 6B). These data suggest that activation of p38 MAPK by IL-13 is required for maximal Stat1 and Stat3 DNA binding activities.
FIG. 6.
p38 MAPK regulates Stat1 and Stat3 DNA binding. Monocytes were either treated with 5 μM SB202190 for 30 min followed by addition of IL-13 for an additional 30 min or treated with IL-13 alone for 30 min. Nuclear proteins were extracted, and 5 μg of proteins was subjected to electrophoretic mobility shift assay analysis with 32P-labeled Stat1- and Stat3-specific probes (A) or the OCT1 probe (Promega, Madison, Wis.) (B). In some experiments, unlabeled probe in 50-fold excess was introduced for competitive inhibition, as indicated. Arrows point to the positions of protein and DNA complexes.
p38 MAPK activity is required for IL-13 signaling pathways leading to 15-LO expression.
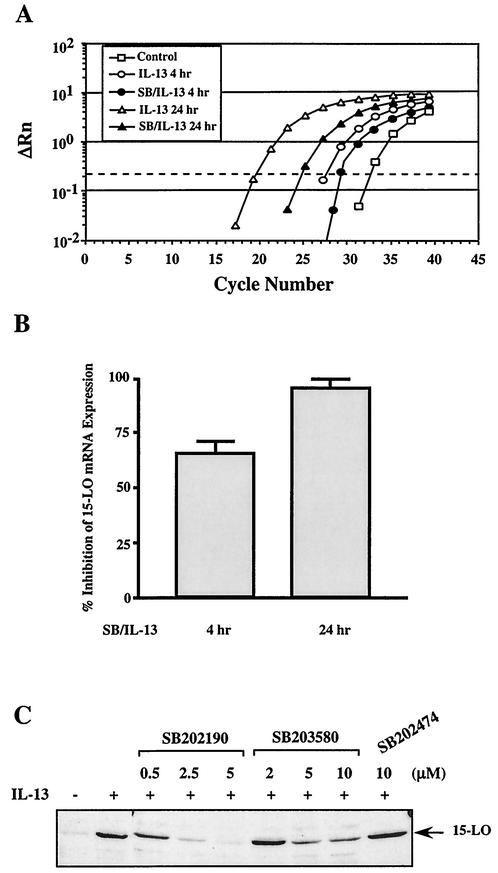
Induction of 15-LO by IL-13 in human monocytes is a controlled event with enormous physiological implications. Since p38 MAPK was shown to regulate IL-13-induced Stat1 and Stat3 serine phosphorylation and their DNA binding activities, we next examined whether p38 MAPK activity was required for the IL-13-induced expression of 15-LO mRNA and protein. For these experiments, monocytes were treated with SB202190 (5 μM) for 30 min and then incubated with IL-13 for an additional 4 or 24 h. Total RNA was extracted and subjected to real-time RT-PCR to quantify the expression of 15-LO mRNA. Treatment with SB202190 had profound effects on 15-LO mRNA levels, causing 71% inhibition of 15-LO mRNA expression at 4 h and 97% inhibition at 24 h (Fig. 7A and B).
FIG. 7.
p38 MAPK regulates IL-13-induced 15-LO expression. (A) Amplification plots of real-time RT-PCR show the inhibitory effects of SB202190 on IL-13-induced 15-LO mRNA expression. Monocytes were treated with IL-13 in the presence or absence of SB202190 for various times as indicated. Total cellular RNA was extracted, and RNA (1 μg) from each sample was used for real-time RT-PCR analysis. (B) Induction of 15-LO mRNA by IL-13 in the absence and presence of SB202190 was normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification. The percent inhibition was calculated from the data generated from three independent experiments. (C) Freshly isolated monocytes (5 × 106/well) were treated for 30 min with p38 MAPK inhibitors SB202190 and SB203580 or their structural analogue SB202474 at various doses, followed by stimulation with 500 pM IL-13 for 24 h. The cells were harvested and lysed, and 15-LO protein expression was detected on Western blots with a 15-LO antibody after running an SDS-8% PAGE gel with the extracts (75 μg/lane) prepared according to the protocols described under Materials and Methods. The arrow indicates the position of 15-LO (68 kDa).
We next evaluated the effect of the p38 MAPK inhibitors SB202190 and SB203580 on IL-13-induced 15-LO protein expression. Monocytes were incubated for 24 h with or without IL-13 in the presence or absence of these drugs. After incubation, the monocytes were harvested and lysed, and 15-LO protein was detected on Western blots. The results, shown in Fig. 7C, indicate that both of the p38 inhibitors suppressed expression of 15-LO protein in a dose-dependent manner. SB202190 treatment of monocytes caused a remarkable ≈98% inhibition of 15-LO induction at 5 μM, whereas SB203580 treatment at the same concentration inhibited induction by 70%. These results are consistent with the prior observation that SB203580 was less effective than SB202190 in inhibiting p38 activity (51). In contrast, SB202474, a negative structural analogue of SB203580 and SB202190, had no effect on the induction of 15-LO by IL-13.
Requirement for Stat1 and Stat3 in IL-13-induced 15-LO gene expression.
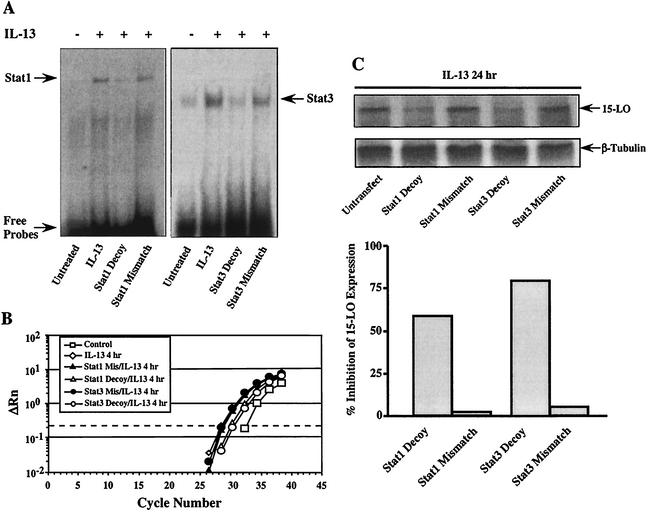
To directly assess the functional role of Stat1 and Stat3 in IL-13-induced 15-LO gene expression, we performed the following experiments with Stat1 and Stat3 decoy oligodeoxyribonucleotides. Monocytes were transfected with either Stat1 or Stat3 decoys (2 μM) for 24 h, followed by IL-13 treatment for an additional 4 h or 24 h. Total cellular RNA or protein was extracted and subjected to real-time RT-PCR or Western blot analysis. As indicated in Fig. 8A, transfection of monocytes with either the Stat1 or Stat3 decoy markedly attenuated Stat1 and Stat3 DNA binding activities induced by IL-13, respectively, compared to that of untransfected cells or cells transfected with mismatched oligodeoxyribonucleotides. Transfection of cells with decoys inhibited IL-13-induced 15-LO mRNA expression up to 65% for Stat1 and 81% for Stat3 compared to the levels in IL-13-treated cells (Fig. 8B). Cells transfected with mismatched oligodeoxyribonucleotides also showed slight inhibition (12% for Stat1 and 17% for Stat3) resulting from the nonspecific effects of transfection. Transfection of decoys inhibited IL-13-induced 15-LO protein expression by 56% for Stat1 and 79% for Stat3 compared to the levels in untransfected monocytes (Fig. 8C), whereas transfection of cells with mismatched oligodeoxyribonucleotides showed minimal inhibition, 1.6% for Stat1 and 7.6% for Stat3. These data provide the first direct evidence that activation of Stat1 and Stat3 by IL-13 is required for 15-LO expression.
FIG. 8.
Stat1 and Stat3 decoys inhibit IL-13-induced 15-LO expression. Monocytes were transfected with either Stat1- or Stat3-specific decoys or their corresponding mismatched double-stranded DNA for 24 h. Cells were then treated with IL-13 for an additional 30 min before harvest. Nuclear proteins were extracted, and 5 μg of protein from each sample was used for electrophoretic mobility shift assay analysis (A). Transfected cells were also treated with IL-13 for 4 or 24 h for 15-LO expression. Total cellular RNA or protein was extracted and subjected to real-time RT-PCR or Western blot analysis (B and C, respectively). (B) Amplification plots of real-time RT-PCR show the inhibitory effects of Stat1 and Stat3 decoys on IL-13-induced 15-LO mRNA expression. (C) Western blot analysis with 50 μg of protein from each sample and anti-15-LO antibody is presented in the upper panel. The blots were stripped and reprobed with anti-β-tubulin antibody as internal controls. The percent inhibition of 15-LO protein expression compared to the untransfected control is shown in the lower panel. Data shown are representative of two independent experiments.
DISCUSSION
IL-4 and IL-13 are the only cytokines that have been shown to induce both the expression and activity of 15-LO in monocytes/macrophages (11, 35, 44). Although 15-LO protein expression in reticulocytes is primarily regulated by posttranscriptional mechanisms (30, 36, 37), our data indicate that in human monocytes, IL-13 significantly and profoundly regulates 15-LO expression at the level of transcription. With the real-time RT-PCR technique, we were able to detect 15-LO mRNA in untreated monocytes. The induction of 15-LO mRNA by IL-13 occurred as early as 1 h after treatment, suggesting that early signaling events are involved in the regulation of 15-LO gene expression. Furthermore, IL-13 caused a profound increase in 15-LO mRNA levels of >4,000-fold after 24 h of treatment (Fig. 1). Because real-time PCR measures amplified products during the log phase of the reaction versus the plateau phase of the reaction used by conventional PCR, our approach provides a more quantitative and sensitive detection of 15-LO mRNA than earlier studies showing detection of 15-LO mRNA at 4 h after incubation of monocytes with IL-13 (35).
Previously, we reported that IL-13-mediated induction of 15-LO requires phosphorylation of Jak2 and Tyk2 (44) and also involves the participation of a heterodimeric receptor as well as tyrosine phosphorylation of selected members of the Stat family of proteins (43). In this study, we observed that IL-13-induced 15-LO expression was markedly inhibited by H7 (Fig. 2B). In addition, we found that IL-13 induced both Stat1 and Stat3 Ser 727 phosphorylation in a time-dependent manner (Fig. 3). Treatment of cells with H7 inhibited both Stat1 and Stat3 Ser 727 phosphorylation induced by IL-13. These data suggest that serine-threonine kinases may also play an important role in 15-LO expression through regulation of Stat1 and Stat3 activities.
While the Jak kinases are involved in tyrosine phosphorylation of Stat proteins, a variety of serine-threonine kinases, mostly the components of the MAPK signaling cascade, have been implicated in Ser phosphorylation of Stats. In human monocytes, we showed that p38 MAPK was phosphorylated/activated by IL-13 as early as 15 min of treatment and that H7 inhibited p38 MAPK activation (Fig. 4), indicating that p38 MAPK may be one of the key components involved in the early signaling events induced by IL-13. Although recent studies reported that IL-4 induces p38 MAPK phosphorylation/activation in dermal fibroblasts (25), our results provide the first evidence demonstrating that IL-13 can induce p38 MAPK activation in human monocytes.
Activation of p38 MAPK by IL-13 was involved in the phosphorylation of Stat1 and Stat3 on serine 727. Previous studies have indicated that p38 MAPK may be involved in Stat serine phosphorylation and transcriptional activation in different cell systems. In Ewing's sarcoma cells, IFN-β-induced Stat1 Ser 727 phosphorylation was observed with concomitant activation of p38 MAPK (46). In T cells, Gollob et al. (20) showed that the functional synergy between IL-12 and IL-2 was dependent upon the activation of p38 MAPK, which correlated with Stat serine phosphorylation. They proposed that this Ser 727 Stat phosphorylation induced by IL-12 and IL-2 might be mediated by p38 MAPK. Earlier, Goh et al. (19) suggested that p38 MAPK may regulate Stat1 serine phosphorylation and transcriptional activity in response to IFN-α and IFN-γ in HeLa S3 cells. While our results are in good agreement with the works by Goh et al. (19) and Gollob et al. (20), it is worth noting that in other systems, p38 MAPK regulates IFN-α-dependent gene transcription without affecting Stat1 and Stat3 serine phosphorylation, whereas protein kinase Cδ mediates Ser 727 phosphorylation on Stat1 (54, 56). These discrepant findings likely result from differences in cell-specific signaling pathways used by various stimuli. More studies are needed to sort out the various signal transduction cascades mediated by different cytokines.
In addition to its effects on Stat1 and Stat3 serine phosphorylation, treatment with SB202190 also markedly reduced Stat1 and Stat3 DNA binding activities (Fig. 6). These findings are not likely due to nonspecific effects of the inhibitor because Western blot analysis with the same cell lysates showed that treatment of cells with SB202190 did not alter IL-13-induced Stat1 and Stat3 tyrosine phosphorylation (Fig. 5C). Previous studies with various cell lines indicated that while Ser 727 phosphorylation of Stat1 and Stat3 is important for maximal transcriptional activation, this modification appeared to have minimal effects on their DNA binding activities (13, 20). The results from our study and others (5), however, suggest that in primary human monocytes, p38 MAPK activity is required for both Stat1 and Stat3 Ser 727 phosphorylation and their DNA binding activities. These findings may reflect the difference in signaling pathways between primary cells and cell lines. In cell lines, there may be multiple signaling pathways mediating Stat serine phosphorylation, whereas primary monocytes may only use selected pathways for their specifically committed biological functions. Multiple signaling pathways in cell lines may be involved in serine phosphorylation in addition to Ser 727 of Stat proteins or other targets that may facilitate Stat DNA binding activity. Therefore, inhibition of Stat Ser 727 phosphorylation or introduction of point-mutants of Stat Ser 727 into cell lines may not affect Stat DNA binding activities if other signaling cascades are able to compensate for the defect. The precise mechanisms of how Ser 727 phosphorylation affects Stat1 and Stat3 DNA binding in monocytes remains to be clarified.
The composition of the IL-13 receptor complex varies among various cell types. Differential signaling pathways induced in response to IL-13 and IL-4 have been reported in cell lines of various origins with varying expression of receptor components and related signaling components. It appears that when IL-2 receptor γC is part of the receptor complex and becomes phosphorylated upon exposure to IL-4, Jak1 and Jak3 are tyrosine phosphorylated in response to IL-4 (32, 45). In contrast, Jak2 and Tyk2 have been shown to be phosphorylated in cells where IL-2 receptor γC is not phosphorylated upon IL-13 exposure (for example, in monocytes) (43, 44). Earlier reports indicated Jak2 phosphorylation by IL-4 and IL-13 in cells not expressing γC or γC that is not associated with IL-4 receptor α in forming the receptor complex (33, 39). A significant role of Jak2 leading to activation of p38 MAPK (64) and the requirement for Jak2, Ras, and Raf for activation of ERK/MAPK (59) have also been described. Thus, the possibility of involvement of MAPK in the IL-13 signaling pathway seemed reasonable in human monocytes. In this study, we further proved that activation of p38 MAPK is required for IL-13-induced 15-LO expression. With pharmacological inhibitors specific to p38 MAPK, we found that the induction of 15-LO mRNA and protein by IL-13 was substantially blocked, with compatible degrees of inhibition (Fig. 7). Although we cannot rule out some contribution of translational regulation, our data strongly suggest that IL-13-induced 15-LO expression is primarily regulated at the transcriptional level in human monocytes.
Recently, two reports suggested that p38 MAPK is required for transcriptional responses of Stat target genes independently of Stat serine phosphorylation (40, 41). These findings may raise an alternative interpretation of our current results; however, there are fundamental differences between our study and theirs. First, p38 MAPK is not activated by IFN-γ (41) or by IL-4 alone (40) in the cell lines used in these reports, whereas p38 MAPK is immediately activated upon IL-13 stimulation in human monocytes (Fig. 4). Second, IFN-γ-induced Stat1 Ser 727 phosphorylation is mediated through kinases other than p38 MAPK (41) and Stat6 is not a direct substrate for p38 MAPK (40). In human monocytes, however, p38 MAPK is required for IL-13-induced Stat1/Stat3 Ser 727 phosphorylation and their DNA binding activities. These data again reflect the unique signaling pathways used by IL-13 in primary human monocytes.
Transcription factor decoy technology has recently been developed to inhibit the expression of several important genes with synthetic double-stranded oligodeoxyribonucleotides containing the consensus binding sequence of a transcription factor. Previous studies reported that Stat1 and Stat3 decoy treatment significantly inhibited the advanced glycation end product-induced DNA binding activities of Stat1 and Stat3 and cellular mitogenesis in NRK-49F cells (24). The results of our decoy experiments indicated that both Stat1 and Stat3 are mediated in IL-13-induced 15-LO expression (Fig. 8). Although the majority of attention has focused on the role of Stat6 in 15-LO expression (12, 23, 30, 49), our data provide novel evidence that Stat1 and Stat3 are also crucial players in the regulation of 15-LO expression.
Future studies pursuing IL-13 signal transduction pathways and the regulation of 15-LO expression in primary monocytes are somewhat constrained in this cell type. Since primary monocytes are essentially nontransfectable by cDNA, traditional approaches of expressing mutant Stats or dominant negative/constitutively active p38 are not feasible. Existing monocytic cell lines unfortunately do not respond to IL-13 in terms of induction of 15-LO expression. The only cell line that has been documented to possess this unique response is A549, an epithelial lung carcinoma. We found, however, that the IL-13-mediated signaling pathways differ between primary human monocytes and A549 cells because IL-13-induced 15-LO expression is insensitive to SB202190 in A549 cells (B. Xu and M. K. Cathcart, unpublished observations), whereas SB202190 markedly inhibits IL-13-induced 15-LO expression in monocytes (Fig. 7). Although murine bone marrow-derived macrophages do not express 15-LO upon exposure to murine IL-13 (A. Bhattacharjee and M. K. Cathcart, unpublished observations), we are currently investigating whether murine blood monocytes respond similarly to human monocytes. This would offer additional tools for evaluating in greater detail the mechanisms involved in IL-13 induction of 15-LO as well as allow us to evaluate the contributions of p38 MAPK and Stats in 15-LO expression and pathogenesis of atherosclerosis in vivo.
In summary, we report here the transcriptional regulation of 15-LO by IL-13 and the involvement of p38 MAPK in the IL-13 signaling pathway of human monocytes. Our results indicate that IL-13 activates p38 MAPK in a time-dependent manner and that this activation has a role in serine phosphorylation of Stats 1 and 3 on serine 727 as well as 15-LO expression. Finally, with the Stat1 and Stat3 decoys, our results suggest a direct link between Stat activation and IL-13-induced 15-LO expression in human monocytes. These data confirm that the JAK-STAT and MAPK signaling pathways interact at the level of Stat proteins and that this interaction is necessary for regulating 15-LO expression in IL-13-stimulated primary human monocytes. The results of this study provide new insights into how 15-LO expression is regulated by inflammatory cytokines in human monocytes and will help us to better understand the role of 15-LO and cytokines in the pathogenesis of asthma and atherosclerosis.
Acknowledgments
B. Xu and A. Bhattacharjee contributed equally to this study and share first authorship.
These studies were supported by NIH grant HL51068 awarded to M.K.C.
REFERENCES
- 1.Abe, K., M. Hirai, K. Mizuno, N. Higashi, T. Sekimoto, T. Miki, T. Hirano, and K. Nakajima. 2001. The YXXQ motif in gp 130 is crucial for STAT3 phosphorylation at Ser727 through an H7-sensitive kinase pathway. Oncogene 20:3464-3474. [DOI] [PubMed] [Google Scholar]
- 2.Beadling, C., J. Ng, J. W. Babbage, and D. A. Cantrell. 1996. Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway. EMBO J. 15:1902-1913. [PMC free article] [PubMed] [Google Scholar]
- 3.Bode, J. G., P. Gatsios, S. Ludwig, U. R. Rapp, D. Haussinger, P. C. Heinrich, and L. Graeve. 1999. The mitogen-activated protein (MAP) kinase p38 and its upstream activator MAP kinase kinase 6 are involved in the activation of signal transducer and activator of transcription by hyperosmolarity. J. Biol. Chem. 274:30222-30227. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, C. J., G. Steel, J. D. Gilbert, and W. A. Harland. 1971. Lipids of human atheroma. 4. Characterisation of a new group of polar sterol esters from human atherosclerotic plaques. Atherosclerosis 13:223-237. [DOI] [PubMed] [Google Scholar]
- 5.Burysek, L., T. Syrovets, and T. Simmet. 2002. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J. Biol. Chem. 277:33509-33517. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, G. S., D. J. Meyer, R. Raz, D. E. Levy, J. Schwartz, and C. Carter-Su. 1995. Activation of acute phase response factor (APRF)/Stat3 transcription factor by growth hormone. J. Biol. Chem. 270:3974-3979. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, J. S., R. Seger, J. D. Graves, L. M. Graves, A. M. Jensen, and E. G. Krebs. 1994. The Map kinase cascade. Raven Press, Baltimore, Md. [DOI] [PubMed]
- 8.Cathcart, M. K., and V. A. Folcik. 2000. Lipoxygenases and atherosclerosis: protection versus pathogenesis. Free Radic. Biol. Med. 28:1726-1734. [DOI] [PubMed] [Google Scholar]
- 9.Chung, J., E. Uchida, T. C. Grammer, and J. Blenis. 1997. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 17:6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb, M. H., and E. J. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 11.Conrad, D. J., H. Kuhn, M. Mulkins, E. Highland, and E. Sigal. 1992. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 89:217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad, D. J., and M. Lu. 2000. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am. J. Respir. Cell Mol. Biol. 22:226-234. [DOI] [PubMed] [Google Scholar]
- 13.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 14.de Waal Malefyt, R., C. G. Figdor, R. Huijbens, S. Mohan-Peterson, B. Bennett, J. Culpepper, W. Dang, G. Zurawski, and J. E. de Vries. 1993. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J. Immunol. 151:6370-6381. [PubMed] [Google Scholar]
- 15.Enslen, H., J. Raingeaud, and R. J. Davis. 1998. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273:1741-1748. [DOI] [PubMed] [Google Scholar]
- 16.Folcik, V. A., R. A. Nivar-Aristy, L. P. Krajewski, and M. K. Cathcart. 1995. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J. Clin. Investig. 96:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foltz, I. N., J. C. Lee, P. R. Young, and J. W. Schrader. 1997. Hemopoietic growth factors with the exception of interleukin-4 activate the p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 272:3296-3301. [DOI] [PubMed] [Google Scholar]
- 18.Frank, D. A., S. Mahajan, and J. Ritz. 1997. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J. Clin. Investig. 100:3140-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh, K. C., S. J. Haque, and B. R. Williams. 1999. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18:5601-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gollob, J. A., C. P. Schnipper, E. A. Murphy, J. Ritz, and D. A. Frank. 1999. The functional synergy between IL-12 and IL-2 involves p38 mitogen-activated protein kinase and is associated with the augmentation of STAT serine phosphorylation. J. Immunol. 162:4472-4481. [PubMed] [Google Scholar]
- 21.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 22.Harland, W. A., J. D. Gilbert, G. Steel, and C. J. Brooks. 1971. Lipids of human atheroma. 5. The occurrence of a new group of polar sterol esters in various stages of human atherosclerosis. Atherosclerosis 13:239-246. [DOI] [PubMed] [Google Scholar]
- 23.Heydeck, D., L. Thomas, K. Schnurr, F. Trebus, W. E. Thierfelder, J. N. Ihle, and H. Kuhn. 1998. Interleukin-4 and -13 induce upregulation of the murine macrophage 12/15-lipoxygenase activity: evidence for the involvement of transcription factor STAT6. Blood 92:2503-2510. [PubMed] [Google Scholar]
- 24.Huang, J. S., J. Y. Guh, W. C. Hung, M. L. Yang, Y. H. Lai, H. C. Chen, and L. Y. Chuang. 1999. Role of the Janus kinase (JAK)/signal transducters and activators of transcription (STAT) cascade in advanced glycation end-product-induced cellular mitogenesis in NRK-49F cells. Biochem. J. 342:231-238. [PMC free article] [PubMed] [Google Scholar]
- 25.Ihn, H., K. Yamane, Y. Asano, M. Kubo, and K. Tamaki. 2002. IL-4 up-regulates the expression of tissue inhibitor of metalloproteinase-2 in dermal fibroblasts via the p38 mitogen-activated protein kinase dependent pathway. J. Immunol. 168:1895-1902. [DOI] [PubMed] [Google Scholar]
- 26.Jain, N., T. Zhang, W. H. Kee, W. Li, and X. Cao. 1999. Protein kinase C delta associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J. Biol. Chem. 274:24392-24400. [DOI] [PubMed] [Google Scholar]
- 27.Khan, K. D., K. Shuai, G. Lindwall, S. E. Maher, J. E. Darnell, Jr., and A. L. Bothwell. 1993. Induction of the Ly-6A/E gene by interferon alpha/beta and gamma requires a DNA element to which a tyrosine-phosphorylated 91-kDa protein binds. Proc. Natl. Acad. Sci. USA 90:6806-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovarik, P., M. Mangold, K. Ramsauer, H. Heidari, R. Steinborn, A. Zotter, D. E. Levy, M. Muller, and T. Decker. 2001. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. EMBO J. 20:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovarik, P., D. Stoiber, P. A. Eyers, R. Menghini, A. Neininger, M. Gaestel, P. Cohen, and T. Decker. 1999. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc. Natl. Acad. Sci. USA 96:13956-13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn, H., D. Heydeck, R. Brinckman, and F. Trebus. 1999. Regulation of cellular 15-lipoxygenase activity on pretranslational, translational, and posttranslational levels. Lipids 34:S273-279. [DOI] [PubMed] [Google Scholar]
- 31.Kyriakis, J. M., P. Banerjee, E. Nikolakaki, T. Dai, E. A. Rubie, M. F. Ahmad, J. Avruch, and J. R. Woodgett. 1994. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156-160. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki, T., A. Kawahara, H. Fujii, Y. Nakagawa, Y. Minami, Z. J. Liu, I. Oishi, O. Silvennoinen, B. A. Witthuhn, J. N. Ihle, and et al. 1994. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266:1045-1047. [DOI] [PubMed] [Google Scholar]
- 33.Murata, T., P. D. Noguchi, and R. K. Puri. 1996. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J. Immunol. 156:2972-2978. [PubMed] [Google Scholar]
- 34.Nakagami, H., R. Morishita, K. Yamamoto, Y. Taniyama, M. Aoki, K. Matsumoto, T. Nakamura, Y. Kaneda, M. Horiuchi, and T. Ogihara. 2001. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension 37:581-586. [DOI] [PubMed] [Google Scholar]
- 35.Nassar, G. M., J. D. Morrow, L. J. Roberts 2nd, F. G. Lakkis, and K. F. Badr. 1994. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J. Biol. Chem. 269:27631-27634. [PubMed] [Google Scholar]
- 36.Ostareck, D. H., A. Ostareck-Lederer, M. Wilm, B. J. Thiele, M. Mann, and M. W. Hentze. 1997. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell 89:597-606. [DOI] [PubMed] [Google Scholar]
- 37.Ostareck-Lederer, A., D. H. Ostareck, N. Standart, and B. J. Thiele. 1994. Translation of 15-lipoxygenase mRNA is inhibited by a protein that binds to a repeated sequence in the 3′ untranslated region. EMBO J. 13:1476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paccani, S. R., M. Boncristiano, C. Ulivieri, M. M. D'Elios, G. Del Prete, and C. T. Baldari. 2002. Nonsteroidal anti-inflammatory drugs suppress T-cell activation by inhibiting p38 MAPK induction. J. Biol. Chem. 277:1509-1513. [DOI] [PubMed] [Google Scholar]
- 39.Palmer-Crocker, R. L., C. C. Hughes, and J. S. Pober. 1996. IL-4 and IL-13 activate the JAK2 tyrosine kinase and Stat6 in cultured human vascular endothelial cells through a common pathway that does not involve the gamma c chain. J. Clin. Investig. 98:604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesu, M., S. Aittomaki, K. Takaluoma, A. Lagerstedt, and O. Silvennoinen. 2002. p38 Mitogen-activated protein kinase regulates interleukin-4-induced gene expression by stimulating STAT6-mediated transcription. J. Biol. Chem. 277:38254-38261. [DOI] [PubMed] [Google Scholar]
- 41.Ramsauer, K., I. Sadzak, A. Porras, A. Pilz, A. R. Nebreda, T. Decker, and P. Kovarik. 2002. p38 MAPK enhances STAT1-dependent transcription independently of Ser-727 phosphorylation. Proc. Natl. Acad. Sci. USA 99:12859-12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen, R. L., K. D. Winestock, G. Chen, X. Liu, L. Hennighausen, and D. S. Finbloom. 1996. Granulocyte-macrophage colony-stimulating factor preferentially activates the 94-kDa STAT5A and an 80-kDa STAT5A isoform in human peripheral blood monocytes. Blood 88:1206-1214. [PubMed] [Google Scholar]
- 43.Roy, B., A. Bhattacharjee, B. Xu, D. Ford, A. L. Maizel, and M. K. Cathcart. 2002. IL-13 signal transduction in human monocytes: phosphorylation of receptor components, association with Jaks, and phosphorylation/activation of Stats. J. Leukoc. Biol. 72:580-589. [PubMed] [Google Scholar]
- 44.Roy, B., and M. K. Cathcart. 1998. Induction of 15-lipoxygenase expression by IL-13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J. Biol. Chem. 273:32023-32029. [DOI] [PubMed] [Google Scholar]
- 45.Russell, S. M., J. A. Johnston, M. Noguchi, M. Kawamura, C. M. Bacon, M. Friedmann, M. Berg, D. W. McVicar, B. A. Witthuhn, O. Silvennoinen, et al. 1994. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266:1042-1045. [DOI] [PubMed] [Google Scholar]
- 46.Sanceau, J., J. Hiscott, O. Delattre, and J. Wietzerbin. 2000. IFN-beta induces serine phosphorylation of Stat-1 in Ewing's sarcoma cells and mediates apoptosis via induction of IRF-1 and activation of caspase-7. Oncogene 19:3372-3383. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 17:6419.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuringa, J. J., L. V. Dekker, E. Vellenga, and W. Kruijer. 2001. Sequential activation of Rac-1, SEK-1/MKK-4, and protein kinase Cdelta is required for interleukin-6-induced STAT3 Ser-727 phosphorylation and transactivation. J. Biol. Chem. 276:27709-27715. [DOI] [PubMed] [Google Scholar]
- 49.Shankaranarayanan, P., P. Chaitidis, H. Kuhn, and S. Nigam. 2001. Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase-1 gene. J. Biol. Chem. 276:42753-42760. [DOI] [PubMed] [Google Scholar]
- 50.Shuai, K., C. M. Horvath, L. H. Huang, S. A. Qureshi, D. Cowburn, and J. E. Darnell, Jr. 1994. Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell 76:821-828. [DOI] [PubMed] [Google Scholar]
- 51.Singh, R. P., P. Dhawan, C. Golden, G. S. Kapoor, and K. D. Mehta. 1999. One-way cross-talk between p38(MAPK) and p42/44(MAPK). Inhibition of p38(MAPK) induces low density lipoprotein receptor expression through activation of the p42/44(MAPK) cascade. J. Biol. Chem. 274:19593-19600. [DOI] [PubMed] [Google Scholar]
- 52.Su, B., E. Jacinto, M. Hibi, T. Kallunki, M. Karin, and Y. Ben-Neriah. 1994. JNK is involved in signal integration during costimulation of T lymphocytes. Cell 77:727-736. [DOI] [PubMed] [Google Scholar]
- 53.Turkson, J., T. Bowman, J. Adnane, Y. Zhang, J. Y. Djeu, M. Sekharam, D. A. Frank, L. B. Holzman, J. Wu, S. Sebti, and R. Jove. 1999. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol. Cell. Biol. 19:7519-7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uddin, S., F. Lekmine, N. Sharma, B. Majchrzak, I. Mayer, P. R. Young, G. M. Bokoch, E. N. Fish, and L. C. Platanias. 2000. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J. Biol. Chem. 275:27634-27640. [DOI] [PubMed] [Google Scholar]
- 55.Uddin, S., B. Majchrzak, J. Woodson, P. Arunkumar, Y. Alsayed, R. Pine, P. R. Young, E. N. Fish, and L. C. Platanias. 1999. Activation of the p38 mitogen-activated protein kinase by type I interferons. J. Biol. Chem. 274:30127-30131. [DOI] [PubMed] [Google Scholar]
- 56.Uddin, S., A. Sassano, D. K. Deb, A. Verma, B. Majchrzak, A. Rahman, A. B. Malik, E. N. Fish, and L. C. Platanias. 2002. Protein kinase C-delta (protein kinase C-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J. Biol. Chem. 277:14408-14416. [DOI] [PubMed] [Google Scholar]
- 57.Wen, Z., and J. E. Darnell, Jr. 1997. Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 25:2062-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]
- 59.Winston, L. A., and T. Hunter. 1995. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J. Biol. Chem. 270:30837-30840. [DOI] [PubMed] [Google Scholar]
- 60.Yokogami, K., S. Wakisaka, J. Avruch, and S. A. Reeves. 2000. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 10:47-50. [DOI] [PubMed] [Google Scholar]
- 61.Zauberman, A., D. Zipori, M. Krupsky, and R. Ben-Levy. 1999. Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene 18:3886-3893. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, X., J. Blenis, H. C. Li, C. Schindler, and S. Chen-Kiang. 1995. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science 267:1990-1994. [DOI] [PubMed] [Google Scholar]
- 63.Zhong, Z., Z. Wen, and J. E. Darnell, Jr. 1994. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95-98. [DOI] [PubMed] [Google Scholar]
- 64.Zhu, T., and P. E. Lobie. 2000. Janus kinase 2-dependent activation of p38 mitogen-activated protein kinase by growth hormone. Resultant transcriptional activation of ATF-2 and CHOP, cytoskeletal reorganization and mitogenesis. J. Biol. Chem. 275:2103-2114. [DOI] [PubMed] [Google Scholar]