Abstract
ES cell-tetraploid (ES) mice are completely derived from embryonic stem cells and can be obtained at high efficiency upon injection of hybrid ES cells into tetraploid blastocysts. This method allows the immediate generation of targeted mouse mutants from genetically modified ES cell clones, in contrast to the standard protocol, which involves the production of chimeras and several breeding steps. To provide a baseline for the analysis of ES mouse mutants, we performed a phenotypic characterization of wild-type B6129S6F1 ES mice in relation to controls of the same age, sex, and genotype raised from normal matings. The comparison of 90 morphological, physiological, and behavioral parameters revealed elevated body weight and hematocrit as the only major difference of ES mice, which exhibited an otherwise normal phenotype. We further demonstrate that ES mouse mutants can be produced from mutant hybrid ES cells and analyzed within a period of only 4 months. Thus, ES mouse technology is a valid research tool for rapidly elucidating gene function in vivo.
The standard protocol to derive mouse mutants currently requires the production of germ line chimeras from heterozygous targeted embryonic stem (ES) cells, followed by at least two breeding steps to obtain homozygous mutants (2). Thus, the production of a mutant strain is a time-intensive task exceeding 12 months prior to the analysis of adult mutants. In addition, substantial resources are required for the breeding and genotyping of several hundred mice involved in a typical knockout project. Besides this classical approach, conditional gene targeting through Cre/LoxP-mediated recombination is increasingly used as it allows the spatial and temporal control of gene inactivation (13, 21). Given that a Cre transgene needs to be introduced via additional breeding steps, the production of conditional knockout mice involves four reproductive cycles requiring at least 16 months before the target gene's function can be analyzed in vivo.
Due to these extensive timelines, the impact of targeted mutants in high-throughput functional genome analysis is currently limited, creating a demand for a time-saving single-step procedure. Cloning of mice does not provide a viable alternative because the nuclear transplantation procedure is inefficient and a variety of abnormalities have been described in cloned mice which likely result from incomplete genome reprogramming (8, 17, 25, 30). Alternatively, ES cell-tetraploid (ES) mice can be produced in a single step through the introduction of diploid ES cells into tetraploid blastocysts (16). The latter provide an initial host environment for the differentiation of ES cells but do not contribute to the embryo at later developmental stages. Although the methodology to produce ES mice from inbred ES cell lines was described more than a decade ago, its application was limited due to the extremely low frequency at which viable ES pups are recovered (16).
Recently, this technology was significantly improved through the discovery that ES cell lines derived from hybrid mouse strains support the development of viable ES mice at a 50-fold higher rate than inbred ES cells (4). Importantly, the production of ES mice is technically not more demanding than the generation of chimeras (15). Thus, ES mouse technology now offers the opportunity to efficiently produce targeted mouse mutants directly from hybrid ES cell clones within a single mouse generation and without the requirement for further breeding. With this approach, classical as well as conditional mouse mutants can be obtained in less than half the time compared to the current knockout protocol. The technical feasibility of this novel approach is demonstrated by the recent report that hybrid ES cell lines tolerate multiple consecutive gene-targeting cycles without loosing their potency for tetraploid blastocyst complementation (5, 23).
The biological characteristics of ES cell-tetraploid mice have not yet been fully described. This aspect is of particular interest because faulty expression of imprinted genes and increased body weight have been documented for both cloned neonates and ES mice (9). A validation of the biological characteristics of ES mice is therefore required to provide a baseline for the phenotyping of ES mouse mutants.
We report here an extensive phenotypic characterization of adult B6129S6F1 ES mice derived from wild-type ES cells in relation to controls of the same age, sex, and genotype raised by normal breeding. The comparison of multiple morphological, physiological, and neurological parameters revealed elevated body weights and hematocrits of ES mice as the only differences and an otherwise normal phenotype. We further demonstrate that ES mouse mutants can be produced from mutant hybrid ES cells and analyzed within a period of only 4 months. Our results indicate that ES mouse technology provides a useful research tool which expedites the generation and analysis of designed mouse mutants for functional genome analysis.
MATERIALS AND METHODS
Cell culture.
ES cells were cultured in Dulbecco's modified Eagle's medium with 15% fetal calf serum containing 2,000 U of leukemia inhibitory factors (LIF) (Chemicon International, Hofheim, Germany) per ml on mitomycin C-treated embryonic fibroblasts as previously described (27). For the establishment of wild-type ES lines and adenomatous polyposis coli multiple intestinal neoplasia (APCMin) ES cell lines, blastocysts were collected 3.5 days postcoitum from C57BL/6B6JRj females (Janvier, Le Genest St Isle, France) mated to 129S6/SvEvTac@Bom males (M&B, Ry, Denmark) or from C57BL/6J-APCMin females (Jackson Laboratories, Bar Harbor, Maine) mated to 129S2/SvPasIcoCrlBR males (Charles River Laboratories, Sulzfeld, Germany), respectively. Blastocysts were cultured in ES cell medium on a feeder layer; at day 5 the outgrowth was dissociated by pipetting in trypsin solution, and the cell suspension was replated on a fresh feeder layer. These plates were screened 3 days later for the presence of ES cell colonies. About half of the dissociated blastocysts developed into ES cell lines, which were further expanded.
All ES cell lines were controlled for a correct karyotype by chromosome counts on Giemsa-stained metaphases, and their sex was determined by hybridization of Southern-blotted genomic DNA with a Y chromosome-specific probe (1). ES cell lines derived from mice heterozygous for the APCMin mutation were further characterized for the presence or absence of the APCMin allele with a PCR assay of genomic DNA with primers 5′-GCCATCCCTTCACGTTAG-3′ (0.02 μM), 5′-TTCCACTTTGGCATAAGGC-3′ (1.0 μM), and 5′-TTCTGAGAAAGACAGAAGTTA-3′ (3.5 μM) and cycling conditions of 94°C for 5 min, 56°C for 2 min, and 72°C for 3 min for 28 cycles, followed by a final extension step at 94°C for 5 min.
Production of ES and control mice.
ES mice were produced by tetraploid embryo complementation as previously described (4, 15). Briefly, embryo culture was carried out in microdrops on standard bacterial petri dishes (Falcon) under mineral oil (Sigma). Modified CZB medium was used for embryo culture unless otherwise noted. HEPES-buffered CZB was used for room temperature operations. After administration of hormones, superovulated B6D2F1 females were mated with B6D2F1 males (Janvier). Fertilized oocytes were isolated from the oviduct, and any remaining cumulus cells were removed with hyaluronidase. After overnight culture, two-cell embryos were electrofused with the CF-150B cell fusion instrument (BLS Ltd., Budapest, Hungary) to produce tetraploid embryos. Embryos that had not undergone fusion within 1 h were discarded. Embryos were then cultured in vitro to the blastocyst stage. For microinjection, 10 to 20 blastocysts were placed in a drop of Dulbecco's modified Eagle's medium with 15% fetal calf serum under mineral oil. A flat-tipped piezo-actuated microinjection pipette with an internal diameter of 12 to 15 μm was used to inject 20 ES cells into each tetraploid blastocyst. Prior to blastocyst injection, ES cells were trypsinized, resuspended in ES cell medium, and plated for 30 min to remove feeder cells and debris. After recovery, 10 injected blastocysts were transferred to each uterine horn of pseudopregnant NMRI females 2.5 days post coitum. Recipient mothers were sacrificed at day of embryonic development 19.5 (E 19.5), and pups were quickly removed and cross-fostered to lactating NMRI females.
B6129S6F1 ES mice were generated with the wild-type ES cell line ART4/12 derived from a male B6129S6F1 blastocyst. Control males of the B6129S6F1 genotype were raised from matings of 129S6/SvEvTac@Bom males (M&B) with C57BL/B6JRj females (Janvier). ES and control mice were born in the same week and raised under the same housing conditions. A second group of normal mice (in vitro controls) were raised from B6129S6F1 zygotes that were treated like B6D2F1 tetraploid blastocysts except that electrofusion and ES cell injections were omitted. B6129S6F1 zygotes were cultured to the blastocyst stage in modified CZB medium and transferred into pseudopregnant NMRI females. Pups of the in vitro control group were recovered like ES mice by caesarean section at E 19.5 and cross-fostered to lactating NMRI females.
B6129S2F1-APCMin ES mice were generated with the ES cell line ART/APCMin-8, established from a male B6129S2F1-APCMin blastocyst. Control males of the B6129S2F1-APCMin genotype were obtained from matings of 129S2/SvPasIcoCrlBR males (Charles River Laboratories) with C57BL/6J-APCMin females (Jackson Laboratories). All mice were typed for the presence of the APCMin allele with a specific PCR assay with tail DNA as described above. ES and control mice were born in the same week and raised under the same housing conditions. Both groups were maintained on the high-fat diet US17 as described previously (19).
The analysis of glycosylphosphatidylinositol (GPI) isoforms was performed exactly as described previously (15). Briefly, tissues from 8- to 12-week-old B6D2F1 control or B6129S6F1 ES mice were homogenized in sample buffer and centrifuged. Aliquots of the supernatants were applied to Titan-III cellulose-acetate plates with the Super Z applicator kit and run for 90 min at 300 V in a zip zone chamber with Supreheme buffer (all reagents from Helena Laboratories Inc., Beaumont, Tex.). Next, the plates were overlaid with an agarose-staining solution mixture, incubated for 10 min in the dark, and fixed in acetic acid-glycerol before being photographed.
Phenotype analysis.
An experienced veterinarian pathologist performed the external examination and necropsy of B6129S6F1 ES and control mice. For the preparation of histological sections, mice were perfused in the heart with Bouin's solution, and the organs were embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Brains were stained in addition with a combined Nissl/Luxol fast blue stain. Images were recorded with a Leica DME microscope connected to a Hitachi HVC20 M camera with the Diskus imaging program (C. Hilgers, Königswinter, Germany). Tumors in the complete small intestine of APCMin ES and control mice were counted and also subjected to histological analysis.
For measurement of hematological parameters, blood-EDTA samples were collected from the retrobulbar venous plexus from each animal for determination of complete blood counts, including differentiation of white cells. Hematology parameters were measured from EDTA-blood with an automatic electronic cell counter (CD3500; Abbott Diagnostics, Baar, Switzerland). For clinical biochemistry tests, serum was prepared immediately after blood coagulation and analyzed in a Cobas Integra 700 instrument (Roche Diagnostics, Rotkreuz, Switzerland) with Roche reagent kits under the measurement conditions specified by the International Federation of Clinical Chemistry at 37°C. Preceding the hematological and biochemical measurements, the CD3500 and the Cobas Integra instruments were tested for accuracy and precision with quality control EDTA-blood and serum samples, respectively. Data analysis was performed with a Mann-Whitney U test, and the level of significance was set at P < 0.05. All analyses were performed by Frimorfo Ltd. (Fribourg, Switzerland). Body weights were measured at the age of 9 to 30 weeks with a standard laboratory electronic balance; data analysis was performed with a Student's t test, and the level of significance was set at P < 0.05.
For the behavioral assessment of 10-week-old ES (n = 5) and control (n = 5) mice, mice were housed individually per cage and maintained in an incubator with controlled temperature (21 to 22°C) and a reversed light-dark cycle (12 h/12 h) with food and water available ad libitum. All experiments were carried out by Neurofit S.A. (Illkirch, France) in accordance with institutional guidelines. The test battery was based on a modified Irwin screen (10). All parameters were scored to provide a quantitative assessment. Aggressiveness and convulsions when the animals were handled were recorded. To assess normal behavior, each animal was placed in a glass viewing jar 17 cm in height and 21 cm in diameter for 5 min. On the back of the jar, a sheet of white absorbent paper was placed. The jar was placed in a room with red lights. Without disturbing the animal, the spontaneous activity, respiration rate, and tremors were recorded, and the amount of urination or defecation was measured at the end of the observation period.
Afterwards, each animal was transferred from the viewing jar to an open field without being handled. The observation was performed in a Plexiglas (52 by 52 by 40 cm) open field divided into nine equal squares, placed in a dark room with red light. During the transfer into the new environment, transfer arousal was noted, and palpebral closure was recorded immediately after the transfer. Within the open field, the locomotor activity, tail elevation, touch escape, and startle response (90-dB noise) were recorded. Finally, the animal was removed from the open field to record visual placing, grip strength, body tone, and corneal and righting reflexes as described previously (10). The skin color was recorded from the plantar surface and digits of forelimbs. Data analysis was performed with the Mann-Whitney U test. The level of significance was set at P < 0.05.
Serum insulin and leptin levels were determined by enzyme-linked immunosorbent assay (ELISA) with serum from fed and fasted mice, respectively. Blood was collected from the tail vein, and plasma was separated by centrifugation at 4°C. The ELISAs were performed according to the manufacturer's protocol (Crystal Chem. Inc.). Glucose and insulin tolerance tests were performed on animals that had been fasted overnight. Blood glucose values were determined from tail venous blood with an automatic glucose reader (Glucomen sensor; A. Menarini Diagnostics). For the glucose tolerance test, animals were injected with 2 mg of d-glucose per g of body weight into the peritoneal cavity. Blood glucose levels were measured before and 15, 30, 60, and 120 min after the administration of glucose. For the insulin tolerance test, animals were injected with 1 IU of human insulin (Novo Nordisk Pharma) per kg of body weight. Blood glucose levels were measured before and 15, 30, and 60 min after intraperitoneal administration of insulin. For measurement of white adipose tissue mass, the peritoneal cavity was opened and epididymal fat pads were completely removed and weighed. Data analysis was performed with a Student's t test, and the level of significance was set at P < 0.05.
RESULTS
Generation of wild-type ES mice.
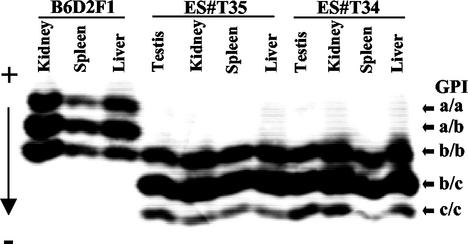
To generate ES mice from wild-type ES cells, we established hybrid ES cell lines from blastocysts of the (C57BL/6 × 129S6)F1 genotype (B6129S6F1). Upon injection into tetraploid B6D2F2 blastocysts, ES cell lines of this genotype generated ES pups at an efficiency of 10 to 15%, comparable to the results obtained with other hybrid ES lines (4). The ES cell origin of these mice was confirmed by the analysis of glucose phosphate isomerase (GPI) isoenzymes (15) in tissue lysates. Figure 1 shows a comparison of samples from a B6D2F1 control (GPI-a/b) with two mice derived from tetraploid B6D2F2 blastocysts (GPI a/a, a/b, or b/b) injected with cells of the B6129S6F1 ES line ART4/12 (GPI-b/c). The presence of the GPI-c isoform as either the c/c homodimer or c/b heterodimer in all samples of ES mice confirmed their origin from ART4/12 ES cells. GPI c/c dimers are unstable and show a less intense signal than GPI b/b dimers (18, 29). The GPI a isoform was not detected in lysates from ES mice (Fig. 1), excluding a contribution from GPI a/a or GPI a/b host blastocysts; only a small fraction (one-eighth) of tetraploid blastocysts are expected to exhibit only the GPI b isoform. The same results were obtained from the analysis of six additional ES mice (R. Kühn, unpublished data).
FIG. 1.
Analysis of GPI isoenzymes in tissue lysates of ES and control mice. Lysates of the indicated tissues of a B6D2F1 (GPI a/b) control mouse and two ES mice (ES#T35, ES#T34) derived from the ART4/12 ES cell line (GPI b/c) were separated by electrophoresis on a cellulose-acetate gel. The gel was stained for GPI enzyme activity and fixed. The run positions of the GPI homo- and heterodimers are indicated by arrows. The anode (+) and cathode (−) positions are indicated.
For the phenotypic characterization of ES mice, we selected a group of 10 males derived from the B6129S6F1 ES cell line ART4/12 through tetraploid blastocyst complementation and an age-matched control group of the same genotype raised by normal breeding. To control for potential effects of the embryo culture and transfer procedure, we raised a third group of mice from B6129S6F1 zygotes that were cultivated like tetraploid blastocysts and transferred into pseudopregnant females (in vitro controls).
Morphological and metabolic analysis of ES and control mice.
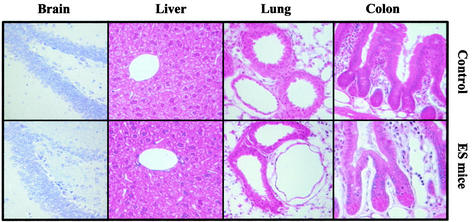
The morphology analysis program for B6129S6F1 ES mice and controls included skeleton radiography, external examination, body weight measurement, macroscopic examination of body cavities, organs, and tissues (necropsy), and pathological diagnosis based on histological sections of various organs. Inspection of all external and internal organs and the skeleton of five 10-week-old ES mice and five controls revealed no visible abnormalities in either group. The histological examination of sections prepared from liver, lung, and intestine (Fig. 2) as well as heart, kidney, and brain also showed no difference between ES and control mice. These results indicate that the embryonic and postnatal development of organs and tissues in ES mice proceeds normally.
FIG. 2.
Histological analysis of ES and control mice. Tissues of 10-week-old B6129S6F1 ES mice and normal mated controls were fixed, paraffin embedded, sectioned, and stained with either cresyl violet (brain) or hematoxylin and eosin (liver, lung, and colon). Magnification, ×40.
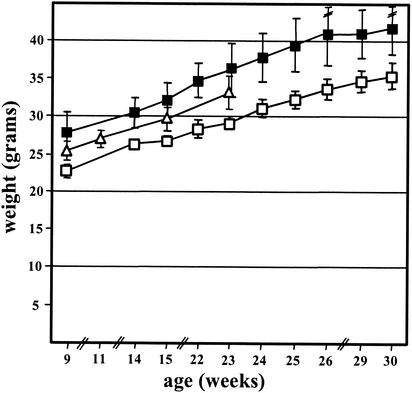
As expected from previous studies (4), adult ES mice and controls from in vitro-cultured embryos exhibited elevated body weight relative to controls derived from natural matings (Fig. 3). The relative weights of ES mice versus normal controls did not increase but were stable over the time measured (9 to 30 weeks), with a mean elevation of 21%. The same result was obtained for ES mice derived from an independent B6129S6F1 ES cell line (R. Kühn, unpublished data). We did not measure the birth weight of ES mice and controls, but an earlier study reported ≈20% elevated birth weight for ES neonates (4).
FIG. 3.
Body weights of B6129S6F1 ES and control mice. The body weights of ES males (solid squares), control mice from normal matings (open squares), and control mice from in vitro-cultured embryos (open triangles) were measured at the indicated ages. Results are expressed as mean values ± standard deviations.
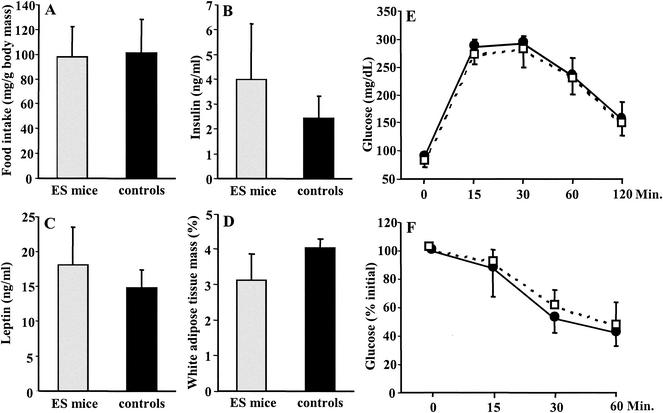
In order to characterize whether the elevated weight of ES mice resulted from the development of obesity, we measured food intake, white adipose tissue mass, plasma insulin, and leptin levels of five 11-month-old B6129S6F1 ES mice and five normal controls. We found no significant differences between these groups for any of these parameters (Fig. 4A to D). To further characterize glucose metabolism in ES mice, we performed insulin and glucose tolerance tests with five 11-month-old B6129S6F1 ES mice and five normal controls of the same age and genotype. These studies revealed that ES mice showed a normal blood glucose response upon challenge with insulin or glucose (Fig. 4E and F). We conclude that ES mice, unlike mice cloned by nuclear transfer (25), have a normal glucose and lipid metabolism and do not become obese.
FIG. 4.
Metabolic parameters of B6129S6F1 ES and control mice. All assays were performed with groups of five 11-month-old adult mice as indicated. (A) Food intake. Daily food intake of ES mice (left bar) and F1 controls (right bar). (B) Insulin levels. Plasma insulin concentrations were determined by ELISA with tail venous blood. (C) Leptin levels. Plasma leptin concentrations were determined by ELISA with tail venous blood of fasted mice. (D) Body fat. White adipose tissue mass was expressed as a percentage of total body weight. (E) Glucose tolerance test. Blood glucose levels were measured before and after intraperitoneal administration of glucose (2 mg/g of body weight). Results are expressed as mean glucose levels of ES mice (solid circles) and controls (open squares) ± standard error of the mean. (F) Insulin tolerance test. Animals were injected with 1.0 IU of human insulin/kg of body weight and analyzed for blood glucose levels at the indicated time points. Results are expressed as a percentage of the initial glucose level ± standard error of the mean of ES mice (solid circles) and controls (open squares). Glucose and insulin tolerance tests were performed on animals that had been fasted overnight.
Hematological analysis of ES and control mice.
To further assess the health status of ES mice, we performed a hematological analysis and determined levels of metabolites, enzymes, and electrolytes in the serum of five B6129S6F1 ES and five control mice at the age of 10 weeks. The concentrations of four metabolites, three enzymes, and seven electrolytes under study showed no significant differences between ES and control mice (Table 1), indicative of a normal metabolism and normal liver and kidney functions in ES mice. The numbers of blood lymphocytes, monocytes, basophils, eosinophils, and neutrophils showed no significant differences between the groups (Table 1), suggesting a normal immune cell lineage differentiation in ES mice. The only differences found in the ES mouse group were mildly enhanced hematocrit values and erythrocyte numbers.
TABLE 1.
Hematology and clinical biochemistry of ES and control mice
| Parameter | Value for:
|
Pa | |||
|---|---|---|---|---|---|
| Controls
|
ES mice
|
||||
| Mean | SD | Mean | SD | ||
| Metabolites | |||||
| Glucose (mmol/liter) | 7.84 | 2.13 | 9.86 | 1.78 | 0.17 |
| Albumin (g/liter) | 25.8 | 1.30 | 26.2 | 1.64 | 0.91 |
| Cholesterin (mmol/liter) | 2.4 | 0.12 | 2.6 | 0.19 | 0.08 |
| Triglycerides (mmol/liter) | 1.46 | 0.39 | 1.5 | 0.65 | 0.75 |
| Enzymes | |||||
| Alkaline phosphatase (U/liter) | 130.2 | 10.4 | 122.4 | 13.2 | 0.47 |
| Aspartate amino transferase (U/liter) | 68.8 | 2.9 | 62.8 | 15.9 | 0.92 |
| Alanine amino transferase (U/liter) | 39.2 | 16.6 | 42.8 | 10.4 | 0.60 |
| Electrolytes | |||||
| Ca2+ (mmol/liter) | 2.4 | 0.05 | 2.45 | 0.07 | 0.25 |
| Mg (mmol/liter) | 1.41 | 0.09 | 1.37 | 0.09 | 0.21 |
| Fe (μmol/liter) | 31.6 | 2.5 | 33.9 | 2.1 | 0.25 |
| Phosphorus (mmol/liter) | 2.65 | 0.19 | 2.71 | 0.17 | 0.56 |
| Na (mmol/liter) | 167.6 | 3.9 | 16.7 | 1.6 | 0.83 |
| K (mmol/liter) | 4.78 | 0.40 | 4.96 | 0.59 | 0.60 |
| Cl (mmol/liter) | 122.8 | 2.8 | 122.8 | 2.0 | 0.83 |
| Erythrocytes | |||||
| Hematocrit (%) | 47.2 | 1.4 | 49.6 | 1.1 | 0.03 |
| Hemoglobin (g/dl) | 15.6 | 0.5 | 16.2 | 0.3 | 0.02 |
| Erythrocytes (106/μl) | 9.8 | 0.39 | 10.6 | 0.3 | 0.01 |
| Mean corpuscular hemoglobin (pg) | 1.6 | 0 | 15.2 | 0.5 | 0.04 |
| Mean corpuscular hemoglobin content (g/dl) | 33.2 | 0.5 | 32.8 | 0.5 | 0.35 |
| Mean corpuscular volume (fl) | 4.8 | 0.7 | 46.8 | 0.8 | 0.60 |
| Leukocytes | |||||
| Leukocytes (103/μl) | 4.1 | 0.8 | 5 | 0.8 | 0.08 |
| Neutrophils/μl | 1,410 | 2,060 | 70.2 | 15.8 | 0.25 |
| Eosinophils/μl | 9.4 | 5.2 | 8.1 | 3.1 | 0.34 |
| Basophils/μl | 24 | 23 | 2.8 | 26 | >0.99 |
| Monocytes/μl | 5.6 | 5.1 | 10.8 | 7.9 | 0.34 |
| Lymphocytes/μl | 3,424 | 81.5 | 4,073 | 69.0 | 0.60 |
P values were calculated by using the Mann-Whitney U test; the level of significance was set at a P value of ≤0.05.
Behavioral analysis of ES and control mice.
To compare the behavioral and neurological functions of B6129S6F1 ES mice and normal mated controls, five mice each were assessed through the behavioral observation profile described by Irwin (10). As shown in Table 2, ES mice performed like normal mated controls for all 17 parameters of the test battery without statistically significant differences between the groups. ES mice exhibited normal responses to environmental stimuli, including social, exploratory, and avoidance behavior, indicating that their muscle and motor neuron, spinocerebellar, and sensory functions were within the normal range. Furthermore, the autonomic functions and reflexes of the ES mice were indistinguishable from those of the controls; bizarre behavior and convulsions were not observed in any of the groups. Upon mating to wild-type C57BL/6 females, all ES males tested (n = 11) proved to be fertile, with an average first litter size of six pups (range, three to nine), indicating normal mating behavior.
TABLE 2.
Behavioral analysis of ES and control mice
| Behavioral or physiologic parameter | Response of ES mice and controls | Pa |
|---|---|---|
| Aggressivity | Normal | >0.99 |
| Spontaneous activity | Normal | >0.99 |
| Respiration rate | Normal | >0.99 |
| Defecation | Normal | 0.89 |
| Urination | Normal | 0.74 |
| Palpebral closure | Normal | >0.99 |
| Locomotor activity | Normal | 0.26 |
| Tail elevation | Normal | 0.12 |
| Touch escape | Normal | 0.67 |
| Startle response | Normal | 0.60 |
| Struggle response | Normal | >0.99 |
| Visual placing | Normal | >0.99 |
| Grip strength | Normal | >0.99 |
| Body tone | Normal | >0.99 |
| Corneal reflex | Normal | >0.99 |
| Righting reflex | Normal | >0.99 |
| Skin color | Normal | >0.99 |
P values were calculated by using the Mann-Whitney U test; the level of significance was set at a P value of ≤0.05.
Tumor development in APCMin mutant ES mice.
ES mouse technology allows the assessment of mutant phenotypes within a short time, as mice can be produced directly from genetically modified ES cells through tetraploid embryo complementation. To demonstrate the feasibility of this approach, we generated ES mice and control mice harboring the Min allele of the adenomatous polyposis coli (APC) tumor suppressor gene, an established genetic model for colorectal cancer in humans (6, 24). Mutant ES mice were produced with a male ES cell line (ART/APCMin-8), established from a blastocyst of the (C57BL/6-APCMin × 129S2)F1 genotype (B6129S2F1-APCMin). Tumor development in mice of this genetic background has been described previously (7).
Upon injection of ART/APCMin-8 ES cells into tetraploid blastocysts, ES mice were obtained at normal frequency (10%). A control group of B6129S2F1-APCMin males was raised from contemporaneous normal matings. At 3 months of age, the small intestines of three B6129S2F1-APCMin ES mice and two normal mated controls were analyzed for the presence of tumors. All mice under study exhibited intestinal tumors typical of the APCMin mutation (five to nine tumors in ES mice, 7 to 17 tumors in controls). While the small groups do not allow quantitative comparison of tumorigenesis, the phenotype of the APCMin ES mice demonstrates that ES mouse mutants are suitable for assaying tumor suppressor gene function. With the availability of genetically modified ES cells, we produced and analyzed adult mutants within a period of 4 months. In addition, ART/APCMin-8 ES cells provide a useful tool allowing us to inactivate other putative tumor modifiers in the APCMin background to assess the phenotype of compound mutants in a time-saving manner.
DISCUSSION
The recent finding that viable ES mice can be efficiently produced with hybrid ES cells (4), even after multiple rounds of gene targeting (5, 23), led us to study the utility of these mice for biological studies. To assess the phenotype of hybrid ES mice, we studied a variety of morphological, physiological, and neurological parameters able to indicate abnormal embryonic or postnatal development as well as disease states of the adult. We found that adult B6129S6F1 ES mice, despite their full in vitro origin, are apparently normal and healthy. The elevated body weight of ES mice did not result from obesity or diabetes. A similar weight increase was found for normal control mice derived from in vitro-cultured embryos. Thus, weight increase is not unique to ES mice but likely results from the common experimental procedure used to derive the ES and control mice. In particular, the specific pre- and postnatal nursing conditions of ES mice and in vitro controls may be of critical importance, since both were raised by outbred (NMRI) females upon embryo transfer. In contrast, the F1 controls were derived from normal matings by inbred (C57BL/6) mothers. It is well known that the offspring's body weight can be increased through uterine heterosis, depending on the mother's genotype (3, 20, 22).
Our results are consistent with an earlier report showing that ES pups and neonates derived from in vitro-cultured blastocysts exhibit ≈20% elevated birth weight (4). In addition, a great variability in the expression of imprinted genes that are frequently involved in fetal and placental growth (8, 31) was documented in ES cell lines and neonatal ES mice (9). The in vitro culture of murine preimplantation embryos has also been shown to cause the altered expression of growth-related imprinted genes (12). Thus, the deregulated expression of such genes could also contribute, besides uterine heterosis, to the increased body weight of ES mice and pups derived from in vitro-cultured embryos. Presently we cannot determine which of these explanations is the main cause for the observed weight increase.
The elevated weight of ES mice and control mice may be distinguished from the neonatal overgrowth found in cloned mice as a direct consequence of the cloning procedure. Newborn mice cloned from ES cell nuclei were reported to exhibit a 60% weight increase, and placental weights were more than doubled (4). Cloned mice also exhibit abnormal imprinted gene expression (9), and severe health impairments such as reduced life span, frequent pneumonia, and obesity were described (17, 25, 30). It was recently shown that genome reprogramming in mice cloned from both nuclei of cultured ES cells and freshly isolated cumulus cells is associated with a broadly disturbed expression profile in the placenta, representing at least 4% of all expressed genes (8). The majority of these genes were common to both types of clones. Gene expression changes in the livers of cloned pups were less pronounced than in the placentas and affected a largely distinct set of genes (8). Even surviving clones may not be normal at birth or later in life as a result of severe placental dysfunction during gestation. In contrast to clones, the extra-embryonic tissues of ES mice, such as the placenta, are largely derived from the tetraploid host blastocysts rather then the donor cells (16). It was further shown that neither the placenta nor the birth weight of ES pups exhibited overgrowth and did not differ from those of neonates derived from in vitro-cultured control embryos (4). Vice versa, neonatal overgrowth and other abnormalities may be reduced if cloned mice were derived by tetraploid embryo complementation with ES cell lines established from cloned blastocysts.
Apart from the increased body weight, our results show that all other studied biological characteristics of B6129S6F1 ES mice fell into the wild-type range as defined by isogenic controls derived from normal matings. The histological, physiological, and neurological parameters reported in this work provide a phenotypic baseline for adult hybrid ES mice and and confirm their general suitability for the analysis of mutant phenotypes. We believe that these results strongly encourage the future use of this technology for the rapid production of targeted mouse mutants. It is beyond the scope of a single study to investigate all biological features of ES mice and controls. Our results, however, will also stimulate further characterization of ES mice in more specialized disciplines.
ES mouse technology offers the benefit of producing adult mutants directly from genetically modified ES cells without the requirement for breeding. Mutants are thus available for analysis in less than half the time required by the current methodology involving the generation of germ line chimeras and multiple breeding cycles. In an earlier report, we confirmed the technical feasibility of this approach through the production of ES mice from homozygous mutant ES cell clones generated by gene targeting (23). In a proof-of-principle experiment, with an ES cell clone harboring a biologically relevant mutant tumor suppressor gene, we now demonstrate that the phenotypic analysis of adult ES mouse mutants, including their production from ES cells, can be completed within 4 months. In addition, this ES cell line provides a tool to expedite the mutagenesis of other tumor modifiers to assess the phenotype of compound mutants.
Besides the production of classical knockout mice, ES mouse technology will also allow the direct, time-saving production of conditional mutants with hybrid ES cell lines established from Cre recombinase transgenic mouse strains. Conditional alleles may be introduced into such ES cells by two sequential gene targeting cycles followed by the removal of selection markers flanked by FLP recombinase recognition sites prior to blastocyst injection. As a prerequisite for this approach, we recently established B6129S6F1 ES cell lines harboring cell type-specific or inducible Cre transgenes, able to derive ES mice at the same frequency as wild-type ES lines (R. Kühn, F. Schwenk, and B. Zevnik, unpublished data).
Since ES mice are efficiently produced from hybrid but not inbred ES cell lines, mutants derived from targeted F1 ES cells necessarily exhibit a hybrid genetic background. Provided that B6129F1 ES lines are employed (B6129S6F1 [Art4/12] cells in this study), ES mouse mutants can be studied in a genetic background that has been frequently used for phenotype analysis. So far, most targeted mutations have been introduced into 129-derived inbred ES cells, and mutant strains have been established through the cross of chimeras to C57BL/6 females and subsequent intercrosses resulting in homozygous mouse mutants in a mixed 129 × C57BL/6 background (14). In contrast, ES mouse technology can deliver homozygous mutants at a defined F1 (129 × C57BL/6) genetic background, since further intercrossing is not required. However, ES mouse technology is not suitable for the generation of mutants which require phenotype analysis on an inbred background.
The production of homozygous mutant ES mice requires two sequential transfection rounds to target both copies of an autosomal gene in hybrid cells, e.g., B6129F1 ES cells. Thus, F1 ES cells must maintain their pluripotency during prolonged in vitro culture, and gene targeting vectors should recombine efficiently with the C57BL/6- and 129-derived allele of the target gene. In earlier reports we have shown that B6129F1 ES cells (including line Art4/12) tolerate up to three consecutive gene targeting cycles without losing the ability to complement tetraploid blastocysts (5, 23). We have also reported that both alleles of an autosomal gene (Rosa 26) can be targeted efficiently in B6129F1 ES cells by using gene targeting vectors with identical, 129-derived homology arms (23). In our experience, about 75% of the targeting vectors that we have tested recombined at comparable efficiency with C57BL/6- and 129-derived alleles with a single set of homology regions derived from one of these strains (R. Kühn and F. Schwenk, unpublished results).
Specific genes, such as the retinoblastoma gene, which exhibit high sequence diversity in different mouse strains can be targeted in inbred ES cells only with isogenic homology arms derived from the same inbred strain (26). The targeting of such genes in B6129F1 ES cells would require the use of two independent gene targeting vectors, one derived from C57BL/6 genomic DNA and the other from the respective 129 substrain. However, with the availability of the complete sequences of the C57BL/6 genome and several 129 strains (11, 28), it is now possible to predict beforehand from the sequence diversity of a given gene whether a single set of homology arms is sufficient to target both alleles in B6129F1 ES cells.
Taken together, our results indicate that ES mouse technology provides a useful research tool to expedite the generation and analysis of mouse mutants in a hybrid background. Since this approach is simple and technically no more demanding than the current gene targeting protocols, we expect it to become a widely used tool in reverse mouse genetics.
Acknowledgments
We thank I. Falkner, A. Hortz, D. Schulz, and D. Thiel for excellent technical assistance, J. Löhler for comments, and G. Stott and L. Jackson-Grusby for critically reading the manuscript.
This work was supported by Artemis Pharmaceuticals GmbH and the German Ministry for Education and Science (BMBF, grants 0311956 and ZMMKTV2).
F. Schwenk and B. Zevnik contributed equally to this work.
REFERENCES
- 1.Bishop, C. E., and D. Hatat. 1987. Molecular cloning and sequence analysis of a mouse Y chromosome RNA transcript expressed in the testis. Nucleic Acids Res. 15:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capecchi, M. R. 1989. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 5:70-76. [DOI] [PubMed] [Google Scholar]
- 3.Cowley, D. E., D. Pomp, W. R. Atchley, E. J. Eisen, and D. Hawkins-Brown. 1989. The impact of maternal uterine genotype on postnatal growth and adult body size in mice. Genetics 122:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggan, K., H. Akutsu, J. Loring, L. Jackson-Grusby, M. Klemm, W. M. Rideout III, R. Yanagimachi, and R. Jaenisch. 2001. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl. Acad. Sci. USA 98:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggan, K., A. Rode, I. Jentsch, C. Samuel, T. Hennek, H. Tintrup, B. Zevnik, J. Erwin, J. Loring, L. Jackson-Grusby, M. R. Speicher, R. Kuehn, and R. Jaenisch. 2002. Male and female mice derived from the same embryonic stem cell clone by tetraploid embryo complementation. Nat. Biotechnol. 20:455-459. [DOI] [PubMed] [Google Scholar]
- 6.Fodde, R., and R. Smits. 2001. Disease model: familial adenomatous polyposis. Trends Mol. Med. 7:369-373. [DOI] [PubMed] [Google Scholar]
- 7.Gould, K. A., C. Luongo, A. R. Moser, M. K. McNeley, N. Borenstein, A. Shedlovsky, W. F. Dove, K. Hong, W. F. Dietrich, and E. S. Lander. 1996. Genetic evaluation of candidate genes for the Mom1 modifier of intestinal neoplasia in mice. Genetics 144:1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humpherys, D., K. Eggan, H. Akutsu, A. Friedman, K. Hochedlinger, R. Yanagimachi, E. S. Lander, T. R. Golub, and R. Jaenisch. 2002. Abnormal gene expression in cloned mice derived from embryonic stem cell and cumulus cell nuclei. Proc. Natl. Acad. Sci. USA 99:12889-12894. [DOI] [PMC free article] [PubMed]
- 9.Humpherys, D., K. Eggan, H. Akutsu, K. Hochedlinger, W. M. Rideout III, D. Biniszkiewicz, R. Yanagimachi, and R. Jaenisch. 2001. Epigenetic instability in ES cells and cloned mice. Science 293:95-97. [DOI] [PubMed] [Google Scholar]
- 10.Irwin, S. 1968. Comprehensive observational assessment. Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 13:222-257. [DOI] [PubMed] [Google Scholar]
- 11.Kerlavage, A., V. Bonazzi, M. di Tommaso, C. Lawrence, P. Li, F. Mayberry, R. Mural, M. Nodell, M. Yandell, J. Zhang, and P. Thomas. 2002. The Celera discovery system. Nucleic Acids Res. 30:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khosla, S., W. Dean, D. Brown, W. Reik, and R. Feil. 2001. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol. Reprod. 64:918-926. [DOI] [PubMed] [Google Scholar]
- 13.Kwan, K. M. 2002. Conditional alleles in mice: Practical considerations for tissue-specific knockouts. Genesis 32:49-62. [DOI] [PubMed] [Google Scholar]
- 14.Mak, T. W. 1998. The gene knockout facts book. Academic Press, London, UK.
- 15.Nagy, A. 2000. Production and analysis of ES cell aggregation chimaeras, p. 177-206. In A. L. Joyner (ed.), Gene targeting: a practical approach, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 16.Nagy, A., E. Gocza, E. M. Diaz, V. R. Prideaux, E. Ivanyi, M. Markkula, and J. Rossant. 1990. Embryonic stem cells alone are able to support fetal development in the mouse. Development 110:815-821. [DOI] [PubMed] [Google Scholar]
- 17.Ogonuki, N., K. Inoue, Y. Yamamoto, Y. Noguchi, K. Tanemura, O. Suzuki, H. Nakayama, K. Doi, Y. Ohtomo, M. Satoh, A. Nishida, and A. Ogura. 2002. Early death of mice cloned from somatic cells. Nat. Genet. 30:253-254. [DOI] [PubMed] [Google Scholar]
- 18.Padua, R. A., G. Bulfield, and J. Peters. 1978. Biochemical genetics of a new glucosephosphate isomerase allele (Gpi-1c) from wild mice. Biochem. Genet. 16:127-143. [DOI] [PubMed] [Google Scholar]
- 19.Petrik, M. B., M. F. McEntee, B. T. Johnson, M. G. Obukowicz, and J. Whelan. 2000. Highly unsaturated (n-3) fatty acids, but not alpha-linolenic, conjugated linoleic or gamma-linolenic acids, reduce tumorigenesis in Apc(Min/+) mice. J. Nutr. 130:2434-2443. [DOI] [PubMed] [Google Scholar]
- 20.Pomp, D., D. E. Cowley, E. J. Eisen, W. R. Atchley, and D. Hawkins-Brown. 1989. Donor and recipient genotype and heterosis effects on survival and prenatal growth of transferred mouse embryos. J. Reprod. Fertil. 86:493-500. [DOI] [PubMed] [Google Scholar]
- 21.Rajewsky, K., H. Gu, R. Kuhn, U. A. Betz, W. Muller, J. Roes, and F. Schwenk. 1996. Conditional gene targeting. J. Clin. Investig. 98:600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhees, B. K., C. A. Ernst, C. H. Miao, and W. R. Atchley. 1999. Uterine and postnatal maternal effects in mice selected for differential rate of early development. Genetics 153:905-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seibler, S., B. Zevnik, B. Küter-Luks, S. Andreas, H. Kern, T. Hennek, A. Rode, C. Heimann, N. Faust, R. Jaenisch, K. Rajewsky, R. Kühn, and F. Schwenk. 2003. Rapid generation of inducible mouse mutants. Nucleic Acids Res. in press. [DOI] [PMC free article] [PubMed]
- 24.Sieber, O. M., I. P. Tomlinson, and H. Lamlum. 2000. The adenomatous polyposis coli (APC) tumour suppressor—genetics, function and disease. Mol. Med. Today 6:462-469. [DOI] [PubMed] [Google Scholar]
- 25.Tamashiro, K. L., T. Wakayama, H. Akutsu, Y. Yamazaki, J. L. Lachey, M. D. Wortman, R. J. Seeley, D. A. D'Alessio, S. C. Woods, R. Yanagimachi, and R. R. Sakai. 2002. Cloned mice have an obese phenotype not transmitted to their offspring. Nat. Med. 8:262-267. [DOI] [PubMed] [Google Scholar]
- 26.te Riele, H., E. R. Maandag, and A. Berns. 1992. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc. Natl. Acad. Sci. USA 89:5128-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres, R. M., and R. Kühn. 1997. Laboratory protocols for conditional gene targeting. Oxford University Press, Oxford, UK.
- 28.Waterston, R. H., K. Lindblad-Toh, E. Birney, J. Rogers, J. F. Abril, P. Agarwal, R. Agarwala, R. Ainscough, M. Alexandersson, P. An, S. E. Antonarakis, J. Attwood, R. Baertsch, J. Bailey, K. Barlow, S. Beck, E. Berry, B. Birren, T. Bloom, P. Bork, M. Botcherby, N. Bray, M. R. Brent, D. G. Brown, S. D. Brown, C. Bult, J. Burton, J. Butler, R. D. Campbell, P. Carninci, S. Cawley, F. Chiaromonte, A. T. Chinwalla, D. M. Church, M. Clamp, C. Clee, F. S. Collins, L. L. Cook, R. R. Copley, A. Coulson, O. Couronne, J. Cuff, V. Curwen, T. Cutts, M. Daly, R. David, J. Davies, K. D. Delehaunty, J. Deri, E. T. Dermitzakis, C. Dewey, N. J. Dickens, M. Diekhans, S. Dodge, I. Dubchak, D. M. Dunn, S. R. Eddy, L. Elnitski, R. D. Emes, P. Eswara, E. Eyras, A. Felsenfeld, G. A. Fewell, P. Flicek, K. Foley, W. N. Frankel, L. A. Fulton, R. S. Fulton, T. S. Furey, D. Gage, R. A. Gibbs, G. Glusman, S. Gnerre, N. Goldman, L. Goodstadt, D. Grafham, T. A. Graves, E. D. Green, S. Gregory, R. Guigo, M. Guyer, R. C. Hardison, D. Haussler, Y. Hayashizaki, L. W. Hillier, A. Hinrichs, W. Hlavina, T. Holzer, F. Hsu, A. Hua, T. Hubbard, A. Hunt, I. Jackson, D. B. Jaffe, L. S. Johnson, M. Jones, T. A. Jones, A. Joy, M. Kamal, E. K. Karlsson, et al. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420:520-562. [DOI] [PubMed] [Google Scholar]
- 29.West, J. D., and J. H. Flockhart. 1989. Non-additive inheritance of glucose phosphate isomerase activity in mice heterozygous at the Gpi-1s structural locus. Genet. Res. 54:27-35. [DOI] [PubMed] [Google Scholar]
- 30.Wilmut, I. 2002. Are there any normal cloned mammals? Nat. Med. 8:215-216. [DOI] [PubMed] [Google Scholar]
- 31.Young, L. E., K. Fernandes, T. G. McEvoy, S. C. Butterwith, C. G. Gutierrez, C. Carolan, P. J. Broadbent, J. J. Robinson, I. Wilmut, and K. D. Sinclair. 2001. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 27:153-154. [DOI] [PubMed] [Google Scholar]