Abstract
Inhibin and activin are members of the transforming growth factor β (TGF-β) family of ligands produced and secreted primarily by the gonads and adrenals. Inhibin-null (INH−/−) mice develop gonadal tumors and—when gonadectomized—adrenocortical carcinoma. The mechanisms leading to adrenal tumorigenesis have been proposed to involve the lack of a gonadal factor and/or a compensatory increase in gonadotropins. In order to achieve elevation of gonadotropins without the concomitant loss of a gonadal hormone, we crossed INH−/− mice with a transgenic mouse strain that has chronically elevated luteinizing hormone (LH) levels (LH-CTP). Compound INH−/−-LH-CTP mice die within 6 weeks of age from severe cancer cachexia induced by large, activin-secreting ovarian tumors. Unexpectedly, INH−/−-LH-CTP mice not only fail to develop adrenal tumors but have smaller adrenals, with a regressed x zone, indicating that elevated LH levels are not sufficient to induce adrenal tumor formation. However, following gonadectomy, INH−/−-LH-CTP mice develop large, sex steroid-producing adrenal tumors that arise from the x zone, indicating a growth-promoting effect of high levels of LH on the adrenal cortex in the absence of ovarian tumors. In addition, in vivo and in vitro data indicate that activin induces apoptosis specifically in the adrenal x zone. The restricted expression of activin receptor subunits and Smad2 in cells of the adrenal x zone, together with the elevated activin levels in INH−/−-LH-CTP mice, supports the conclusion that activin inhibits adrenal tumor growth by inducing x-zone regression.
Adrenocortical carcinoma is a rare but highly malignant endocrine tumor entity with a worldwide incidence of approximately two new cases per million persons per year (23). Epidemiological evidence supports a bimodal age distribution with a peak incidence in early childhood (49). In children, adrenocortical neoplasms occur in the context of clinical syndromes such as the Li-Fraumeni syndrome, which is caused by germ line mutations in the p53 tumor suppressor gene and represents a familial aggregation of neoplasms including adrenocortical carcinoma (24). The Beckwith-Wiedemann syndrome, a congenital overgrowth disorder characterized by a high risk of development of childhood tumors, is also distinguished by a high incidence of adrenocortical carcinoma (47). This syndrome, which is associated with overproduction of insulin-like growth factor II or loss of the cyclin-dependent kinase inhibitor p57KIP2, is often accompanied by nonneoplastic enlargement of the adrenal gland caused by cytomegalic hyperplasia of the fetal cortex (34). The majority of adrenocortical cancers in children, however, occur sporadically with no defined underlying disorder.
Regulation of adrenal growth and differentiation is a complex process that requires a diverse array of specific transcription factors and conserved signaling cascades. Compelling examples of this paradigm are the transforming growth factor β (TGF-β) family members inhibin and activin. While initially identified as opposing endocrine regulators of pituitary follicle-stimulating hormone secretion (46), both inhibin and activin have since been shown to play a critical role as paracrine and autocrine factors that regulate growth and differentiation in a number of organs, including the gonads and the adrenal gland (43). Much of this evidence has come from studies of the inhibin-null (INH−/−) mouse, which spontaneously develops primary gonadal tumors and adrenocortical carcinomas upon gonadectomy (26). In situ hybridization studies with rat embryos have detailed the developmental expression of inhibin and activin subunits in reproductive tissues (38, 39). Post partum, two different transgenic animals have demonstrated transgene expression driven by the α-inhibin promoter in the murine adrenal gland (12, 15). In humans, inhibin is specifically localized to the fetal zone of the developing adrenal cortex, provoking speculation that the growth dynamics of the fetal zone are regulated at least in part by this hormone (2). Since inhibin and activin have a beta subunit in common, targeted deletion of the inhibin alpha subunit in INH−/− mice not only removes inhibin but leads to dysregulation of activin expression. Like inhibin, activin has essential functions in the regulation of both organ development and tumor growth, where it exerts its effects by modulating cellular differentiation, survival, proliferation, and apoptosis (3). In the human adrenal, activin has been shown to induce apoptosis specifically in cells of the fetal zone and has been considered to be a mediator of physiological fetal-zone regression (44).
While both gonadotropins (18, 19) and androgens (41) have been proposed to contribute to the ovarian cancer phenotype in INH−/− mice, the precise mechanisms underlying dysregulated adrenocortical growth and ultimate carcinoma formation in gonadectomized INH−/− mice have yet to be elucidated. An increasing body of evidence from both mouse models and patients indicating the direct effect of luteinizing hormone (LH) on adrenal function (17, 20, 35) has predicted a role of LH on adrenal tumorigenesis in INH−/− mice. However, the defined contribution of gonadectomy to the induction of adrenal tumorigenesis in this mouse model remains uncertain. While a compensatory increase in pituitary LH following gonadectomy may promote the induction of adrenal tumorigenesis, it is equally plausible that the loss of a gonadal factor such as activin is responsible for the adrenal phenotype.
Genetic intercrossing of INH−/− mice and LH-overproducing transgenic mice, coupled with surgical gonadectomy, results in a spectrum of defined disturbances in inhibin, activin, and LH action. A detailed characterization of the resulting gonadal and adrenal phenotype has allowed us to define the individual roles of these factors, as well as the complex interplay between them, in gonadal and adrenal growth in the context of development and cancer in vivo.
MATERIALS AND METHODS
Experimental animals.
All experiments involving animals were performed in accordance with institutionally approved and current animal care guidelines. Generation and genotyping of mice with a targeted deletion of the α-inhibin gene (27) and mice harboring the LH-CTP transgene (a chimeric protein derived from the β subunit of bovine LH and a fragment of the β subunit of human chorionic gonadotropin) under the control of the bovine LH α-subunit promoter has been described previously (37). To obtain a time course of the ovarian and adrenal phenotype of gonadectomized and nongonadectomized wild-type and INH−/− mice, a group of mice (n = 3 to 6) of both genotypes was euthanized at 9, 12, 15, and 18 weeks of age while another group (n = 3 to 5) of each genotype was gonadectomized at 6 weeks of age by standard procedures and euthanized at 9, 12, 15, 18, 21, and 26 weeks of age. Trunk blood for hormonal measurements and adrenals and ovaries were collected. Following microdissection, adrenal and ovarian weights were measured and the tissues were snap-frozen for protein or RNA extraction or immersed in paraformaldehyde for histologic analysis.
The LH-CTP transgene was introduced into the INH−/− background by genetic intercrossing. Both parent inhibin-deficient mice and parent transgenic LH-CTP mice were recruited from extensively outbred colonies. Although generated in the breeding process, no heterozygous INH knockout (INH+/−) animals were used for the in vivo studies. Likewise, only animals hemizygous for the LH-CTP transgene were generated. The body weights of the resulting wild-type, LH-CTP, INH−/−, and compound INH−/−-LH-CTP mice were monitored twice a week between 2 and 5 weeks of age, when all animals were sacrificed. Another group of 1.5-week-old mice of all four genotypes was subjected to gonadectomy by standard procedures. Body weights were monitored thereafter on a weekly basis, and all mice were euthanized 17 weeks after gonadectomy. Blood samples and adrenal and ovarian tissues were collected as described above.
Immunoblotting.
Immunoblotting was done as described in detail previously (1), by using primary antibodies against Smad2 (1:1000; Zymed Laboratories, San Francisco, Calif.) and β-actin (1:5000; Sigma, St. Louis, Mo.). Experiments were performed at least three times with adrenal samples from at least two different animals.
Morphology and calculation of adrenal areas.
Tissues for morphological evaluation were dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) by following standard protocols. H&E-stained adrenal sections from four animals of each genotype obtained both before and after gonadectomy were examined with a standard light microscope at a magnification of ×50. Areas of the total cortex and the adrenal x zone were quantified with the Quantity One software (version 4.2.0; Bio-Rad, Hercules, Calif.). To ensure a reliable comparison between specimens, three adjacent sections from the middle portion of each individual adrenal were examined. In order to control for the spherical shape of the mouse adrenal gland, the x-zone area was normalized to the total cortical area and expressed as the x-zone/total cortical area ratio.
Immunohistochemical analysis.
PCNA (proliferating-cell nuclear antigen) immunohistochemical analysis was performed as described previously (1). Smad2 immunostaining was done accordingly by using a rabbit polyclonal antibody (1:100; Zymed Laboratories, San Francisco, Calif.).
In vitro activin treatment, organ culture, and TUNEL assay.
For in vitro activin experiments, primary adrenal cell cultures were prepared from nongonadectomized 6-week-old wild-type female mice, 6-week-old INH−/− female mice, and 30-week-old INH+/− male mice (n = 4 per genotype), as well as from an established adrenal tumor from a gonadectomized INH−/− mouse, as described in detail elsewhere (17). Cells were incubated in Dulbecco modified Eagle medium-F12-10% fetal calf serum with and without recombinant human activin A (100 ng/ml; R&D Systems, Minneapolis, Minn.) in triplicate for 16 h. Cell counts were expressed as the percent change from untreated cells. For organ culture experiments, mice were euthanized by decapitation and adrenals were injected with medium only or with medium with 100 ng of recombinant human activin A per ml. Adrenals were removed, immediately placed in prewarmed medium, and incubated for 3 or 6 h. Adrenal sections were assayed for apoptosis by fluorescent terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay with the DeadEnd kit (Promega, Madison, Wis.) as recommended by the manufacturer.
In situ hybridization.
Templates for in situ hybridization probes for LH-R and P450c17 were cloned from a mouse testicular cDNA library by PCR amplification with the Advantage 2 polymerase kit (Clontech, Palo Alto, Calif.) and restriction site-linked primers (Table 1). PCR fragments were directionally cloned into pcDNA3 vector (Invitrogen, Carlsbad, Calif.), and RNA probes were synthesized with a digoxigenin (DIG) RNA labeling kit (Roche, Indianapolis, Ind.) as suggested by the manufacturer. Probe templates for ActR-IA and ActR-IIB were amplified with primers with T7 and SP6 binding sites (Table 1), and the gel-purified amplification product was used directly as the template for RNA synthesis. Sections were permeabilized with 0.3% Triton X-100-phosphate-buffered saline (PBS)-20 μg of proteinase K per ml and then washed in 100 mM glycine-PBS before being refixed in 4% paraformaldehyde. Tissues were then acetylated with 0.25% acetic anhydrite-triethanolamine buffer and prehybridized under plastic coverslips for 2 h at 55°C. DIG-labeled RNA probes were boiled and hybridized for 14 to 16 h at 55°C. After removal of excess probe with 50% formamide-0.5× SCC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.5× SCC and blocking of the sections with 2% BSA-10% sheep serum-PBS, detection of the hybridized probe was done with alkaline phosphatase-conjugated anti-DIG Fab fragments and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside-nitroblue tetrazolium substrate (Roche, Indianapolis, Ind.) for 16 to 24 h as suggested by the manufacturer.
TABLE 1.
Primer sequences used for RT-PCR and in situ hybridization, respectivelya
| Amplification product | Sequence | Nucleotides | Annealing temp (°C) |
|---|---|---|---|
| Inhibin alpha (fwd) | GTAAGCTTCATCAGGGCAAGTGAACTATGG | −16-1105 | 58 |
| Inhibin alpha (rev) | GATCTAGAGGTGCTTTTAGATACAAGCACAGTG | ||
| Activin betaA (fwd) | ATGTGGAGATAGAGGACGACATT | 298-1223 | 55 |
| Activin betaA (rev) | GATGTTTTGACCATCATCGTAATA | ||
| Activin betaB (fwd) | ATCGACTTTCGGCTCATCGG | 1360-1635 | 58 |
| Activin betaB (rev) | CACGATCATGTTGGGCACATC | ||
| ActRIA (fwd) | CTGGCCAAGCTGTGGAGTGCTGCCAA | 259-829 | 55 |
| ActRIA (rev) | GTACTGGAGTGTCTAGAGGTCATGT | ||
| ActRIB (fwd) | GGAAAGCTTATGGCGGAGTCGGCCGGA | −7-553 | 55 |
| ActRIB (rev) | GATCGTAGACGAGATCCTGGAGCGT | ||
| ActRIIA (fwd) | CGACGACATTGTTTTGCTACC | 168-455 | 55 |
| ActRIIA (rev) | CCCCGCAATTAACATAAGTGG | ||
| ActRIIB (fwd) | GGATGTATCGTCATCGGAAAC | 472-1239 | 55 |
| ActRIIB (rev) | CCATGGCGTACATGTCGATAC | ||
| GAPDH (fwd) | GAGATTGTTGCCATCAACGAC | 78-962 | 55 |
| GAPDH (rev) | CCTGTTGCTGTAGCCGTATTC | ||
| LH-R (fwd) | ATGGATCCCTCTCACCTATCTCCCTGT | 176-878 | 62 |
| LH-R (rev) | AGTCTAGATCTTTCTTCGGCAAATTCCTG | ||
| P450c17 (fwd) | CGAAGCTTGGAACTTGTGGGTCTCTTGC | 6-513 | 62 |
| P450c17 (rev) | CGCTCGAGAACCTCAACCTGTGCATCCT | ||
| P450arom (fwd) | GACACATCATGCTGGACACC | 575-1297 | 62 |
| P450arom (rev) | CAAAGCCAAAAGGCTGAAAG | ||
| T7-ActRIA (fwd) | GTAATACGACTCACTATAGGGAAAGCCGGTTATACAATGGTC | −14-437 | 58 |
| SP6-ActRIA (rev) | GCATTTAGGTGACACTATGATTGCCTCTCTTAAACTTCCTG | ||
| T7-ActRIIB (fwd) | GTAATACGACTCACTATAGGGAGATGACTTCAATTGCTACGAC | 237-496 | 58 |
| SP6-ActRIIB (rev) | GCATTTAGGTGACACTATGGAGGTTTCCGATGACGATAC |
Underlined sequences denote restriction sites or binding sites for RNA polymerases. Nucleotide positions are given relative to the start codon of the respective open reading frame. For further details, see text. fwd; forward; rev; reverse.
Reverse transcription (RT)-PCR.
Individual adrenals from each group were used for RNA extraction with the Qiagen RNA mini kit (Qiagen, Valencia, Calif.) in accordance with the instructions of the manufacturer. cDNA was created with an RT kit (Ambion Inc., Houston, Tex.) and 1.0 μg of total RNA. Aliquots of the cDNA samples were subjected to the subsequent PCRs, which were performed with the primer pairs and annealing temperatures described in Table 1. Amplification products were separated on a 1% agarose gel and stained with ethidium bromide.
Northern blot analysis.
Total RNA from adrenal glands from wild-type and INH−/− mice at 3, 12, and 25 weeks after gonadectomy, as well as from adrenal glands from two individual animals per genotype (wild-type, LH-CTP, INH−/−, and INH−/−-LH-CTP mice at 5 weeks of age and 17 weeks after gonadectomy), was extracted as described above. For detection of the activin beta A subunit, LH-R, P450c17, and P450arom mRNAs by Northern blot assay, DNA probes from the RT-PCRs described above were used. Northern blotting was done as described in detail earlier (1).
Plasma hormone measurements.
LH was assayed by radioimmunoassay with reagents from the National Hormone and Pituitary Program. Activin A, estradiol, and testosterone levels were measured with a mouse activin A immunoassay (Quantikinine M kit; R&D Systems Inc.), an ultrasensitive estradiol radioimmunoassay (DSL, Webster, Tex.), and a testosterone enzyme-linked immunosorbent assay (ELISA) kit (ICN, Costa Mesa, Calif.), respectively. All hormone measurements were performed on single aliquots because of the small amount of plasma available.
Statistical analysis.
All results are expressed as the mean ± the standard error of the mean. Statistical comparisons were analyzed by analysis of variance and Fisher's protective least-significant-difference test. Statistical significance was defined as P < 0.05 and is indicated by asterisks in the figures.
RESULTS
Development of activin-secreting ovarian tumors in inhibin-deficient mice is accompanied by adrenal x-zone regression.
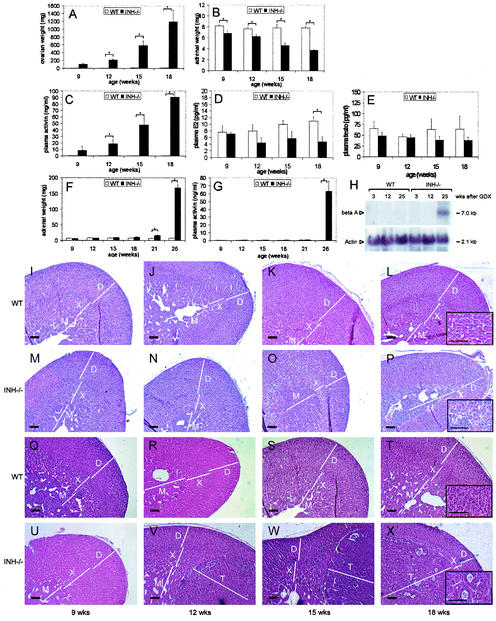
In order to determine direct or indirect effects of the presence of ovarian tumors on the adrenal phenotype, we undertook a time course study by sacrificing groups of female wild-type and INH−/− mice (three to six per group) between 9 and 18 weeks of age. As expected, INH−/− mice developed progressive ovarian tumors during the observation period (combined ovarian weights: 9 weeks, 101.7 ± 25.6 mg; 12 weeks, 210.3 ± 27.5 mg; 15 weeks, 587.7 ± 115.7 mg; 18 weeks, 1,193.3 ± 300.8 mg [Fig. 1A ]). However, the adrenal weights of INH−/− mice decreased with increasing ovarian tumor weights (9 weeks, 6.8 ± 0.5 mg; 12 weeks, 6.3 ± 0.3 mg; 15 weeks, 4.6 ± 0.5 mg; 18 weeks, 3.7 ± 0.1 mg) whereas no changes in adrenal weight were observed in wild-type animals (9 weeks, 8.2 ± 0.2 mg; 12 weeks, 7.7 ± 0.3 mg; 15 weeks, 7.8 ± 0.7; 18 weeks, 7.8 ± 0.3 mg [Fig. 1B]). Morphologically, the decrease in adrenal weight in INH−/− mice was accompanied by gradual and eventually complete regression of the x zone, leaving a fibrous connective tissue band in 18-week-old mice (Fig. 1P). In contrast, no x-zone regression was observed during the observation period in wild-type female animals (Fig. 1L). Whereas wild-type mice did not show changes in plasma activin levels over the time course, increasing ovarian tumor weights in INH−/− mice were accompanied by increasing plasma activin levels (9 weeks, 8.58 ± 6.2 ng/ml; 12 weeks, 18.8 ± 6.6 ng/ml; 15 weeks, 48.0 ± 10.5 ng/ml; 18 weeks, 90.6 ± 8.0 ng/ml [Fig. 1C]). In contrast, the presence of ovarian tumors in INH−/− mice did not result in higher estradiol or testosterone levels compared to wild-type animals (Fig. 1D and E). Since ovarian sex steroids (10) and activin (44) are factors considered to contribute to x-zone regression, these data suggest that the observed x-zone regression in INH−/− is potentially induced by high activin levels but does not require excessive ovarian sex steroid production.
FIG. 1.
Time course of ovarian and adrenal phenotype in nongonadectomized and gonadectomized wild-type (WT) and INH−/− animals. The expected increase in ovarian tumor weight in INH−/− mice (A) was accompanied by an increase in plasma activin levels (C) and a decrease in adrenal weight (B). Estradiol (D) and testosterone (E) were not elevated in the presence of ovarian tumors. Morphological examination of wild-type (I to L) and INH−/− adrenals (M to P) at 9 (I and M), 12 (J and N), 15 (K and O), and 18 (L and P) weeks of age revealed x-zone regression in INH−/− mice in the presence of ovarian tumors (O and P). When gonadectomized at 6 weeks of age, INH−/− mice developed adrenal tumors with a significant increase in adrenal weight at 21 weeks of age and thereafter (F). Adrenal tumor development was associated with a significant increase in plasma activin levels (G) and expression of activin beta A subunit mRNA in late-stage adrenal tumors (H). Adrenal morphology in gonadectomized wild-type (Q to T) and INH−/− (U to X) mice at 9 (Q and U), 12 (R and V), 15 (S and W), and 18 (T and X) weeks of age revealed a prominent x zone in the 9-week-old INH−/− mice (U) and, consecutively, development of well-circumscribed x-zone tumors (V to X) surrounded by a morphologically intact zona glomerulosa and fasciculata. The inserts in panels L, P, T, and X represent high magnifications of the x-zone area or x-zone tumor, respectively. Bars in panels I to X, including high-magnification inserts, 20 μm. D, definitive zone; X, x zone; M, medulla; T, tumor.
Gonadectomized inhibin knockout mice develop adrenal tumors suggestive of an x-zone origin.
In an attempt to further characterize adrenal tumors in INH−/− mice and delineate their cellular origin, we undertook a time course experiment in which INH−/− mice and wild-type animals were gonadectomized at 6 weeks of age and subsequently euthanized at 9, 12, 15, 18, 21, and 26 weeks of age. As expected, INH−/− mice developed adrenal tumors, which led to a cumulative adrenal weight increase (9 weeks, 6.2 ± 0.7 mg; 12 weeks, 7.4 ± 0.2 mg; 15 weeks, 9.0 ± 1.0 mg; 18 weeks, 10.8 ± 1.0 mg; 21 weeks, 15.8 ± 2.8 mg; 26 weeks, 168.0 ± 18.5 mg [Fig. 1F]) that was accompanied by increasing activin levels (9 weeks, 0.29 ± 0.03 ng/ml; 12 weeks, 0.42 ± 0.07 ng/ml; 15 weeks, 0.38 ± 0.07 ng/ml; 18 weeks, 0.62 ± 0.18 ng/ml; 21 weeks, 0.95 ± 0.21 ng/ml; 26 weeks, 63.20 ± 12.75 ng/ml [Fig. 1G]). In accordance with the plasma activin levels measured by ELISA, increased expression of the activin beta A subunit was demonstrated by Northern blot assay in late-stage adrenal tumors (Fig. 1H).
Morphological examination revealed a prominent x zone in 9-week-old INH−/− mice with no apparent tumor growth (Fig. 1U). However, although not accompanied by a significant adrenal weight increase, adrenal tumor growth was evident already in 12-week-old INH−/− mice. These well-circumscribed early-stage tumors were surrounded by a morphologically intact zona glomerulosa and fasciculata (Fig. 1V and W). Consistently, morphological examinations at later time points showed a stretched and narrowed but intact definitive zone (Fig. 1X) until large tumor masses eliminated any detectable normal adjacent adrenal tissue. Taken together, these data are suggestive of an x-zone origin of adrenal tumors in gonadectomized INH−/− mice.
Compound INH−/−-LH-CTP mice develop massive ovarian tumors, have highly elevated activin levels, and die of cachexia at an early age.
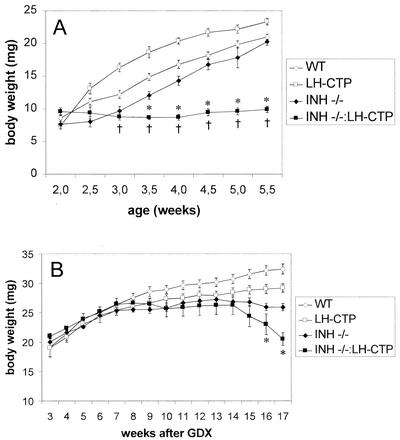
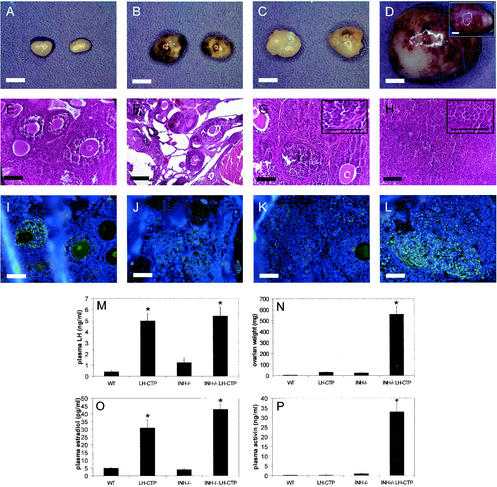
An early indicator of ovarian tumor development in INH−/− mice is severe weight loss and a cachexia-like wasting syndrome that eventually results in the death of the animal at 16 to 20 weeks of age (4). By monitoring of body weight, it became evident that compound INH−/−-LH-CTP mice failed to gain weight after 3.5 weeks of age and eventually died of severe cachexia at 6 weeks of age (Fig. 2A). In contrast to INH−/− mice, which expectedly had developed only small ovarian tumors at this time point (25.3 ± 4.4 mg [n = 15]), compound INH−/−-LH-CTP mice showed massive bilateral ovarian tumors (559.6 ± 73.6 mg [n = 10, P < 0.0001 versus each other genotype] [Fig. 3N ]). Morphological examination of the ovarian tumors of INH−/− mice at this early time point demonstrated preservation of the follicular architecture with an increase in endocrine cell mass compared to that of wild-type ovaries. Tumorlets with occasional apoptotic bodies were evident; however, proliferation was not prominent (Fig. 3G). In contrast, the morphology of ovarian tumors of INH−/−-LH-CTP mice was characterized by loss of follicular architecture. The ovarian mass consisted predominantly of solid collections of proliferating endocrine cells. Mitotic activity was evident by readily identifiable mitotic figures. While generally lacking structure, occasional cystic changes were observed. Occasional primitive rosettes were also present, suggesting evidence of endocrine differentiation (Fig. 3H). Consistent with more-aggressive ovarian tumor growth, immunohistochemical examination revealed widespread expression of the proliferation marker PCNA in ovarian tumors from INH−/−-LH-CTP mice (Fig. 3L).
FIG. 2.
(A) Body weights of nongonadectomized compound INH−/−-LH-CTP mice were significantly lower than those of wild-type (WT) mice at 3 weeks of age (†) and INH−/− at 3.5 weeks of age (*). All INH−/−-LH-CTP mice died of severe cachexia before or at 6 weeks of age. (B) When gonadectomized at 1.5 weeks of age, compound INH−/−-LH-CTP mice started developing cachexia-like symptoms at 14 weeks after gonadectomy (GDX) and had significantly lower body weights than gonadectomized INH−/− mice at 16 weeks of age and thereafter (*).
FIG. 3.
Ovarian phenotypes and hormonal profiles of 5-week-old mice. Gross anatomy (A, B, C, and D), H&E-stained sections (E, F, G, and H), and PCNA immunohistochemistry (I, J, K, and L) of ovaries from wild-type (WT) (A, E, and I), LH-CTP (B, F, and J), INH−/− (C, G, and K), and compound INH−/−-LH-CTP animals (D [contralateral ovary inserted], H, and L). Whereas INH−/− mice developed only small ovarian tumors with retained follicular architecture, INH−/−-LH-CTP mice had massive, undifferentiated ovarian tumors with abundant PCNA expression. As expected, the presence of the LH-CTP transgene led to a significant increase in plasma LH (M) and estradiol (O) levels. The significantly higher tumor weight (N) in INH−/−-LH-CTP mice was accompanied by considerably higher plasma activin levels (P). See the text for a full statistical evaluation. Bars for gross anatomy represent 2 mm; bars for histology, including high-magnification inserts, represent 20 μm.
As expected, the presence of the LH-CTP transgene resulted in a considerable increase in LH (LH-CTP, 4.9 ± 0.8 ng/ml [n = 10]; INH−/−-LH-CTP, 5.4 ± 0.9 ng/ml [n = 10]) and estradiol (LH-CTP, 30.9 ± 5.4 pg/ml [n = 10]; INH−/−-LH-CTP, 42.9 ± 3.6 pg/ml [n = 9]) compared to the littermates without the transgene (LH: wild type, 0.4 ± 0.1 ng/ml [n = 23, P < 0.0001]; INH−/−, 1.2 ± 0.4 ng/ml [n = 18, P < 0.0001] [Fig. 3M]) (estradiol: wild type, 5.0 ± 0.3 pg/ml [n = 9, P < 0.0001]; INH−/−, 4.1 ± 0.7 pg/ml [n = 5, P < 0.0001] [Fig. 3O]). Consistent with the greater ovarian tumor burden, compound INH−/−-LH-CTP mice also proved to have significantly higher estradiol levels (P = 0.03) in comparison to LH-CTP mice. In accordance with a more severe phenotype and larger ovarian tumors, compound INH−/−-LH-CTP mice had considerably higher plasma activin A levels (33.0 ± 5.3 ng/ml [n = 9]) than INH−/− mice (0.97 ± 0.19 ng/ml [n = 5; P < 0.0001] [Fig. 3P]). Although the presence of the LH-CTP transgene led to higher plasma activin A levels in the context of inhibin deficiency, chronically elevated LH levels alone did not affect activin levels in mice with intact inhibin (LH-CTP, 0.28 ± 0.01 ng/ml [n = 10] versus the wild type, 0.26 ± 0.02 ng/ml [n = 9]; P = 0.99). Taken together, these results demonstrate that chronic elevation of LH levels exacerbates ovarian tumorigenesis in INH−/− mice, resulting in higher activin levels, an earlier and more severe wasting syndrome, and premature death of the animal.
High levels of LH are not sufficient to induce adrenal tumorigenesis in inhibin-deficient mice.
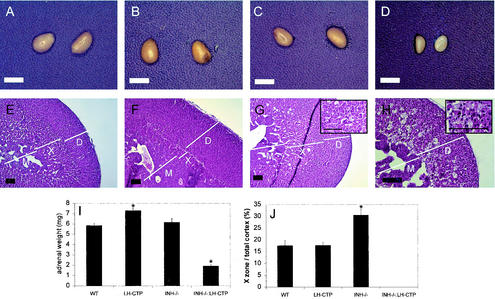
The early death of compound INH−/−-LH-CTP mice secondary to the development of ovarian tumors precluded an evaluation of adrenal morphology at time points later than 5 weeks. Macroscopic evaluation of the adrenal glands at 5 weeks revealed that adrenals of INH−/−-LH-CTP mice did not develop tumors and in fact were significantly smaller (1.9 ± 0.1 mg [n = 10, P < 0.0001 versus each other genotype]) than the adrenals of wild-type animals (5.9 ± 0.2 mg [n = 20]), LH-CTP mice (7.3 ± 0.3 mg [n = 13]), and INH−/− mice (6.2 ± 0.4 mg [n = 15] [Fig. 4I ]). Even when normalized for body weight, the adrenal weight remained significantly lower in INH−/−-LH-CTP mice than in mice of the other genotypes (data not shown), supporting a specific decrease in adrenal size that is not the consequence of a loss of body mass due to the overall cachexia. Histological examination of the adrenals of compound INH−/−-LH-CTP mice demonstrated maintained cortical and medullary architecture with a nearly completely absent x zone. Mixed in the remaining x zone were vacuolated cells representing macrophages. While devoid of significant nuclear debris or hemosiderin, these were indicative of a resorptive process. No incipient neoplasms were detectable (Fig. 4H). These data indicate that high levels of LH are not sufficient to induce adrenal tumorigenesis in the context of inhibin deficiency. However, quantification of the x-zone area in 5-week-old INH−/− mice in the presence of only small ovarian tumors revealed a significantly enlarged x zone (30.6 ± 1.6 [n = 4]; P < 0.0001 versus the wild type [17.6 ± 1.4; n = 4] and LH-CTP [17.8 ± 0.8; n = 4]; Fig. 4J), suggesting that inhibin may function as a negative regulator of x-zone growth. More importantly, however, the observations of an unaltered x-zone size in LH-CTP mice, an enlarged x zone in INH−/− animals, and a regressed x zone in compound INH−/−-LH-CTP mice indicate the involvement of a factor(s) other than inhibin and LH in the regulation of x-zone growth in this model.
FIG. 4.
Gross appearance and morphological characterization of adrenals from 5-week-old mice. Gross anatomy (A, B, C, and D) and H&E-stained sections (E, F, G, and H) from adrenals of wild-type (WT) (A and E), LH-CTP (B and F), INH−/− (C and G), and compound INH−/−-LH-CTP (D and H) mice. Adrenals from compound INH−/−-LH-CTP mice were significantly smaller than those from mice of the other genotypes (I). In addition, morphological examination revealed an almost complete lack of the x zone. In contrast, the x zone of INH−/− mice was significantly larger than that of wild-type and LH-CTP mice (J), indicating that inhibin deficiency alone is not sufficient to induce x-zone regression. See the text for a full statistical evaluation. Inserts in panels G and H represent high magnifications of the x-zone area. Bars for gross anatomy represent 2 mm; bars for histology, including high-magnification inserts, represent 20 μm. Note the higher magnification in panel H. D, definitive zone; X, x zone; M, medulla.
Gonadectomized compound INH−/−-LH-CTP mice develop larger adrenal tumors than do INH−/− mice, accompanied by higher levels of activin and an earlier onset of cachexia.
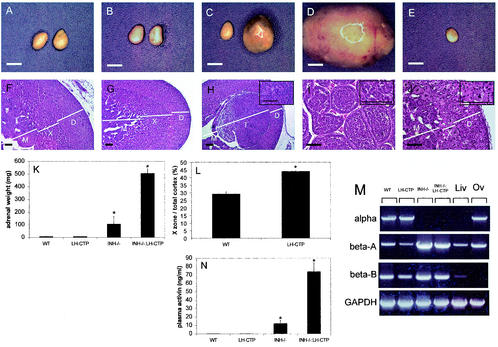
To explore whether the loss of a gonadal factor is required to manifest any necessary role of LH in adrenal tumor induction, gonadectomy was performed on mice of the four genotypes. When body weight was monitored in gonadectomized mice on a weekly basis, it became evident that compound INH−/−-LH-CTP mice began to develop cachexia at 16 weeks after gonadectomy (Fig. 2B). As a result, all animals were sacrificed 17 weeks after gonadectomy. Whereas adrenal weights in LH-CTP mice (7.7 ± 0.3 mg [n = 7]) were not different from those of wild-type animals (8.4 ± 0.5 mg [n = 8, P = 0.98]), INH−/− mice had developed adrenal tumors (107.2 ± 59.8 [n = 4, P = 0.029 versus the wild type, P = 0.032 versus LH-CTP]), as expected. Compound INH−/−-LH-CTP mice, however, had large adrenal tumors with considerably higher weights than INH−/− mouse adrenals (506.4 ± 34.3 mg [n = 6, P < 0.0001 versus all other genotypes] [Fig. 5K ]). Histological examination of a characteristic adrenal from a compound INH−/−-LH-CTP mouse (Fig. 5I) revealed a large, circumscribed, noninvasive mass with biphasic histology. Small, primitive blastic cells with sparse cytoplasm were arranged in cords and nests scattered in focal collections close to the capsule. Occasional areas of stromal cells were also present. The bulk of the tumor consisted of large nests of larger cells with abundant pink cytoplasm. These areas were composed of lipid-rich adrenocortical cells. Mitotic figures and apoptotic bodies were clearly identifiable. The nodules had a rudimentary alveolar pattern that is reminiscent of what has been described in adrenocortical carcinoma of childhood as an adrenal blastema arising from the fetal zone of the adrenal cortex.
FIG. 5.
Gross appearance and morphological characterization of adrenals from mice 17 weeks after gonadectomy. Gross anatomy (A, B, C, D, and E) and H&E-stained sections (F, G, H, I, and J) from adrenals of wild-type (WT) (A and F), LH-CTP (B and G), INH−/− (C and H), and compound INH−/−-LH-CTP (D, I, E, and J) mice. While inhibin deficiency was necessary for the induction of adrenal tumor growth (C and D), the presence of the LH-CTP transgene resulted in a significantly larger x zone (L) and bigger adrenal tumors, respectively. The higher adrenal weights of INH−/− and INH−/−-LH-CTP mice (K) were paralleled by significantly higher plasma activin levels, as measured by ELISA (N), and adrenal activin beta A and beta B subunit expression, as demonstrated by RT-PCR (M). Notably, the contralateral adrenal from the same INH−/−-LH-CTP animal (E) was small and showed a small x zone consisting of large, multinucleated cells (J). See the text for a full statistical evaluation. The inserts in panels H, I, and J represent high magnifications of the x-zone area or x-zone tumor, respectively. Bars for gross anatomy represent 2 mm; bars for histology, including high-magnification inserts, represent 20 μm. Note the higher magnification in panels I and J. D, definitive zone; X, x zone; T, tumor; M, medulla; Liv, liver; Ov, ovary; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Quantification of the x zone in mice with intact inhibin revealed a significantly larger x zone in LH-CTP mice (43.9 ± 0.7 [n = 4]) than in wild-type animals (x-zone/total cortical area ratio, 28.4 ± 1.1 [n = 4, P < 0.0001] [Fig. 5L]). Moreover, comparison of x-zone areas before and after gonadectomy in both wild-type and LH-CTP mice indicated significantly larger x zones after gonadectomy (P < 0.0001 for both genotypes). Taken together, these data are indirect evidence of a growth-promoting effect of elevated levels of LH on x-zone and x-zone-derived tumor cells.
Consistent with the earlier onset of cachexia-like symptoms and the presence of massive adrenal tumors in INH−/−-LH-CTP mice, plasma activin levels were notably higher in compound INH−/−-LH-CTP mice (74.0 ± 10.6 ng/ml [n = 5, P < 0.0001 versus all other genotypes]) than in INH−/− animals (12.4 ± 3.8 ng/ml [n = 4] [Fig. 5M]). In contrast, the presence of the LH-CTP transgene alone (0.22 ± 0.02 ng/ml [n = 7]) did not alter the plasma activin levels compared to those of wild-type mice (0.24 ± 0.03 ng/ml [n = 6, P = 0.99]). In accordance with the prediction that activin is secreted from the adrenal tumors, expression levels of both the activin beta A and beta B subunits were considerably higher in INH−/− and INH−/−-LH-CTP mice than in wild-type and LH-CTP mice (Fig. 5M). In the majority of compound INH−/−-LH-CTP mice (five out of six), adrenal tumors occurred unilaterally, accompanied by a small contralateral adrenal (Fig. 5E and J). Morphological examination of a characteristic contralateral adrenal of a compound INH−/−-LH-CTP mouse revealed alterations similar to those seen in adrenals from INH−/−-LH-CTP mice in the presence of activin-secreting ovarian tumors, namely, nearly complete absence of the x zone. In the location of the x zone were multinucleated giant cells and hemosiderin-laden macrophages indicative of chronic hemorrhage consistent with a destructive process such as apoptosis.
Activin induces apoptosis in the Smad2-expressing adrenal x zone but not in Smad2-negative adrenal tumors of inhibin-deficient mice.
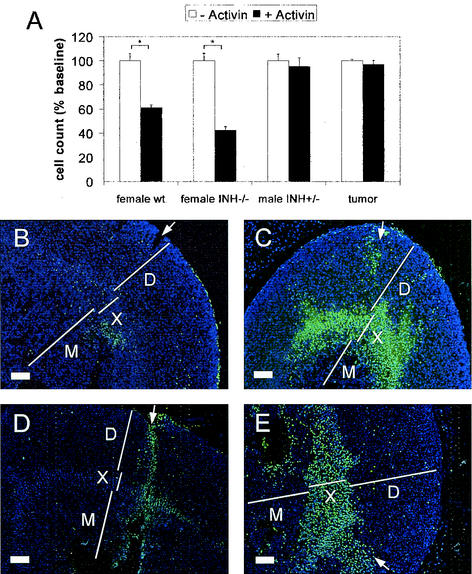
Since activin has been shown to induce apoptosis in the fetal zone of the human adrenal cortex (43), we hypothesized that the site of activin action in the murine adrenal gland could be the x zone. In accordance with this concept, in vitro experiments with primary adrenal cell cultures demonstrated a significant decrease in cell number upon activin treatment only in cultures derived from female wild-type (61.2% ± 2.3% of the untreated control; P < 0.0002) and INH−/− (42.4% ± 3.0%; P < 0.0001) mice that contain x-zone cells. No change in cell numbers was seen in adrenal cells from postpubertal males with a regressed x zone (95.2% ± 7.4%; P = 0.57; Fig. 6A). Similarly, cells derived from an INH−/− adrenal tumor were also nonresponsive to activin treatment (96.9% ± 3.3%; P = 0.68). To further define the site of action of activin within the adrenal gland, injection experiments were performed with intact adrenals. In an assay for apoptosis, injection of activin-containing medium was shown to induce cell death specifically in the adrenal x zone (Fig. 6C and E).
FIG. 6.
Treatment of primary adrenal cell cultures from female wild-type (wt) and INH−/− mice (including x-zone cells) with recombinant activin A led to a significant reduction in cell number, whereas cells from postpubertal male adrenals (with no x zone) and adrenal tumor cells were unaffected (A). TUNEL staining (green; nuclear counterstain in blue) after injection of medium alone (B and D) and activin (C and E) revealed apoptosis after 3 (B and C) and 6 (D and E) h of incubation specifically in the x zone of activin-treated adrenals. White arrows depict injection channels with some adjacent, mechanically induced apoptotic nuclei. Bars for histology represent 20 μm. D, definitive zone; X, x zone; M, medulla.
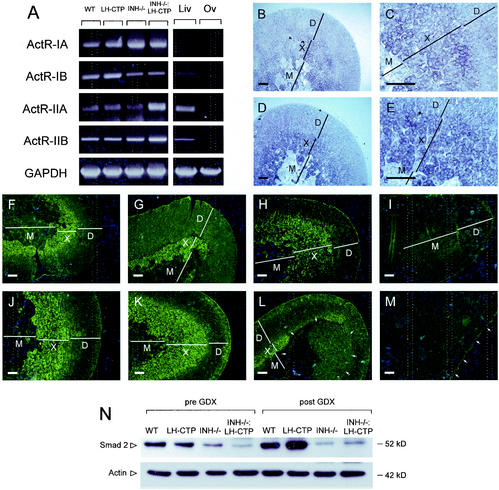
A prerequisite of activin action on the adrenal is the presence of an activin receptor. As confirmed by RT-PCR, all four subunits of activin receptors (ActR-IA, ActR-IB, ActR-IIA, and ActR-IIB) are expressed in the murine adrenal gland (Fig. 7A). Consistent with the x-zone-specific effects of activin, in situ hybridization demonstrated the highest expression of ActR-IA (Fig. 7B and C) and ActR-IIB (Fig. 7D and E) within the x zone of the mouse adrenal gland. Moreover, abundant staining for Smad2, a critical intracellular mediator of the activin-signaling pathway, was also restricted to the x zone (Fig. 7F to M). No specific Smad2 staining was detectable in compound INH−/−-LH-CTP mice, which we demonstrated by morphological criteria to lack an x zone. In adrenal tumors of INH−/− and compound INH−/−-LH-CTP mice, Smad2 staining was found only with low abundance, indicating a potential mechanism for the decreased susceptibility to the apoptosis-inducing action of activin in these tumors. Comparable results demonstrating higher Smad2 expression levels in adrenals with a large x zone and low levels in adrenals with no x zone or adrenal tumors were obtained by immunoblotting (Fig. 7N).
FIG. 7.
RT-PCR panel demonstrating expression of activin receptor subunits (ActR-IA, ActR-IB, ActR-IIA, and ActR-IIB) in adrenals of wild-type (WT), LH-CTP, INH−/−, and compound INH−/−-LH-CTP mice (A). In situ hybridization for both ActR-IA (B, low magnification; C, high magnification of corticomedullary boundary) and ActR-IIB (D and E) revealed the highest expression of both receptors in the x zone. In addition, immunostaining in adrenals of wild-type (F and J), LH-CTP (G and K), INH−/− (H and L), and compound INH−/−-LH-CTP (I and M) mice before (F to I) and after (J to M) gonadectomy revealed strong x-zone-specific staining of Smad2. In contrast, an adrenal tumor in an INH−/− mouse (L, arrows) showed only weak and partial Smad2 staining whereas no specific staining was detectable in a tumor of an INH−/−-LH-CTP mouse (M). Comparable results demonstrating higher Smad2 expression levels in adrenals with a large x zone and low levels in adrenals with no x zone or with adrenal tumors were obtained by immunoblotting (N). Bars represent 20 μm. Liv, liver; Ov, ovary; D, definitive zone; X, x zone; M, medulla; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Adrenal tumors in gonadectomized INH−/− and INH−/−-LH-CTP mice express LH-R, P450c17, and P450arom and are capable of producing estradiol.
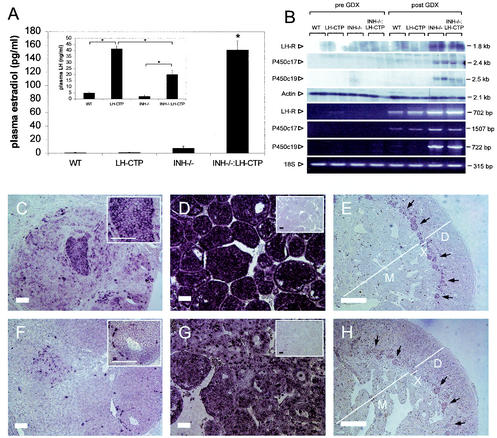
In the initial report on the adrenal phenotype of gonadectomized INH−/− mice with adrenal tumors, a proportion of animals were reported to have elevated estradiol levels indicative of a cellular origin from adrenal sex steroid-producing cells (26). Consistent with this concept, gonadectomized INH−/−-LH-CTP mice had greatly elevated estradiol levels (153.1 ± 13.3; P < 0.0001 versus all other genotypes [Fig. 8A ]). After gonadectomy, the presence of the LH-CTP transgene was able to maintain differences in LH levels compared to their gonadectomized littermates without the transgene (LH-CTP, 41.5 ± 3.0 ng/ml [n = 7], versus the wild type, 5.1 ± 1.0 ng/ml [n = 7, P < 0.0001], and INH−/−-LH-CTP, 15.5 ± 2.6 ng/ml [n = 5], versus INH−/−, 2.3 ± 1.3 ng/ml [n = 5, P = 0.001]). However, LH levels were significantly lower in compound INH−/−-LH-CTP mice than in LH-CTP mice (P < 0.0001), suggesting an estradiol-induced negative feedback effect on LH secretion (Fig. 8A, insert).
FIG. 8.
Adrenal tumors in inhibin-deficient mice are capable of producing estradiol and express LH receptor, P450c17, and P450arom. Pronounced elevation of estradiol levels in gonadectomized INH−/−-LH-CTP mice (A) was accompanied by partial suppression of elevated LH levels (A, insert). Northern blotting and RT-PCR demonstrated the presence of LH receptor, P450c17, and P450arom transcripts in adrenal tumors of inhibin-deficient mice (B; for details, see text). In situ hybridization for LH receptor (C, D, and E) and P450c17 (F, G, and H) revealed expression of LH receptor and P450c17 in adrenal tumors of gonadectomized INH−/− mice (C and F) and more pronounced expression of LH receptor and P450c17 in compound INH−/−-LH-CTP mice (D and G). Adrenals contralateral to the adrenal tumors in INH−/−-LH-CTP mice (E and H) showed staining for LH receptor and P450c17 transcripts specifically in cells of the remaining x-zone cells (black arrows). Control reactions with a sense probe are shown as inserts in panels D and G. Bars represent 20 μm. Note the higher magnification in panels E and H. WT, wild type; GDX, gonadectomy.
Expression of LH-R in the adrenal cortex of mice has been shown to be induced by chronically elevated serum LH levels (17). In accordance with these findings, LH-R transcripts were detected by RT-PCR in 5-week-old LH-CTP mice and in all four groups of mice after gonadectomy, although the levels of LH-R mRNA were high enough to be detectable by Northern blot assay or in situ hybridization only in adrenal tumors from INH−/− mice and compound INH−/−-LH-CTP mice (Fig. 8 and data not shown).
P450c17 and P450arom, two of the key steroidogenic enzymes required for the production of sex steroids, are not present in the adrenal glands of adult mice under normal physiological circumstances (16). In accordance with these findings, P450c17 and P450arom transcripts could not be detected in adrenals from wild-type, LH-CTP, INH−/−, or INH−/−-LH-CTP mice by Northern blot assay, RT-PCR, or in situ hybridization before gonadectomy (Fig. 8 and data not shown). However, as predicted from the elevated estradiol levels, the presence of the P450c17 and P450arom mRNAs was demonstrated in adrenal tumors from both INH−/− and compound INH−/−-LH-CTP mice. Although only a few scattered P450c17-positive cells were found in the adrenal tumors of INH−/− mice by in situ hybridization, widespread expression was found in tumors and contralateral narrow x zones of compound INH−/−-LH-CTP mice.
DISCUSSION
On the basis of epidemiological and clinical data, together with genetic evidence demonstrating a unique pattern of genetic alterations in childhood adrenocortical cancer, it has been proposed that these tumors represent a distinct embryologic tumor derived from the fetal adrenal cortex (13). Under normal circumstances, the primate fetal adrenal cortex, which is required to establish the intrauterine estrogenic milieu of pregnancy, undergoes apoptosis soon after birth (30). Mice possess an x zone that develops after birth and regresses at puberty in the male (11) and during the first pregnancy in the female (10). Although the fetal zone in humans and the x zone in mice differ in the timing of development, several lines of evidence suggest that these two zones are analogous structures. They are located in the same position adjacent to the adrenal medulla, and they both have ultrastructural features of steroid-producing cells. Furthermore, loss of function of the Dax-1 gene (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome) in humans (32) and mice (50) results in lack of regression of the fetal and x zones, respectively. The data presented herein support the concept of dysregulation of adrenal x-zone growth as the basis of the adrenocortical pathology in INH−/− mice, a feature shared by the mouse model of Beckwith-Wiedemann syndrome (7) and a mouse model using inhibin promoter-driven, T-antigen-induced adrenal tumorigenesis (15). While the presence of ovarian tumors in inhibin-deficient mice leads to premature regression of the x zone, gonadectomy results in unopposed x-zone growth and finally tumorigenesis. Furthermore, we provide evidence that, in addition to inhibin, activin and LH are key factors in the complex regulatory network of x-zone growth.
A number of earlier studies have attempted to define factors that induce growth and regression of the x zone. Gonadectomy prevents x-zone regression (11), indicating that the x zone is preserved upon the withdrawal of a gonadal factor and/or the compensatory increase in gonadotropins following gonadectomy. These studies led to the hypothesis that degeneration of the x zone is contingent upon the presence of sex steroids. However, the x zone still degenerates in tfm mutant mice, which have a defect in the androgen receptor gene, suggesting that the degeneration process does not require androgens (40). Two pieces of evidence presented herein support this notion: (i) the adrenal x zone in nulliparous INH−/− female mice only regresses in the presence of ovarian tumors, and (ii) ovarian tumors in simple INH−/− mice do not secrete larger amounts of estradiol or testosterone than wild-type ovaries. Taken together, these data indicate that sex steroids are not necessary for x-zone regression and suggest that an additional gonadal factor and/or gonadotropins are involved in adrenal x-zone regression in this model.
Activins are widely expressed during mouse development and are highly conserved during vertebrate evolution. As evident in mice with targeted deletions of activin subunits and activin receptor type II, activins are also required for female fertility and gonadotroph function (28, 29). However, the adrenal phenotype of these murine models has not been reported to date. In humans, an increasing body of evidence suggests a significant impact of activin on the regulation of fetal-zone growth. Specifically, activin has been shown to inhibit proliferation and induce apoptosis in the adrenal fetal zone (44). Introduction of the LH-CTP transgene into INH−/− mice, which results in pronounced acceleration of ovarian tumor growth accompanied by profound and early elevation of activin levels, has enabled us to explore effects of high levels of activin on adrenal growth and differentiation in vivo. Our data support the concept that activin is also involved in the induction of x-zone regression in the mouse. Increasing activin levels in nongonadectomized INH−/− mice are associated with lower adrenal weight and cumulative x-zone regression. Likewise, high levels of activin in compound INH−/−-LH-CTP mice secreted by ovarian tumors or unilateral adrenal tumors are accompanied by regression of the adrenal x zone. In vitro treatment of primary adrenal cell cultures with activin is accompanied by a decrease in cell number only in cultures that contain x-zone cells. Finally, injection of activin into intact adrenals results in induction of apoptosis specifically in the x zone. In summary, our data indicate that when the adrenal is exposed to high levels of activin, the x zone invariably undergoes regression.
Although activin exerts its pleomorphic effects on a variety of cells and tissues mostly as a paracrine factor, activin acts also as a secreted hormone that stimulates pituitary follicle-stimulating hormone secretion (22, 46) and—at high levels—can have systemic effects leading to tumor cachexia (5). The activin-signaling pathway requires the action of two integral membrane receptor serine/threonine kinases (referred to as activin receptor types I and II), as well as the intracellular Smad family proteins, in order to transduce a signal from the membrane to the nucleus. Binding of radiolabeled activin A to the rat adrenal has been demonstrated (48), while activin results in apoptosis of human adrenal fetal-zone cells (44). As we demonstrate herein, activin type I and II receptors (ActR) are present in the mouse adrenal, with ActR-IA and ActR-IIB being predominantly expressed in the x zone. Moreover, Smad2, which has been shown to play a pivotal role in the transduction of activin signaling (14), is also mainly expressed in the x zone. Thus, this distinct expression profile is consistent with the sensitivity of x-zone cells to activin, as observed in our in vitro and in vivo assays. If activin does play a role in the suppression of adrenal tumorigenesis in inhibin-deficient mice, however, one has to propose that the adrenal tumor itself escapes the growth-inhibiting autocrine effects of activin. As we show herein, cells derived from INH−/− adrenal tumors are, in fact, unresponsive to in vitro activin treatment. Cancer cells can acquire resistance to the antiproliferative effect of TGF-β and activin by a number of different mechanisms, including defects in the specific cell surface receptors and mutational inactivation of shared downstream effector components of the signaling pathway (25). Loss of expression or inactivating mutations of Smad2 have been reported in a variety of different tumors, including head and neck squamous cell carcinoma (31), colorectal carcinoma (8), lung carcinoma (45), and serous ovarian carcinoma (21). Intriguingly, Smad2 levels are greatly diminished in adrenal tumors of both INH−/− and compound INH−/−-LH-CTP mice, suggesting a possible mechanism of escape from activin-dependent growth inhibition.
Hypophysectomy of both male and female mice causes degeneration of all adrenocortical zones, including the x zone; while LH replacement prevents x-zone degeneration, suggesting that LH is required for maintenance of the x zone (6). Accordingly, we demonstrate that gonadectomy in both wild-type and LH-CTP mice is accompanied by a significant increase in x-zone size. In addition, gonadectomized LH-CTP mice have a significantly larger x zone than do gonadectomized wild-type animals. Furthermore, in gonadectomized, inhibin-deficient mice, the presence of the LH-CTP transgene results in considerably faster growth of adrenal tumors. Whether LH exerts its growth-promoting effects on the x zone directly or indirectly remains to be determined. Direct LH action on adrenal growth and steroidogenesis through classical LH-dependent pathways presupposes the presence of LH receptor expression in the target tissue. In accordance with this concept, the LH receptor is expressed at low levels in the adrenals of all four genotypes upon gonadectomy. However, since in situ hybridization demonstrates abundant LH receptor expression only within the adrenal tumor and not in the adjacent normal adrenal, additional regulatory cascades presumably altered through somatic mutations within the tumor could be involved in the induction of LH receptor expression in this model.
While the function of the x zone is still unknown, x-zone cells contain lipid droplets, characteristic mitochondrial complexes, and a smooth endoplasmic reticulum, which are characteristics of steroid-secreting cells (9). Although the x zone has been proposed to synthesize adrenal sex steroids (33), expression of P450c17 or P450arom (aromatase), key steroidogenic enzymes required for the production of sex steroids, is not detectable in the adrenal glands of adult mice (16). In contrast and as predicted by the elevated estradiol levels, we demonstrate expression of P450c17 and P450arom in adrenal tumors of gonadectomized, inhibin-deficient mice. The distinct morphology of the tumors and illicit expression of receptors and enzymes required for sex steroid production suggest that the adrenal tumors in inhibin-deficient mice adopt functional and morphological features normally restricted to gonadal tissues. It is intriguing to speculate that these characteristics unravel common properties of gonadal and x-zone cells that reflect the common origin of gonadal and adrenal precursor cells from adjacent areas of the mesonephros during early development (42). Accordingly, expression of P450c17 has been demonstrated in a specific spatio-temporal pattern in a distinct cell population of the developing mouse adrenal (16), highlighting the concept of closely related progenitor cells in adrenal and gonadal development. Virilizing symptoms due to overproduction of sex steroids are the most common clinical presentation in children with adrenocortical carcinoma (36). While the cellular origin of these tumors has not been elucidated in detail, it has been proposed that they are derivatives of the fetal zone of the adrenal cortex (13). Given the strikingly similar aspects of childhood adrenocortical cancer and adrenal tumorigenesis in inhibin-deficient mice, it will be intriguing to explore the roles of activin, LH, and downstream effectors in childhood adrenal cancer in future studies.
Acknowledgments
We are indebted to M. Matzuk (Baylor College of Medicine, Houston, Tex.) for the generous gift of the inhibin alpha-null mice and to T. Giordano (University of Michigan, Ann Arbor) for the review of the histological specimen.
This work was supported by National Institutes of Health grants DK 02393 and DK 58124 to G.D.H. and Emmy Noether grant BE2177/3-1 awarded by the Deutsche Forschungsgemeinschaft to F.B.
REFERENCES
- 1.Beuschlein, F., C. Mutch, D. L. Bavers, Y. M. Ulrich-Lai, W. C. Engeland, C. Keegan, and G. D. Hammer. 2002. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology 143:3122-3135. [DOI] [PubMed]
- 2.Billiar, R. B., M. G. Leavitt, P. Smith, E. D. Albrecht, and G. J. Pepe. 1999. Functional capacity of fetal zone cells of the baboon fetal adrenal gland: a major source of α-inhibin. Biol. Reprod. 61:142-146. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y. G., H. M. Lui, S. L. Lin, J. M. Lee, and S. Y. Ying. 2002. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp. Biol. Med. (Maywood) 227:75-87. [DOI] [PubMed] [Google Scholar]
- 4.Cipriano, S. C., L. Chen, K. H. Burns, A. Koff, and M. M. Matzuk. 2001. Inhibin and p27 interact to regulate gonadal tumorigenesis. Mol. Endocrinol. 15:985-996. [DOI] [PubMed] [Google Scholar]
- 5.Coerver, K. A., T. K. Woodruff, M. J. Finegold, J. Mather, A. Bradley, and M. M. Matzuk. 1996. Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol. Endocrinol. 10:534-543. [DOI] [PubMed] [Google Scholar]
- 6.Deacon, C. F., W. Mosley, and I. C. Jones. 1986. The X zone of the mouse adrenal cortex of the Swiss albino strain. Gen. Comp. Endocrinol. 61:87-99. [DOI] [PubMed] [Google Scholar]
- 7.Eggenschwiler, J., T. Ludwig, P. Fisher, P. A. Leighton, S. M. Tilghman, and A. Efstratiadis. 1997. Mouse mutant embryos overexpressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes Dev. 11:3128-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eppert, K., S. W. Scherer, H. Ozcelik, R. Pirone, P. Hoodless, H. Kim, L. C. Tsui, B. Bapat, S. Gallinger, I. L. Andrulis, G. H. Thomsen, J. L. Wrana, and L. Attisano. 1996. MADR2 maps to 18q21 and encodes a TGF-β-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 86:543-552. [DOI] [PubMed] [Google Scholar]
- 9.Hirokawa, N., and H. Ishikawa. 1974. Electron microscopic observations on postnatal development of the X zone in mouse adrenal cortex. Z. Anat. Entwicklungsgesch. 144:85-100. [DOI] [PubMed] [Google Scholar]
- 10.Holmes, P. V., and A. D. Dickson. 1971. X-zone degeneration in the adrenal glands of adult and immature female mice. J. Anat. 108:159-168. [PMC free article] [PubMed] [Google Scholar]
- 11.Howard-Miller, E. 1928. A transitory zone in the adrenal cortex which shows age and sex relationships. Am. J. Anat. 40:251-293. [Google Scholar]
- 12.Hsu, S. Y., R. J. Lai, D. Nanuel, and A. J. Hsueh. 1995. Different 5′-flanking regions of the inhibin-α gene target transgenes to the gonad and adrenal in an age-dependent manner in transgenic mice. Endocrinology 136:5577-5586. [DOI] [PubMed] [Google Scholar]
- 13.James, L. A., A. M. Kelsey, J. M. Birch, and J. M. Varley. 1999. Highly consistent genetic alterations in childhood adrenocortical tumours detected by comparative genomic hybridization. Br. J. Cancer 81:300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanamaru, C., H. Yasuda, and T. Fujita. 2002. Involvement of Smad proteins in TGF-β and activin A-induced apoptosis and growth inhibition of liver cells. Hepatol. Res. 23:211-219. [DOI] [PubMed] [Google Scholar]
- 15.Kananen, K., M. Markkula, M. Mikola, E. M. Rainio, A. McNeilly, and I. Huhtaniemi. 1996. Gonadectomy permits adrenocortical tumorigenesis in mice transgenic for the mouse inhibin α-subunit promoter/simian virus 40 T-antigen fusion gene: evidence for negative autoregulation of the inhibin α-subunit gene. Mol. Endocrinol. 10:1667-1677. [DOI] [PubMed] [Google Scholar]
- 16.Keeney, D. S., C. M. Jenkins, and M. R. Waterman. 1995. Developmentally regulated expression of adrenal 17α-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology 136:4872-4879. [DOI] [PubMed] [Google Scholar]
- 17.Kero, J., M. Poutanen, F. P. Zhang, N. Rahman, A. M. McNicol, J. H. Nilson, R. A. Keri, and I. T. Huhtaniemi. 2000. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Investig. 105:633-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, T. R., G. Palapattu, P. Wang, T. K. Woodruff, I. Boime, M. C. Byrne, and M. M. Matzuk. 1999. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol. Endocrinol. 13:851-865. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, T. R., Y. Wang, and M. M. Matzuk. 1996. Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology 137:4210-4216. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix, A., P. Hamet, and J. M. Boutin. 1999. Leuprolide acetate therapy in luteinizing hormone-dependent Cushing's syndrome. N. Engl. J. Med. 341:1577-1581. [DOI] [PubMed] [Google Scholar]
- 21.Lassus, H., R. Salovaara, L. A. Aaltonen, and R. Butzow. 2001. Allelic analysis of serous ovarian carcinoma reveals two putative tumor suppressor loci at 18q22-q23 distal to SMAD4, SMAD2, and DCC. Am. J. Pathol. 159:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling, N., S. Y. Ying, N. Ueno, S. Shimasaki, F. Esch, M. Hotta, and R. Guillemin. 1986. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321:779-782. [DOI] [PubMed] [Google Scholar]
- 23.Lipsett, M. B., R. Hertz, and G. T. Ross. 1963. Clinical and pathophysiologic aspects of adrenocortical carcinoma. Am. J. Med. 35:374.. [DOI] [PubMed] [Google Scholar]
- 24.Malkin, D., F. P. Li, L. C. Strong, J. F. Fraumeni, Jr., C. E. Nelson, D. H. Kim, J. Kassel, M. A. Gryka, F. Z. Bischoff, and M. A. Tainsky. 1990. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250:1233-1238. [DOI] [PubMed] [Google Scholar]
- 25.Massague, J. 1998. TGF-β signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 26.Matzuk, M. M., M. J. Finegold, J. P. Mather, L. Krummen, H. Lu, and A. Bradley. 1994. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc. Natl. Acad. Sci. USA 91:8817-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matzuk, M. M., M. J. Finegold, J. G. Su, A. J. Hsueh, and A. Bradley. 1992. α-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313-319. [DOI] [PubMed] [Google Scholar]
- 28.Matzuk, M. M., T. R. Kumar, and A. Bradley. 1995. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 374:356-360. [DOI] [PubMed] [Google Scholar]
- 29.Matzuk, M. M., T. R. Kumar, A. Vassalli, J. R. Bickenbach, D. R. Roop, R. Jaenisch, and A. Bradley. 1995. Functional analysis of activins during mammalian development. Nature 374:354-356. [DOI] [PubMed] [Google Scholar]
- 30.Mesiano, S., and R. B. Jaffe. 1997. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 18:378-403. [DOI] [PubMed] [Google Scholar]
- 31.Muro-Cacho, C. A., K. Rosario-Ortiz, S. Livingston, and T. Munoz-Antonia. 2001. Defective transforming growth factor β signaling pathway in head and neck squamous cell carcinoma as evidenced by the lack of expression of activated Smad2. Clin. Cancer Res. 7:1618-1626. [PubMed] [Google Scholar]
- 32.Muscatelli, F., T. M. Strom, A. P. Walker, E. Zanaria, D. Récan, A. Meindl, B. Bardoni, S. Guioli, G. Zehetner, and W. Rabl. 1994. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672-676. [DOI] [PubMed] [Google Scholar]
- 33.Nussdorfer, G. G. 1986. Cytophysiology of the adrenal cortex. Int. Rev. Cytol. 98:1-405. [PubMed] [Google Scholar]
- 34.Orozco-Florian, R., J. A. McBride, B. E. Favara, A. Steele, S. J. Brown, and P. Steele. 1991. Congenital hepatoblastoma and Beckwith-Wiedemann syndrome: a case study including DNA ploidy profiles of tumor and adrenal cytomegaly. Pediatr. Pathol. 11:131-142. [DOI] [PubMed] [Google Scholar]
- 35.Pabon, J. E., X. Li, Z. M. Lei, J. S. Sanfilippo, M. A. Yussman, and C. V. Rao. 1996. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J. Clin. Endocrinol. Metab. 81:2397-2400. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro, R. C., E. L. Michalkiewicz, B. C. Figueiredo, L. DeLacerda, F. Sandrini, M. D. Pianovsky, G. Sampaio, and R. Sandrini. 2000. Adrenocortical tumors in children. Braz. J. Med. Biol. Res. 33:1225-1234. [DOI] [PubMed] [Google Scholar]
- 37.Risma, K. A., C. M. Clay, T. M. Nett, T. Wagner, J. Yun, and J. H. Nilson. 1995. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc. Natl. Acad. Sci. USA 92:1322-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, V. J., and S. L. Barth. 1994. Expression of messenger ribonucleic acids encoding the inhibin/activin system during mid- and late-gestation rat embryogenesis. Endocrinology 134:914-923. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, V. J., P. E. Sawchenko, and W. Vale. 1991. Expression of inhibin/activin subunit messenger ribonucleic acids during rat embryogenesis. Endocrinology 128:3122-3129. [DOI] [PubMed] [Google Scholar]
- 40.Shire, J. G. 1976. Degeneration of the adrenal X-zone in Tfm mice with inherited insensitivity to androgens. J. Endocrinol. 71:445-446. [DOI] [PubMed] [Google Scholar]
- 41.Shou, W., T. K. Woodruff, and M. M. Matzuk. 1997. Role of androgens in testicular tumor development in inhibin-deficient mice. Endocrinology 138:5000-5005. [DOI] [PubMed] [Google Scholar]
- 42.Smith, C., and S. Mackay. 1991. Morphological development and fate of the mouse mesonephros. J. Anat. 174:171-184. [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer, S. J., S. Mesiano, J. Y. Lee, and R. B. Jaffe. 1999. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J. Clin. Endocrinol. Metab 84:1110-1115. [DOI] [PubMed] [Google Scholar]
- 44.Spencer, S. J., J. Rabinovici, S. Mesiano, P. C. Goldsmith, and R. B. Jaffe. 1992. Activin and inhibin in the human adrenal gland: regulation and differential effects in fetal and adult cells. J. Clin. Investig. 90:142-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida, K., M. Nagatake, H. Osada, Y. Yatabe, M. Kondo, T. Mitsudomi, A. Masuda, and T. Takahashi. 1996. Somatic in vivo alterations of the JV18-1 gene at 18q21 in human lung cancers. Cancer Res. 56:5583-5585. [PubMed] [Google Scholar]
- 46.Vale, W., J. Rivier, J. Vaughan, R. McClintock, A. Corrigan, W. Woo, D. Karr, and J. Spiess. 1986. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 321:776-779. [DOI] [PubMed] [Google Scholar]
- 47.Wiedemann, H. R. 1964. Complexe malformatif familial avec hernie ombilicale et macroglossie—un syndrome nouveau? J. Genet. Hum. 13:223-232. [PubMed] [Google Scholar]
- 48.Woodruff, T., L. Krummen, S. Chen, R. Lyon, S. Hansen, G. DeGuzman, R. Covello, J. Mather, and P. Cossum. 1993. Pharmacokinetic profile of recombinant human (rh) inhibin A and activin A in the immature rat. II. Tissue distribution of [125I]rh-inhibin A and [125I]rh-activin A in immature female and male rats. Endocrinology 132:725-734. [DOI] [PubMed] [Google Scholar]
- 49.Wooten, M. D., and D. K. King. 1993. Adrenal cortical carcinoma: epidemiology and treatment with mitotane and a review of the literature. Cancer 72:3145-3155. [DOI] [PubMed] [Google Scholar]
- 50.Yu, R. N., M. Ito, T. L. Saunders, S. A. Camper, and J. L. Jameson. 1998. Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 20:353-357. [DOI] [PubMed] [Google Scholar]