Abstract
The suppression and eradication of primary tumors and distant metastases is a major goal of alternative treatment strategies for cancer, such as inhibition of angiogenesis and targeted immunotherapy. We report here a synergy between two novel monotherapies directed against vascular and tumor compartments, respectively, a tumor vasculature-specific antiangiogenic integrin αv antagonist and tumor-specific antibody–interleukin 2 (IL-2) fusion proteins. Simultaneous and sequential combination of these monotherapies effectively eradicated spontaneous liver metastases in a poorly immunogenic syngeneic model of neuroblastoma. This was in contrast to controls subjected to monotherapies with either an antiangiogenic integrin αv antagonist or antibody–IL-2 fusion proteins, which were only partially effective at the dose levels applied. Furthermore, simultaneous treatments with the integrin αv antagonist and tumor-specific antibody–IL-2 fusion proteins induced dramatic primary tumor regressions in three syngeneic murine tumor models, i.e., melanoma, colon carcinoma, and neuroblastoma. However, each agent used as monotherapy induced only a delay in tumor growth. A mechanism for this synergism was suggested because the antitumor response was accompanied by a simultaneous 50% reduction in tumor vessel density and a 5-fold increase in inflammatory cells in the tumor microenvironment. Subsequently, tumor necrosis was demonstrated only in animals receiving the combination therapy, but not when each agent was applied as monotherapy. The results suggest that these synergistic treatment modalities may provide a novel and effective tool for future therapies of metastatic cancer.
The generation of new blood vessels, or angiogenesis, plays a key role in the growth of malignant disease and has generated much interest in developing agents that inhibit angiogenesis (1–6). However, the identification of well characterized, vasculature-specific inhibitors of angiogenesis that are synergistic with therapies specifically targeting the tumor compartment may be critical for achieving optimally effective cancer treatment.
Angiogenesis is characterized by invasion, migration, and proliferation of endothelial cells, processes that depend on cell interactions with extracellular matrix components. In this context, the endothelial adhesion receptor integrin αvβ3 was shown to be a key player (7, 8) by providing a vasculature-specific target for antiangiogenic treatment strategies. The requirement for vascular integrin αvβ3 in angiogenesis was demonstrated by several in vivo models in which the generation of new blood vessels by transplanted human tumors was inhibited entirely by systemic administration of peptide antagonists of either integrin αvβ3 or anti-αvβ3 antibody LM609 (7, 9). Such antagonists block the ligation of integrin αvβ3, which promotes apoptosis of the proliferative angiogenic vascular cells and thereby disrupts the maturation of newly forming blood vessels, an event essential for the proliferation of tumors.
A major obstacle for effective treatment of disseminated malignancies includes minimal residual disease characterized by micrometastases that lack a well established vascular supply. In this regard, a novel immunotherapeutic strategy proved very efficient in using tumor compartment-specific mAbs to direct cytokines to the tumor microenvironment. This was achieved by recombinant antibody–cytokine fusion proteins, generated to maintain the unique tumor-specific targeting ability of mAbs and the immunomodulatory functions of cytokines. In fact, the use of an antibody–interleukin 2 (IL-2) fusion protein to direct IL-2 into the tumor compartment induced activation of effector cells invading the tumor microenvironment and resulted in highly efficient eradication of established micrometastases in three different syngeneic mouse tumor models (10–12). Specifically, the daily injection of 10 μg antiganglioside GD2 antibody–IL-2 fusion protein (6×) was effective in eradicating spontaneous liver and bone marrow metastases in a novel syngeneic model of neuroblastoma (20) in contrast to lower doses (5 × 5 μg) used here that were only partially effective. Although quite effective at early stages of tumor metastasis, this tumor compartment-directed approach could only delay growth of metastases at later stages of tumor growth characterized by a fully developed vascular compartment (21). Here, we addressed the question of whether there is a complementary advantage of such specific vascular and tumor compartment-directed treatment strategies being synergistic when used in sequential and simultaneous combinations.
This hypothesis was tested in three syngeneic murine tumor models of colon carcinoma, melanoma, and neuroblastoma, the latter characterized by spontaneous hepatic metastases. All three models exhibit close similarities to the diseases in humans. The melanoma and neuroblastoma models express disialoganglioside GD2, a well established tumor-associated antigen in such neuroectodermal malignancies (13, 14), and the colon carcinoma model is characterized by the expression of the epithelial cell adhesion molecule (Ep-CAM), a target molecule successfully exploited for passive immunotherapy in humans (15). These antigens specifically delineate the tumor compartment in the models targeted by the antibody–IL-2 fusion proteins with human/mouse chimeric anti-GD2 antibody (ch14.18-IL-2) (16) and humanized anti-Ep-CAM antibody (huKS1/4-IL-2) (11, 17), respectively. The vascular compartment of these tumor models, as described in several animal models, is defined by expression of integrin αvβ3 on newly formed blood vessels (7). The data presented here demonstrate a synergistic efficacy of simultaneous and sequential treatments specifically targeting tumor and vascular compartments of primary tumors and distant metastases. A mechanism for this synergism is provided by a decrease in blood vessel formation and an increase in inflammation only in animals treated with the combination therapy. These observations emphasize the beneficial effect of combining antiangiogenic with tumor-specific immunotherapeutic approaches.
MATERIALS AND METHODS
Generation and Characterization of Tumor-Specific Antibody–Cytokine Fusion Proteins and Vasculature-Specific Integrin αv Antagonist.
Construction and characterization of ch14.18-IL-2 and huKS1/4-IL-2 antibody–cytokine fusion proteins were described previously (11, 16). Antigen-binding characteristics of both constructs were identical to those of their respective antibodies, and the specific IL-2 activity was equivalent to commercially available rhIL-2. The integrin αvβ3 antagonistic cyclic peptide EMD121974 [cyclic Arg-Gly-Asp-d-Phe-(N-methyl)Val] and the control peptide EMD135981 [cyclic Arg-β-Ala-Asp-d-Phe(N-methyl)Val] were synthesized and characterized at Merck (Darmstadt, Germany).
Cell Lines and Animal Models.
All cell lines and respective animal models were established in the authors’ laboratories and described previously (10–12). The absence of integrin αvβ3 on NXS2 and CT26-KSA cells was demonstrated with antiintegrin β3 antibody CD61 (1 μg/106 cells) (PharMingen) and by the inability of NXS2 cells to adhere to plastic coated with this antibody. However, all tumor cells express integrin αv by fluorescence-activated cell sorter and adhere on vitronectin, indicating the presence of integrin αvβ5.
For all surgical procedures, mice were anesthetized by ketamine injection (100 mg/kg i.p.) and simultaneous Metofane inhalation (Pitman–Moore, Mundelein, IL). Osmotic pumps (model 2001; Alzet, Palo Alto, CA) for the administration of a saturated solution (18 mg/ml) of the integrin αv antagonist and the control peptide were used at a delivery rate of 17.5 μg/h and achieved effective serum levels of 500–1,000 ng peptide/ml serum. These pumps were implanted in the dorsal s.c. tissue under sterile conditions, replaced on day 7 after implantation, and removed after 10 days of antivascular treatment. All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Histology and Immunohistochemistry.
Acetone-fixed, frozen sections of primary tumors were incubated with 4% goat serum to block nonspecific binding. Incubations with anti-mouse CD31 and CD45 mAbs (PharMingen) (1:100) and subsequent staining with rhodamine-labeled goat anti-rat antibody (1:300) were performed in a humidified chamber at room temperature. Each incubation was followed by washes with PBS (3×). Vessel and white blood cell counts per high-power field were determined microscopically at ×200 magnification (18). Representative areas were photographed at ×200 (vessels) and ×800 (white blood cells), respectively.
RESULTS
Primary Tumors Regress Only in Mice Treated with Integrin αv Antagonist Combined with Antibody–IL-2 Fusion Proteins.
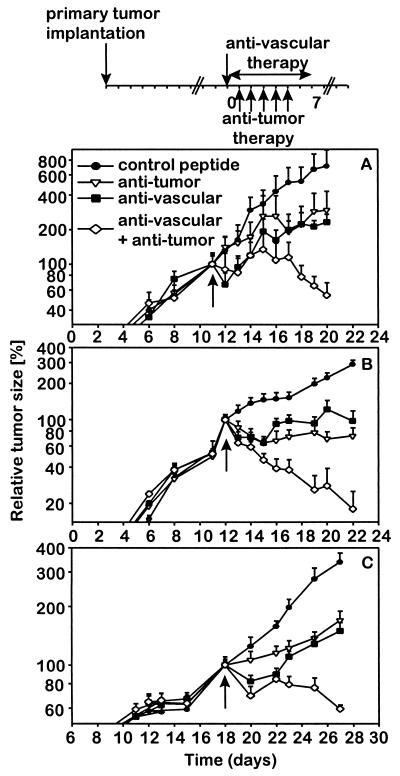
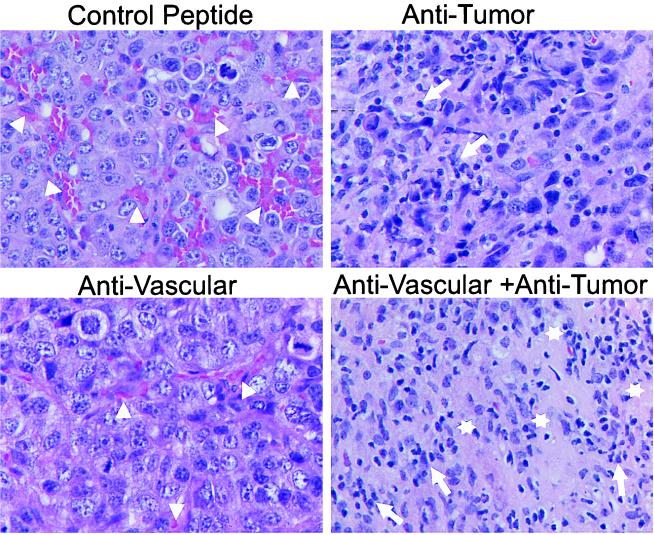
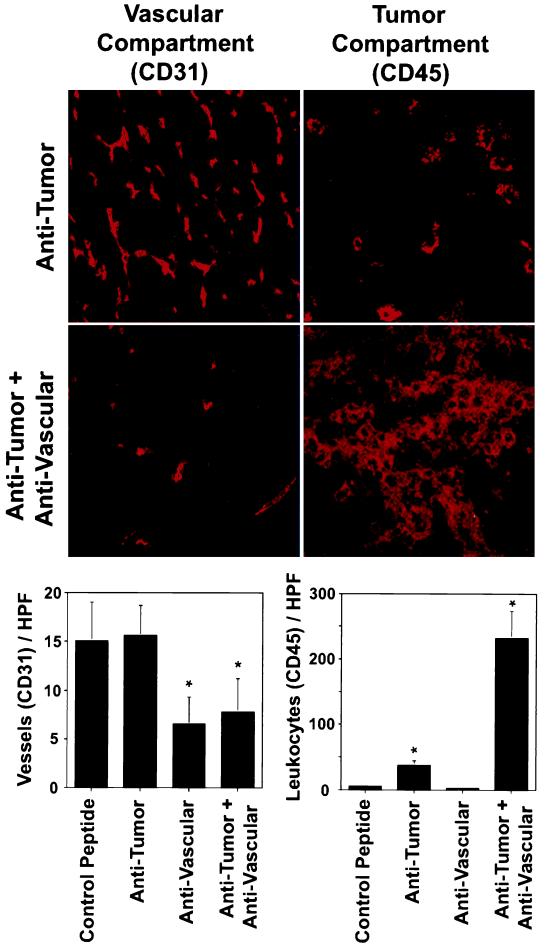
Synergistic effects of integrin αv antagonist and antibody–IL-2 fusion proteins were determined in mice with established s.c. tumors (110–130 μl) in all three syngeneic models (Fig. 1). First, suboptimal amounts for each therapeutic modality were established and their subsequent use in combination was initiated. Only mice treated with the integrin αv antagonist and the IL-2 fusion proteins presented with a 50–90% tumor regression in all three models (P < 0.001). In fact, half the animals inoculated with neuroblastoma and colon carcinoma cells entirely rejected their primary tumors (data not shown). In contrast, each strategy used as monotherapy, at best, delayed growth when compared with controls. Mice receiving the integrin αv antagonist showed a 50% decrease in vascularization (Figs. 2 and 3) coincident with a growth delay of primary tumors, demonstrating effective targeting of the vascular compartment. In this case, the tumor compartment was not affected directly (Figs. 1 and 2). Treatment with nonspecific control peptide affected neither formation of new blood vessels nor s.c. tumor growth when compared with untreated controls (data not shown). Furthermore, the treatment with anti-GD2-IL-2 fusion protein did not affect neovascularization (Fig. 3 Bottom Left), indicating that its effect is mediated entirely by attacking the tumor compartment. In contrast, mice treated only with the anti-GD2-IL-2 fusion protein revealed a distinct leukocytic infiltrate (Figs. 2 and 3), a well established characteristic of this antitumor compartment-directed therapy (10–12), leading to a substantial reduction in s.c. tumor growth (Fig. 1). Importantly, neither the integrin αv antagonist nor the nonspecific peptide control induced a leukocytic infiltrate (Fig. 3 Bottom Right), indicating that the effect of the integrin αv antagonist is attributable solely to an attack on the vascular compartment. However, only mice treated with the combination of integrin αv antagonist and anti-GD2-IL-2 fusion protein revealed a 5-fold increase in leukocytic infiltrate into the tumor compared with mice treated with anti-GD2-IL-2 fusion protein alone and showed a similar decrease in vascularization (Figs. 2 and 3). The leukocytic infiltrate in the tumor microenvironment after treatment with ch14.18-IL-2 is not attributable to the human part of the molecule, because nonspecific IL-2 fusion proteins or mixtures of tumor-specific ch14.18 antibody and human IL-2 elicited neither a leukocytic infiltrate nor an antitumor immune response (10–12). The increase in inflammatory cells, demonstrated by histology (Fig. 2) and immunohistochemistry (Fig. 3), was attributed to an influx of macrophages (data not shown), a pattern frequently seen in necrotic tissues during removal of cellular debris. In fact, such necrotic areas were seen only in tumors after the combination treatment (Fig. 2).
Figure 1.
Effect of a combined therapy with antiangiogenic αv integrin antagonist and antitumor compartment-specific immunotherapy with antibody–IL-2 fusion proteins on primary tumors. Primary tumors were induced by s.c. injection (2 × 106) of each NXS2 neuroblastoma (A), CT26-KSA colon carcinoma (B), and B78-D14 melanoma cells (C). (Top) Treatment of established tumors (110–130 mm3) by daily i.v. injections of tumor-specific antibody–IL-2 fusion proteins huKS1/4-IL-2 (10 μg, colon carcinoma) and ch14.18-IL-2 (5 μg, neuroblastoma; 10 μg, melanoma) (5×) and continuous s.c. infusion of the vasculature-specific integrin αv antagonist or the control peptide with an osmotic pump for 7 days at 17.5 μg/h. The time of treatment initiation is indicated by a solid arrow. The size of the primary tumors of mice in each experimental group (n = 6) was determined by microcaliper measurements (width × length × width/2) (mean ± SE). The regression in primary tumor size of mice receiving the combination treatment compared with the size of established tumors at the time of treatment initiation was statistically significant in the three different syngeneic tumor models (P < 0.001, Wilcoxon Rank–Sum Test) in contrast to all controls (P > 0.05).
Figure 2.
Histology after combined antiangiogenic and tumor-specific immunotherapy of established primary neuroblastoma tumors, surgically removed 20 days after tumor cell inoculation. Formalin-fixed primary tumors were subjected to paraffin embedding and subsequent hematoxylin/eosin staining. Arrowheads delineate blood vessels. Necrotic areas and leukocyte infiltrates are indicated by open stars and arrows, respectively. Representative areas were photographed at ×630.
Figure 3.
Effect of combined antivascular and antitumor therapies on vascularization and antitumor immune response. Mice (n = 6) with established primary neuroblastoma tumors received the combination treatment, as described in Fig. 1, including controls that received each therapy alone. At the end of the treatment, s.c. tumors were removed surgically. Frozen sections of each tumor were analyzed by immunohistochemistry by using antibodies specific for blood vessel endothelial cells (CD31) and for leukocyte infiltration (CD45), respectively. The latter is a well established marker for the tumor compartment-specific immune response induced by the ch14.18-IL-2 fusion protein (10–12). (Left) Blood vessel density of primary tumors after vascular and tumor compartment treatment with either the integrin αv antagonist, ch14.18-IL-2 fusion protein, or a combination thereof (∗, P < 0.001, Student’s t test). (Right) Leukocyte infiltration of primary tumors after vascular and tumor compartment treatments, respectively (∗, P < 0.001, Student’s t test).
Sequential and Simultaneous Vascular and Tumor Targeting Induces Eradication of Spontaneous Hepatic Metastases.
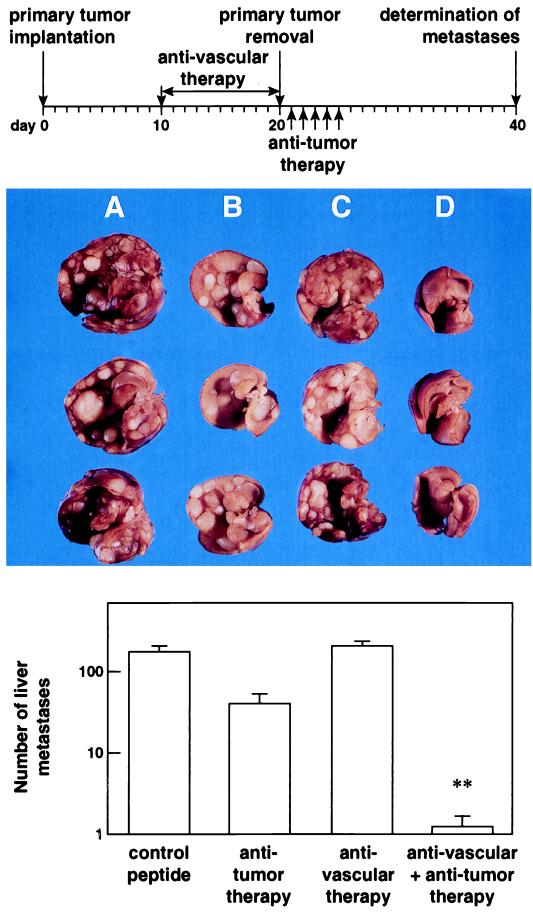
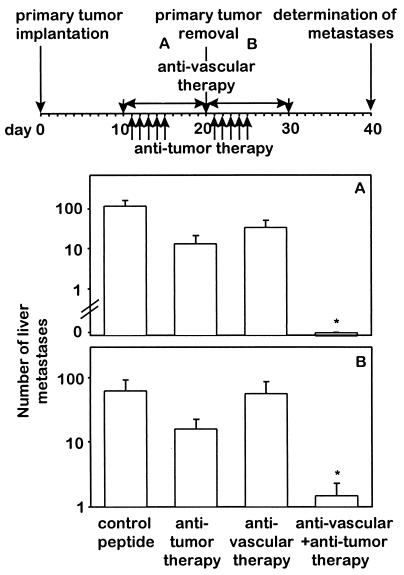
In addition to a successful treatment of primary tumors, the key question is whether distant metastases are affected by such a combined antivascular and anti-tumor-specific treatment strategy. This was addressed in the neuroblastoma model, characterized by spontaneous hepatic metastases. Sequential combination of the antiangiogenic integrin αv antagonist with ch14.18-IL-2 (Fig. 4 Top) resulted in a 1.5- to 2-log decrease in hepatic metastases in contrast to all controls, where treatment with each agent used as monotherapy was ineffective (P < 0.01) (Fig. 4 Bottom). In fact, four of eight mice subjected to the combined therapy revealed a complete absence of hepatic metastases, whereas the remaining animals showed only one to five small metastatic lesions (Fig. 4 A– D). Similar results were obtained by simultaneous combinations of the integrin αv antagonist with the ch14.18-IL-2 fusion protein (Fig. 5 Top). Only mice treated with both agents revealed either a complete absence (Fig. 5A) or a >1.5-log decrease (Fig. 5B) in hepatic metastases (P < 0.01), depending on their administration before or after primary tumor removal.
Figure 4.
Effect of a sequential combination of antiangiogenic integrin αv antagonist and antitumor compartment-specific immunotherapy with antibody–IL-2 fusion protein on spontaneous hepatic neuroblastoma metastases. The antivascular treatment was initiated in mice with established primary tumors, as indicated in Fig. 1, for a total of 10 days (Top). After surgical removal of the primary tumors, mice received the tumor compartment-specific immunotherapy by daily i.v. injections of 5 μg ch14.18-IL-2 fusion protein (5×). Three representative specimens of each treatment group are depicted. (A) Peptide control. (B) Antitumor therapy (ch14.18-IL-2). (C) Antivascular therapy (integrin αv antagonist). (D) Combination of B and C. The number of spontaneous liver metastases was determined by macroscopic counts of liver foci (n = 8) (Bottom) (∗, P < 0.01, Wilcoxon Rank–Sum Test).
Figure 5.
Effect of the simultaneous combination of antiangiogenic integrin αv antagonist and antitumor compartment-specific immunotherapy with antibody–IL-2 fusion protein on spontaneous hepatic neuroblastoma metastases. Spontaneous metastases were induced after induction of primary tumors with 2 × 106 NXS2 neuroblastoma cells s.c. (Top). Treatment with integrin αv antagonist (17.5 μg/h) and tumor-specific ch14.18-IL-2 fusion protein (5 × 5 μg) was initiated before (A) or after (B) removal of the primary tumor. Spontaneous liver metastases were determined by macroscopic counts of liver foci (n = 8) (∗, P < 0.01, Wilcoxon Rank–Sum Test).
DISCUSSION
Disruption of blood vessels in the vascular compartment of malignant tumors is a potentially powerful strategy to combat cancer. By targeting the endothelial cells of the tumor vasculature, the tumor can be treated successfully. A peptide antagonist targeting the vasculature through interaction with αv integrins expressed on angiogenic blood vessels (7, 8) suppressed blood vessel formation and dramatically regressed subsequent tumor growth in three aggressively growing primary tumors and one spontaneously metastasizing tumor. Although the integrin αv antagonist used was directed primarily to αvβ3, it also binds the closely related integrin αvβ5. The colon carcinoma and neuroblastoma tumors examined are clearly lacking αvβ3, but likely express some αvβ5. The melanoma model also expresses αvβ3. However, the effect of this integrin antagonist clearly was restricted to the tumor vasculature in all three animal models, as demonstrated for the neuroblastoma model (Figs. 2 and 3). Importantly, the antitumor effect of targeting the tumor vasculature is amplified by a simultaneous attack on the tumor compartment, which is effective against both primary tumors and spontaneous metastases. This is particularly relevant, because removal of the primary tumor before treatment increased growth and dissemination of neuroblastoma metastases (data not shown), a finding well documented in other tumor models because of a decrease in circulating levels of angiogenesis inhibitors after excision of the primary tumor (1, 2). The simultaneous targeting of the vascular and tumor compartments proved very effective, because it combines a decrease in tumor cell nourishment with the active destruction of tumor cells, leading to a regression of primary tumors and the eradication of distant metastases. Our data, in three clinically relevant tumor models, extend previous findings with a combination approach of tumor- and vasculature-specific targeting in a specially designed model using A-strain C1300 neuroblastoma cells (H-2Kk/H-2Dd) and BALB/c nu/nu mice (H-2Kd/H-2Dd) (19). These C1300 neuroblastoma cells were also genetically engineered to secrete mouse γ-interferon (C1300 Muγ) to increase major histocompatibility complex class II expression (I-Ad/I-Ed) on the BALB/c nu/nu-derived epithelium of the tumor vasculature, which subsequently was used as a vasculature-specific target. The tumor compartment of this model was delineated by the exclusive expression of H-2Kk on the C1300 Muγ tumor allograft in BALB/c nu/nu mice. Treatment of established s.c. tumors of C1300 Muγ neuroblastoma with a combination of ricin-A immunotoxins specific for I-Ad/I-Ed and H-2Kk, delineating the respective vascular and tumor compartments, achieved the eradication of five of eight s.c. tumors in contrast to controls treated with each specific immunotoxin as monotherapy. Importantly, our combination therapy extended these results to both s.c. tumors and disseminated metastases in a relevant syngeneic neuroblastoma model, which, similar to the human disease, is characterized by the natural expression of integrin αvβ3 and ganglioside GD2 in respective vascular and tumor compartments. In our strategy, the tumor compartment-specific response is mediated by inflammatory cells that are activated and directed to the tumor microenvironment by tumor-specific antibody–IL-2 fusion proteins. Importantly, the antiangiogenic strategy, although quite effective in growth suppression of primary tumors with a well established vascular supply, lacks a similar efficacy against distant micrometastases when used as monotherapy (Figs. 4 and 5). However, in such a minimal residual disease setting with small tumor loads characterized by poor vascularization, the antitumor compartment treatment arm used in the combination therapy is quite effective when used as monotherapy (11, 12). In this situation, the role of antiangiogenic treatment strategies could be to suppress micrometastasis-induced neovascularization and subsequent enlargement of metastatic foci (6). This, in turn, would facilitate eradication of such micrometastases by tumor compartment-directed therapies, which are optimally effective in the minimal residual disease setting (10).
In summary, effective treatments of primary tumors and disseminated metastases remain a major challenge in clinical oncology. The results in this report suggest that combinations of specific antiangiogenic therapies and immunotherapies synergize in regression of primary tumors and eradication of micrometastases. Because both treatment modalities, i.e., αv integrin antagonists and antibody–IL-2 fusion proteins, currently are under clinical evaluation as monotherapies, the synergy of their combination may provide a novel and effective tool for cancer therapy.
Acknowledgments
This work was supported by the National Institutes of Health Outstanding Investigator Grants CA-42508 (R.A.R.), CA-45726, and CA-50286 (D.A.C.). H.N.L. and T.M. were supported by training grants from the Deutsche Forschungsgemeinschaft and the Deutsche Krebshilfe, respectively. We thank Lynne Kottel for the preparation of this manuscript. We also thank Merck KGaA, Darmstadt, Germany, for providing the cyclic peptides described in this manuscript. This is The Scripps Research Institute’s manuscript number 11814-IMM.
ABBREVIATION
- IL-2
interleukin 2
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Holmgren L, O’Reilly M S, Folkman J. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel R S. Nature (London) 1997;390:335–336. doi: 10.1038/36978. [DOI] [PubMed] [Google Scholar]
- 5.Boehm T, Folkman J, Browder T, O’Reilly M S. Nature (London) 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 6.Volpert O V, Lawler J, Bouck N P. Proc Natl Acad Sci USA. 1998;95:6343–6348. doi: 10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander M, Brooks P C, Shaffer R W, Kincaid C M, Varner J A, Cheresh D A. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 9.Brooks P C, Montgomery A M, Rosenfeld M, Reisfeld R A, Hu T, Klier G, Cheresh D A. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 10.Becker J C, Pancook J D, Gillies S D, Furukawa K, Reisfeld R A. J Exp Med. 1996;183:2361–2366. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang R, Lode H N, Dolman C S, Dreier T, Varki N M, Qian X, Lo K, Lan Y, Super M, Gillies S D, Reisfeld R A. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]
- 12.Lode H N, Xiang R, Dreier T, Varki N M, Gillies S D, Reisfeld R A. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 13.Irie R F, Matsuki T, Morton D L. Lancet. 1989;1:786–787. doi: 10.1016/s0140-6736(89)92606-8. [DOI] [PubMed] [Google Scholar]
- 14.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, Reuland P, Gillies S D, Reisfeld R A, Neithammer D. Eur J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 15.Riethmuller G, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmaier H, Hirche H, Pichlmayr R, et al. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 16.Gillies S D, Reilly E B, Lo K M, Reisfeld R A. Proc Natl Acad Sci USA. 1992;89:1428–1432. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillies S, Lan Y, Wesolowski J D, Qian X, Reisfeld R A, Holden S, Lo K M. J Immunol. 1998;160:6195–6203. [PubMed] [Google Scholar]
- 18.Brooks P C, Stromblad S, Klemke R, Visscher D, Sarkar F H, Cheresh D A. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burrows F J, Thorpe P E. Proc Natl Acad Sci USA. 1993;90:8996–9000. doi: 10.1073/pnas.90.19.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lode H N, Xiang R, Varki N M, Dolman C S, Gillies S D, Reisfeld R A. J Natl Cancer Inst. 1998;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 21.Becker J C, Varki N, Gillies S D, Furukawa K, Reisfeld R A. Proc Natl Acad Sci USA. 1996;93:7826–7831. doi: 10.1073/pnas.93.15.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]