Abstract
We provide here evidence that supports the occurrence of a biologically dormant form of selectin ligand carbohydrate, the sialyl 6-sulfo Lewis X containing modified sialic acid, in human leukocytes. The modification of sialic acid involves first de-N-acetylation of sialic acid moiety through ubiquitous de-N-acetylation/re-N-acetylation cycle, followed by the dehydrative cyclization of de-N-acetyl sialic acid to form “cyclic sialic acid.” The enzyme involved in the dehydration of de-N-acetyl sialic acid is a calcium-dependent enzyme having neutral–alkaline pH optimum. De-N-acetyl sialyl 6-sulfo Lewis X retained selectin binding activity as well as parental sialyl 6-sulfo Lewis X, but cyclic sialyl 6-sulfo Lewis X was devoid of selectin binding activity. Sialyl 6-sulfo Lewis X carrying the cyclic sialic acid is specifically recognized by the newly generated mAb, G159. The determinant was distributed widely among normal human leukocytes, especially on monocytes and subsets of lymphocytes including NK cells, helper memory T cells, Tcr-γδ T cells, and a part of B cells. The determinant was detected also on several cultured lymphocytic leukemia cell lines and O-tetradecanoylphorbol 13-acetate-activated lymphoid cells. Cyclic sialyl 6-sulfo Lewis X is efficiently formed by the action of the partly membrane-bound calcium-dependent enzyme, tentatively called “sialic acid cyclase,” and a possible physiological significance of this reaction could be a rapid inactivation of selectin binding activity at the cell surface. Conversely, the accumulated intracellular cyclic sialyl 6-sulfo Lewis X determinant may function as a dormant pool of selectin ligands, which, on appropriate stimulation, is hydrolyzed and becomes active in selectin-dependent cell adhesion.
Selectins are cell adhesion molecules that are involved in leukocyte–endothelial and leukocyte–leukocyte interactions (1–3). Sialyl Lewis X has been assumed to be a carbohydrate ligand for selectins, but a considerable molecular heterogeneity is noted in sialyl Lewis X-like determinants expressed on cells and tissues (4–8). Recently, it has been shown that sialyl 6-sulfo Lewis X, but not conventional sialyl Lewis X, serves as the major ligand for L-selectin on high endothelial venules of human lymph nodes by generating a specific mAb, G152, directed to this determinant (9, 10). The sialyl 6-sulfo Lewis X determinant is expressed also on certain subsets of leukocytes, and this determinant is synthesized through a cooperative action of a fucosyltransferase VII (11, 12) and a newly cloned N-acetylglucosamine-6-O-sulfotransferase (13, 14).
During the course of generating mAbs directed to sialyl 6-sulfo Lewis X by using synthetic glycolipid as an immunogen, we obtained one antibody, G159, directed to a by-product of organochemical synthesis, which had a faster TLC mobility than the genuine sialyl 6-sulfo Lewis X. The synthetic by-product seemed to have resulted from an excessive deacetylation under anhydrous alkaline condition at the final step of organochemical synthesis of sialyl 6-sulfo Lewis X.
In our experiences, antibodies directed to such synthetic by-products usually have no reactivity to naturally occurring substances. However, the situation was quite different with this G159 antibody in that the antibody significantly reacted with a wide variety of human peripheral blood leukocytes. In this study, we provide evidence suggesting that the antigenic determinant recognized by this antibody is sialyl 6-sulfo Lewis X, which carries cyclized sialic acid moiety derived from the enzymatic dehydration of de-N-acetyl sialic acid, and we describe the distribution of the determinant among human peripheral leukocytes in relation to its adhesive activity toward selectins.
MATERIALS AND METHODS
Synthetic Carbohydrate Determinants and Generation of mAbs.
Sialyl 6-sulfo Lewis X and genuine sialyl Lewis X were synthesized as described (15, 16). Synthetic sialyl 6-sulfo Lewis X glycolipid had the structure NeuAcα2→3Galβ1→4[Fucα1→3] [SO4−6]GlcNAcβ1→3Galβ1→4Glcβ1→Cer. De-N-acetyl sialyl 6-sulfo Lewis X was synthesized by a strategy essentially similar to that used for sialyl 6-sulfo Lewis X synthesis by using the sialic acid after treating its acetamide group at C-5 position using methansulfonate and protecting the resulting free amino group with trifluoroacetic acid. The structure of the synthetic glycolipid was ascertained by mass spectrometry. Details of the organochemical synthesis procedure for de-N-acetyl sialyl 6-sulfo Lewis X are described elsewhere (S.K., H.I., and M.K., unpublished work). De-N-acetyl sialyl 6-sulfo Lewis X was dehydrated with water soluble carbodiimide, 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (WSC; 0.05 mmol) in 1.0 ml of dichloromethane for 12 hr at room temperature, for generation of sialyl 6-sulfo Lewis X carrying cyclic neuraminic acid.
New hybridoma cells that secrete mAbs directed to 6-sulfated sialyl Lewis X determinants were generated according to the method described by Köhler and Milstein (17) and subsequently adopted for anticarbohydrate antibodies (18). In brief, the synthetic sialyl 6-sulfo Lewis X glycolipid was adsorbed to Salmonella minnesota R595 strain and was used for repeated i.p. immunizations of BALB/c mice. The splenic cells were fused with mouse myeloma P3/X63-Ag8U1. The same glycolipid was used as the antigen in ELISA of hybridoma culture supernatants for the cloning procedures. Two hybridoma clones, G152 (IgM) and G159 (IgG1), were obtained, and G152 turned out to be specific to sialyl 6-sulfo Lewis X. TLC-immunostaining was performed according to the method described (10, 19). Antibody reaction on TLC plates was visualized with ECL Western blotting detection reagents (Amersham).
Flow Cytometric Analysis of G159-Defined Sialyl 6-Sulfo Lewis X Derivative.
Peripheral blood samples were obtained from healthy donors at Kyoto University Hospital. Lymphocytes were isolated from Ficoll/Hypaque by standard methods (20). Cultured human leukemia cell lines used in this study included U937 (a histiocytic leukemia line), Jurkat (a T-cell leukemia line), ED40515-N (an adult T-cell leukemia line), Namalwa (a B-cell leukemia line), and YT (an NK cell-like line). These leukemia cell lines were obtained from the First Division of the Department of Internal Medicine, Kyoto University, except ED40515-N, which was the kind gift of Michiyuki Maeda (Chest Disease Research Institute, Kyoto University). For flow cytometric analysis, cells were harvested at a semiconfluent stage and were stained with the mAb by using purified antibody at 1 μg/ml or culture supernatant at a dilution of 1:5. Cells then were stained with 1:200 dilution of fluorescein isothiocyanate-conjugated goat anti-mouse IgG (heavy and light chain-specific; Silenus Laboratories, Hawthorn, Australia) and were analyzed on a FACScan (Becton Dickinson).
Binding of Recombinant L-Selectin to Sialyl 6-Sulfo Lewis X Derivatives.
For binding assay of recombinant L-selectin-IgG chimera, respective carbohydrate determinant was immobilized on 96-well plates, and to this, a mixture of the recombinant human L-, P-, or E-selectin-IgG chimera (5, 10, and 2.5 μg/ml, respectively; kindly provided by H. Kondo and Y. Inoue, New Drug Research Labs, Kanebo Limited, Osaka), affinity-purified biotinylated goat anti-human IgG antibody (20 μg/ml), and avidin-peroxidase (20 μg/ml) was overlaid and incubated for 60 min at 4°C. The reaction was visualized with 3,3′-diaminobenzidine tetrahydrochloride.
Determination of Enzymatic Activity that Convert de-N-Acetyl Sialyl 6-Sulfo Lewis X to G159-Defined Cyclic Sialyl 6-Sulfo Lewis X.
The enzymatic activity of sialic acid cyclase was determined by ELISA. The standard assay mixture (100 μl) contained 0.1 M glycine-NaOH buffer (pH 9.0) or imidazole buffer (pH 7.5), 5 mM CaCl2, 20–80 ng synthetic de-N-acetyl sialyl 6-sulfo Lewis X immobilized at the bottom of each well in 96-well plates, and sonicate of cells (50–100 μg protein) prepared from ED40515-N cells unless otherwise indicated. Incubation was carried out at 37°C for 3 hr, and the reaction was stopped by adding EDTA (final 20 mM). The amount of cyclic sialyl 6-sulfo Lewis X formed by the reaction was determined by a standard ELISA method as described (19) using the G159 antibody. Peroxidase-conjugated goat anti-mouse IgG (heavy and light chain-specific; Zymet Laboratories, San Francisco) was used for a second antibody. Enzymatic activity was calculated by subtracting the absorbance obtained with heat inactivated enzyme that had been treated at 70°C for 30 min.
RESULTS
Reactivity of G159 Antibody to Synthetic Sialyl 6-Sulfo Lewis X-Related Determinants.
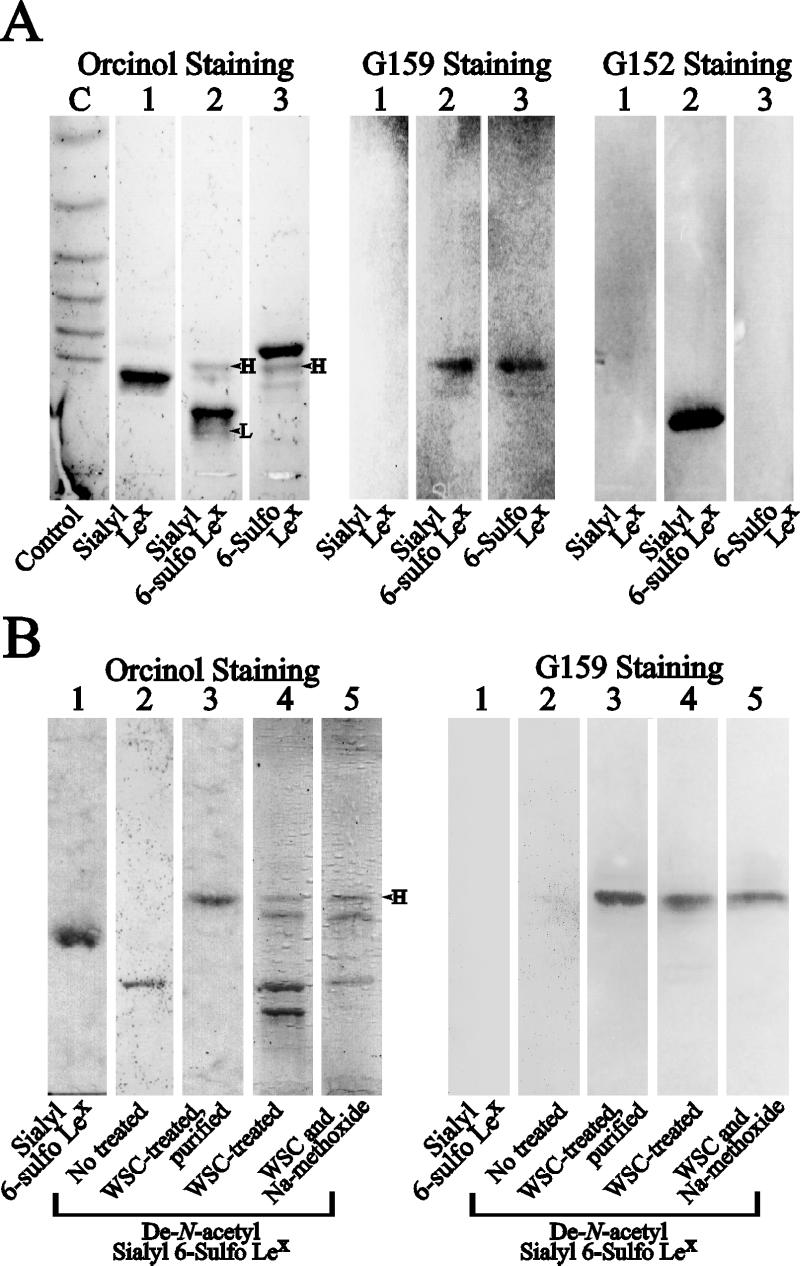
During the course of generating mAbs against synthetic sialyl 6-sulfo Lewis X, the putative carbohydrate capping group of L-selectin ligand carried by high endothelial venules of human lymph nodes, we obtained an antibody G159 having a distinct reactivity. As shown in lane 2 of the left panel in Fig. 1A, the synthetic sialyl 6-sulfo Lewis X determinant contained a small amount of two synthetic by-products; one migrates slower than the main product (band L), and the other migrates faster than the main product (band H). The main band was prone to the sialidase treatment whereas the faster-migrating band H was resistant to the treatment.
Figure 1.
Reactivity of newly generated anti-6-sulfo sialyl Lewis X antibodies G159 in TLC-immunostaining and generation of cyclic sialic acid-containing sialyl 6-sulfo Lewis X from de-N-acetyl 6-sulfo sialyl Lewis X by WSC-treatment. (A) From left, Orcinol/H2SO4 staining, which visualizes all glycolipids, and immunostaining patterns of the same TLC plates with the G159 antibody and a control G152 antibody, respectively. Lanes: C (control), reference laminari-oligosaccharides (a mixture of laminaribiose-laminariheptaose, Seikagaku Kogyo, Tokyo) coupled to cholesteryl aniline serving as TLC-mobility controls; 1, genuine sialyl Lewis X; 2, initially synthesized sialyl 6-sulfo Lewis X, which contained impurities (bands H and L); 3, 6-sulfo Lewis X obtained by a sialidase treatment of initially synthesized sialyl 6-sulfo Lewis X. (B) From left, Orcinol/H2SO4 staining and immunostaining patterns of the same TLC plates with the G159 antibody. Lanes: 1, sialyl 6-sulfo Lewis X purified by preparative TLC, which contained no impurities; 2, newly synthesized de-N-acetyl sialyl 6-sulfo Lewis X; 3, cyclic sialic acid-containing sialyl 6-sulfo Lewis X, which was obtained by the WSC treatment of de-N-acetyl sialyl 6-sulfo Lewis X followed by preparative TLC; 4, crude preparation of WSC-treated de-N-acetyl sialyl 6-sulfo Lewis X before preparative TLC; 5, the same material as in lane 4, treated with Na-methoxide.
The antibody G159 strongly reacted to the band H and showed positive reaction either before or after the sialidase treatment against the band with the same mobility (Fig. 1A Center). This was in a clear contrast to the reactivity of the antibody against genuine sialyl 6-sulfo Lewis X, G152, which was obtained in the same fusion. G152 specifically reacted to the main band and was sensitive to the sialidase treatment (Fig. 1A Right). This suggested that the modification in the fast migrating G159-defined determinant involved alteration occurring in the sialic acid moiety of sialyl 6-sulfo Lewis X.
Production of the G159-Defined Determinant by Dehydrative Condensation of de Novo Synthesized de-N-Acetyl Sialyl 6-Sulfo Lewis X Using Water-Soluble Carbodiimide.
Because these by-products were thought to be produced by an excessive deacetylation under anhydrous condition at the final step of the organochemical synthesis of sialyl 6-sulfo Lewis X, we tried to generate the G159-reactive determinant by the intentional de novo synthesis of de-N-acetyl sialyl 6-sulfo Lewis X and subsequent dehydration with WSC. The TLC mobility of the newly synthesized de-N-acetyl sialyl 6-sulfo Lewis X coincided with that of the initial synthetic by-product having the slower TLC mobility (Fig. 1A, band L) and was not reactive to G159 antibody (Fig. 1B, lane 2). The dehydration of de-N-acetyl sialyl 6-sulfo Lewis X with WSC compound yielded two major bands on TLC; one had the same TLC mobility as the initial fast migrating by-product (band H). After purification by preparative TLC, this band showed one clear spot on TLC and was strongly reactive to the G159 antibody (Fig. 1B, lane 3). The G159 antibody was not reactive to WSC-treated parental sialyl 6-sulfo Lewis X (data not shown), suggesting that the free amino residue in de-N-acetyl sialyl 6-sulfo Lewis X is essential to the formation of the G159-defined determinant.
Dehydrative condensation by a reagent such as WSC usually generates internally cyclized sialic acid. To briefly characterize whether dehydration by WSC resulted in esterification of the carboxyl group with one of the hydroxyl groups (lactone formation) or condensation between the carboxyl and amino groups in sialic acid (lactam formation), a mild alkaline treatment with chloroform/methanol/0.25% Na-methoxide (8:4:3, vol/vol/vol) was performed for 1 hr. This procedure is known to completely convert ganglioside lactones into nonlactonized parent gangliosides without affecting the rest of the carbohydrate structures (21–23). The TLC-mobility and antigenic activity of the G159-reactive band remained unchanged after this treatment, indicating its outstanding tolerance against alkaline treatment (Fig. 1B, lanes 4 and 5). We tentatively conclude the G159-defined determinant to be sialyl 6-sulfo Lewis X carrying a cyclized sialic acid, which is produced by de-N-acetylation of parental sialyl 6-sulfo Lewis X followed by dehydrative cyclization to form, most probably a lactamized sialic acid. Until the lactamized structure is definitely established, we tentatively call thus-modified and G159-defined sialic acid “cyclic sialic acid.”
Distribution of the G159-Defined Cyclic Sialyl 6-Sulfo Lewis X Among Human Peripheral Leukocytes.
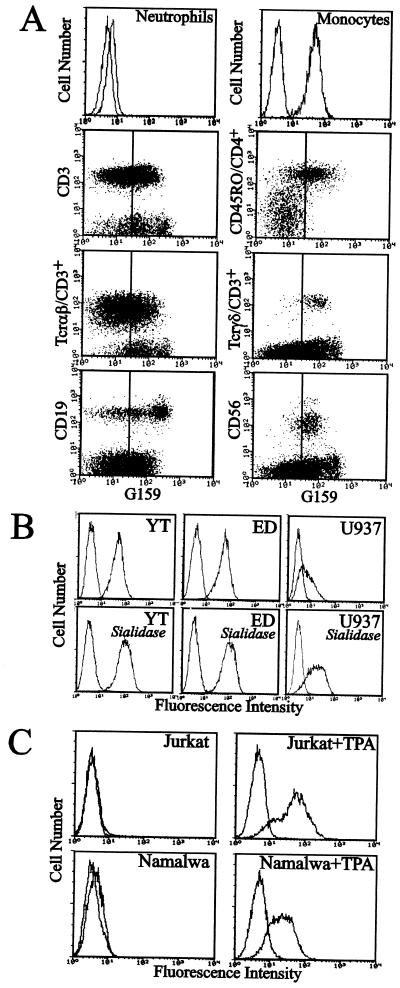
The determinant defined by the G159 antibody was detected in a wide variety of normal human leukocytes. It was expressed moderately on monocytes and strongly on several subsets of lymphocytes but very weakly on neutrophilic granulocytes in flow cytometric analysis (Fig. 2A). The determinant was expressed strongly on NK cells, helper memory T cells, Tcr-γδ T cells, and a part of B cells among lymphocyte populations. This distribution pattern was generally similar to the distribution of selectin ligands among leukocytes, except that neutrophiles expressed the G159 determinant less than expected, and a part of B lymphocytes was strongly G-159-positive.
Figure 2.
Expression of G159-defined cyclic sialyl 6-sulfo Lewis X on human leukocytes as ascertained by flow cytometry. (A) Results on peripheral blood leukocytes prepared from a normal individual. (B) Results on representative cultured human leukemic cell lines, YT (an NK-like cell line), ED40515-N (an adult T-cell leukemia cell line derived from helper memory T-cells), and U937 (a monocytic leukemia cell line), and effect of sialidase treatment. (C) Effect of TPA treatment on the expression of the determinant on Jurkat (leukemic cells derived from helper T-cells) and Namalwa (B-cell leukemia) cells. TPA treatment was performed at 20 ng/ml for 3 days for Jurkat cells and 10 ng/ml for 4 days for Namalwa cells.
Cultured human lymphocytic cell lines ED40515-N cells (derived from helper memory T cells) and YT cells (derived from NK cells) also expressed the G159 determinant very strongly whereas U937 cells derived from monocytic lineage expressed it moderately (Fig. 2B). The expression of the determinant on these cells was sialidase-resistant (Fig. 2B), which was in line with the behavior of the determinant observed on TLC-immunostaining in Fig. 1A, lane 3. Even a considerable increase of the antigenic expression was observed after sialidase treatment. This was in a clear contrast to the expression of sialyl Lewis X and/or sialyl 6-sulfo Lewis X, the expression of which was sensitive to sialidase treatment (not shown). When the Jurkat cells, the cultured human helper T cell leukemia line, or Namalwa cells, the cultured B cell leukemia line, were treated with O-tetradecanoylphorbol 13-acetate (TPA), a significant induction of expression of the G159-defined determinant was observed (Fig. 2C).
Selectin Binding Activity of the Sialyl 6-Sulfo Lewis X Derivatives.
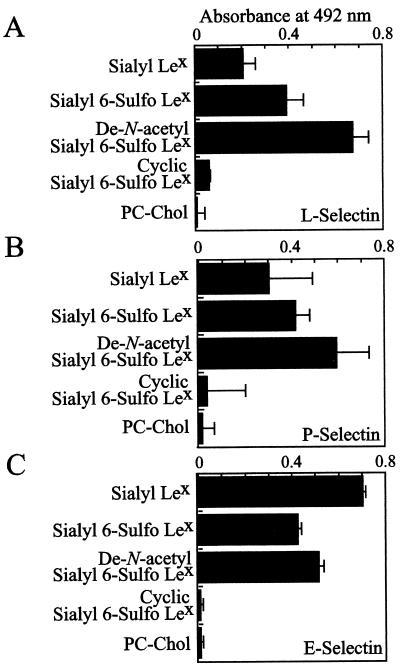
Recombinant L-, P-, and E-selectins significantly bound to both genuine sialyl 6-sulfo Lewis X and de-N-acetyl sialyl 6-sulfo Lewis X, as well as classical sialyl Lewis X, but did not bind to cyclic sialyl 6-sulfo Lewis X (which is WSC-treated de-N-acetyl sialyl 6-sulfo Lewis X after preparative TLC-purification) in conventional ELISA (Fig. 3). This is not unexpected, because the carboxyl group at C-1 position of sialic acid is known to be important in binding to selectins (24, 25), and this compound lacks the group.
Figure 3.
In vitro binding of recombinant selectins to sialyl 6-sulfo Lewis X, de-N-acetyl sialyl 6-sulfo Lewis X, and cyclic sialyl 6-sulfo Lewis X. Binding of recombinant L-selectin (A), P-selectin (B), and E-selectin (C) was tested in ELISA assay by using 40 ng/well immobilized carbohydrate determinants mixed with 100 ng/well egg yolk phosphatidylcholine and 50 ng/well cholesterol. PC-Chol, phosphatidylcholine and cholesterol only.
Binding of selectins to de-N-acetyl sialyl 6-sulfo Lewis X was also not unexpected because the amino residue at C-5 position is known not to be intimately involved in interaction with selectin; even KDN-Lewis X, which lacks the amino group at C-5, is known to bind to selectins, almost as equally as genuine sialyl Lewis X does (26, 27). L- and P-selectins showed preferential binding to sialyl 6-sulfo Lewis X and de-N-acetyl sialyl 6-sulfo Lewis X rather than to classical sialyl Lewis X whereas E-selectin bound better to classical sialyl Lewis X. This finding is in line with the notion that L- and P-selectins favor sulfated carbohydrates. The binding of L- and P-selectins to de-N-acetyl sialyl 6-sulfo Lewis X was 36–70% more than that to genuine sialyl 6-sulfo Lewis X.
Enzymatic Conversion of de-N-Acetyl Sialyl 6-Sulfo Lewis X to the G159-Defined Cyclic Sialyl 6-Sulfo Lewis X.
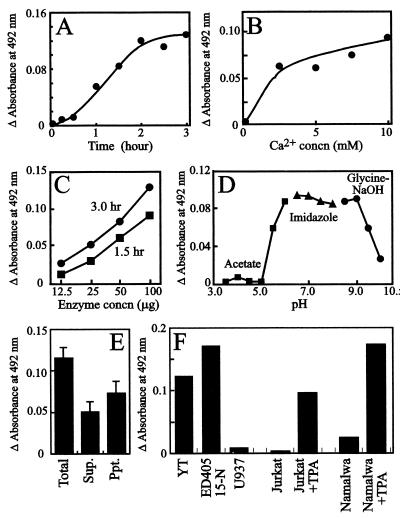
When the enzymatic activity that converts de-N-acetyl sialyl 6-sulfo Lewis X into G159-defined determinant was ascertained in ELISA, the effect of time and protein concentration were found to be stoichiometric and compatible with an enzymatic reaction (Fig. 4 A and C). The enzymatic activity required a millimolar level of calcium concentration for maximal activity and was optimal at pH 6.5–9.0 (Fig. 4 B and D). Nearly 60% of the total activity was recovered in the membrane fraction of ED40515-N cells, indicating that the enzyme is at least partly membrane-bound (Fig. 4E).
Figure 4.
Enzymatic activity that converts de-N-acetyl sialyl 6-sulfo Lewis X to G159-defined cyclic sialyl 6-sulfo Lewis X. The effect of time (A), calcium ion concentration (B), enzyme concentration (C), or pH (D) on the enzymatic activity in ED40515-N homogenates are shown. E indicates subcellular localization of the enzyme; enzymatic activities in a supernatant (Sup.) or precipitate (Ppt.) fraction of ED40515-N homogenates centrifuged at 16,400 × g for 15 min are shown. F indicates the enzymatic activity in the homogenates of representative human leukemic cells and the effect of TPA treatment of the cells. TPA treatment was performed at 20 ng/ml for 3 days for Jurkat cells and 10 ng/ml for 4 days for Namalwa cells.
The cells strongly expressing the G159-defined determinant, such as YT and ED40515-N cells, tended to have an enzymatic activity higher than the cells that only moderately or weakly expressed the determinant, such as U937 and nontreated Jurkat or Namalwa cells (Fig. 4F). A significant increase of the enzymatic activity was observed in TPA-treated cells (Fig. 4F), in parallel to the increased expression of the G159-defined determinant on the cell surface as ascertained by flow cytometry (Fig. 2C). This finding suggests that the enzyme may play a role as a rate-limiting enzyme in the synthesis of G159-defined cyclic sialyl 6-sulfo Lewis X determinant.
DISCUSSION
Almost 10 years have elapsed since the occurrence of de-N-acetyl sialic acid in carbohydrate determinants on the surface of living cells first was described from Hakomori’s laboratory (28). Subsequently, this was proposed to result from an active de-N-acetylation/re-N-acetylation cycle, which works on the amino group at the C-5 position of sialic acid (29, 30) (Fig. 5). The carbohydrate determinants carrying de-N-acetyl sialic acid are thus transient and active metabolic intermediate products, which are present in very small amounts on the cell surface under usual conditions. The amount of the determinant carrying de-N-acetyl sialic acid is considered to be usually only a small percentage of the corresponding parental compound carrying normal sialic acid, evaluated by using specific mAbs (29, 30).
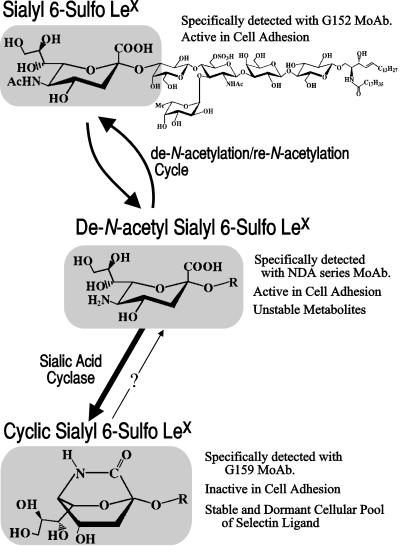
Figure 5.
Schematic diagram showing hypothetical metabolic pathway of sialyl 6-sulfo Lewis X. The specific detection of de-N-acetyl sialyl 6-sulfo Lewis X by the NDA series antibodies is described elsewhere (C.M., S.K., H.I., M.K., and R.K., unpublished work).
The results of the present study suggested that even the sialic acid moieties of the biologically important sialoglycoconjugates such as selectin ligands are involved in the de-N-acetylation/re-N-acetylation cycle. The present study further suggested that an eventual intervention of the de-N-acetylation/re-N-acetylation cycle by an enzyme yields carbohydrate determinants carrying a dehydrated form of de-N-acetyl sialic acid, which we tentatively call cyclic sialic acid. Enough sialyl 6-sulfo Lewis X determinants carrying the cyclic sialic acid were accumulated on freshly prepared human peripheral leukocytes as well as on cultured leukemic cells so as to be readily detected by a specific mAb, G159 (Fig. 5). The exact structure of the cyclic sialic acid is not clear yet and should be studied further. It is a dehydration product of de-N-acetyl sialic acid because the WSC treatment of intentionally synthesized de-N-acetyl 6-sulfo Lewis X quantitatively yielded the G159-defined determinant. Currently available evidence would indicate that it is a sialic acid having a cyclic structure in the form of lactam rather than lactone (Fig. 5). The structural analysis of the G159-defined determinant is now under way in our laboratory, as well as a study of the genetic background for its formation by an expression cloning using the G159 antibody.
Cyclic sialyl 6-sulfo Lewis X is functionally dormant as a ligand for selectins. It is possible that accumulation of this dormant form of the selectin ligand has a biological significance as a rapid inactivating mechanism of selectin binding activity at the cell surface during the leukocyte extravasation. Study of the inactivation mechanism of selectin-mediated cell adhesion is important in understanding the physiological function of the cell adhesion system during leukocyte extravasation. Kishimoto and colleagues recently described the rapid inactivation mechanism of cell surface L-selectin activity through metalloprotease that responds to calcium ion through calmodulin (31). It is noteworthy that the enzyme responsible for the formation of cyclic sialyl 6-sulfo Lewis X, the sialic acid cyclase, is also calcium-dependent and is partly membrane-bound. The significant induction of enzymatic activity by TPA treatment of cells suggests involvement of protein kinase C in its induction.
Another possibility is that the accumulated intracellular cyclic sialyl 6-sulfo Lewis X determinant may serve as a biologically dormant intracellular pool of selectin ligands, hydrolysis of which, on appropriate stimulation, would rapidly yield active ligands for selectins. This would have significance as a rapid and reversible induction mechanism of selectin ligands at the cell surface. Sialyl Lewis X-related selectin ligands are not expressed on most unstimulated normal peripheral T and B lymphocytes but are induced on T and B lymphocytes activated with inflammatory stimuli, and their appearance is known to be accompanied with transcriptional induction of fucosyltransferase Fuc-T VII (5, 32, 33). However, some lymphocytes, including a part of helper memory T cells and/or Tcr-γδ T cells, are known to be recruited routinely to target organs such as skin in the absence of any particular inflammatory challenge (34–37). It is noteworthy that the G159-defined cyclic sialyl 6-sulfo Lewis X determinant is strongly expressed on such populations of lymphocytes, which may undergo routine trafficking between general circulation and the target organs such as skin. Probably a post-translational activation of selectin ligands, which is fully reversible, is more appropriate in performing the routine trafficking for primary immune surveillance rather than the transcriptional induction of Fuc-T VII mRNA followed by the synthesis of enzyme protein, which mainly occurs in the antigen-stimulated lymphocytes on inflammatory stimuli.
We have coincidentally encountered cyclic sialic acid during the course of study of sialyl 6-sulfo Lewis X. However, there is no reason to assume this compound can only be carried by sialyl 6-sulfo Lewis X. If the cyclic sialic acid were to occur in a wide variety of sialoglycoconjugates, the reactivity to other known sialic-acid binding proteins such as CD22, MAG, CD33, or sialoadhesin also could be regulated partly by the de-N-acetylation/re-N-acetylation cycle, followed by the cyclization of the sialic acid moiety in the corresponding carbohydrate ligands. Further study is necessary to elucidate these issues.
Acknowledgments
This work was supported in part by a Grant-in-Aid for the Second Term Comprehensive Ten-Year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan, Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (Grants 10177234 and 09672371), and a grant from the Princess Takamatsu Foundation for the Promotion of Cancer Research.
ABBREVIATIONS
- TPA
O-tetradecanoylphorbol 13-acetate
- WSC
water soluble carbodiimide, 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride
References
- 1.Hakomori S. Histochem J. 1992;24:771–776. doi: 10.1007/BF01046348. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kansas G S. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 4.Sawada M, Takada A, Ohwaki I, Takahashi N, Tateno H, Sakamoto J, Kannagi R. Biochem Biophys Res Commun. 1993;193:337–347. doi: 10.1006/bbrc.1993.1629. [DOI] [PubMed] [Google Scholar]
- 5.Ohmori K, Takada A, Ohwaki I, Takahashi N, Furukawa Y, Maeda M, Kiso M, Hasegawa A, Kannagi M, Kannagi R. Blood. 1993;82:2797–2805. [PubMed] [Google Scholar]
- 6.Furukawa Y, Tara M, Ohmori K, Kannagi R. Cancer Res. 1994;54:6533–6538. [PubMed] [Google Scholar]
- 7.Handa K, Stroud M R, Hakomori S. Biochemistry. 1997;36:12412–12420. doi: 10.1021/bi971181t. [DOI] [PubMed] [Google Scholar]
- 8.Wagers A J, Stoolman L M, Craig R, Knibbs R N, Kansas G S. J Immunol. 1998;160:5122–5129. [PubMed] [Google Scholar]
- 9.Mitsuoka C, Kawakami-Kimura N, Kasugai-Sawada M, Hiraiwa N, Toda K, Ishida H, Kiso M, Hasegawa A, Kannagi R. Biochem Biophys Res Commun. 1997;230:546–551. doi: 10.1006/bbrc.1996.6012. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuoka C, Sawada-Kasugai M, Ando-Furui K, Izawa M, Nakanishi H, Nakamura S, Ishida H, Kiso M, Kannagi R. J Biol Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe E, Ohta S, Hanai N, Nishi T. J Biol Chem. 1994;269:14730–14737. [PubMed] [Google Scholar]
- 12.Natsuka S, Gersten K M, Zenita K, Kannagi R, Lowe J B. J Biol Chem. 1994;269:16789–16794. [PubMed] [Google Scholar]
- 13.Uchimura K, Muramatsu H, Kadomatsu K, Fan Q W, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T. J Biol Chem. 1998;273:22577–22583. doi: 10.1074/jbc.273.35.22577. [DOI] [PubMed] [Google Scholar]
- 14.Uchimura K, Muramatsu H, Kaname T, Ogawa H, Yamakawa T, Fan Q W, Mitsuoka C, Kannagi R, Habuchi O, Yokoyama I, et al. J Biochem (Tokyo) 1998;124:670–678. doi: 10.1093/oxfordjournals.jbchem.a022164. [DOI] [PubMed] [Google Scholar]
- 15.Komba S, Ishida H, Kiso M, Hasegawa A. Bioorg Med Chem. 1996;4:1833–1847. doi: 10.1016/s0968-0896(96)00165-4. [DOI] [PubMed] [Google Scholar]
- 16.Kameyama A, Ishida H, Kiso M, Hasegawa A. Carbohydr Res. 1991;209:c1–c4. doi: 10.1016/0008-6215(91)80171-i. [DOI] [PubMed] [Google Scholar]
- 17.Köhler G, Milstein C. Nature (London) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 18.Kannagi R, Hakomori S. In: Handbook of Experimental Immunology. Weir D M, Herzenberg L, Blackwell C, Herzenberg L A, editors. Vol. 4. Oxford: Blackwell Scientific; 1986. pp. 117.1–117.20. [Google Scholar]
- 19.Hakomori S, Kannagi R. In: Handbook of Experimental Immunology. Weir D M, Herzenberg L, Blackwell C, Herzenberg L A, editors. Vol. 1. Oxford: Blackwell Scientific; 1986. pp. 9.1–9.39. [Google Scholar]
- 20.Ohmori K, Yoneda T, Shigeta K, Hirashima K, Kanai M, Itai S, Sasaoki T, Arii S, Arita H, Kannagi R. Blood. 1989;74:255–261. [PubMed] [Google Scholar]
- 21.Saito T, Hakomori S I. J Lipid Res. 1971;12:257–259. [PubMed] [Google Scholar]
- 22.Riboni L, Sonnino S, Acquotti D, Malesci A, Ghidoni R, Egge H, Mingrino S, Tettamanti G. J Biol Chem. 1986;261:8514–8519. [PubMed] [Google Scholar]
- 23.Bouchon B, Levery S B, Clausen H, Hakomori S. Glycoconj J. 1992;9:27–38. doi: 10.1007/BF00731175. [DOI] [PubMed] [Google Scholar]
- 24.Foxall C, Watson S R, Dowbenko D, Fennie C, Lasky L A, Kiso M, Hasegawa A, Asa D, Brandley B K. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyrrell D, James P, Rao N, Foxall C, Abbas S, Dasgupta F, Nashed M, Hasegawa A, Kiso M, Asa D, et al. Proc Natl Acad Sci USA. 1991;88:10372–10376. doi: 10.1073/pnas.88.22.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandley B K, Kiso M, Abbas S, Nikrad P, Srivasatava O, Foxall C, Oda Y, Hasegawa A. Glycobiology. 1993;3:633–641. doi: 10.1093/glycob/3.6.633. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa A, Kiso M. In: Carbohydrates in Drug Design. Witczak Z J, Nieforth K A, editors. New York: Dekker; 1997. pp. 137–155. [Google Scholar]
- 28.Hanai N, Dohi T, Nores G A, Hakomori S. J Biol Chem. 1988;263:6296–6301. [PubMed] [Google Scholar]
- 29.Manzi A E, Sjoberg E R, Diaz S, Varki A. J Biol Chem. 1990;265:13091–13103. [PubMed] [Google Scholar]
- 30.Sjoberg E R, Chammas R, Ozawa H, Kawashima I, Khoo K H, Morris H R, Dell A, Tai T, Varki A. J Biol Chem. 1995;270:2921–2930. doi: 10.1074/jbc.270.7.2921. [DOI] [PubMed] [Google Scholar]
- 31.Kahn J, Walcheck B, Migaki G I, Jutila M A, Kishimoto T K. Cell. 1998;92:809–818. doi: 10.1016/s0092-8674(00)81408-7. [DOI] [PubMed] [Google Scholar]
- 32.Knibbs R N, Craig R A, Natsuka S, Chang A, Cameron M, Lowe J B, Stoolman L M. J Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraiwa N, Hiraiwa M, Kannagi R. Biochem Biophys Res Commun. 1997;231:183–186. doi: 10.1006/bbrc.1997.6068. [DOI] [PubMed] [Google Scholar]
- 34.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Bergstresser P R, Terstappen L W M M. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 35.Walcheck B, Watts G, Jutila M A. J Exp Med. 1993;178:853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jutila M A, Bargatze R F, Kurk S, Warnock R A, Ehsani N, Watson S R, Walcheck B. J Immunol. 1994;153:3917–3928. [PubMed] [Google Scholar]
- 37.Diacovo T G, Roth S J, Morita C T, Rosat J P, Brenner M B, Springer T A. J Exp Med. 1996;183:1193–1203. doi: 10.1084/jem.183.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]