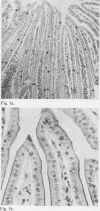
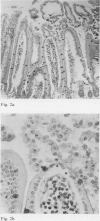
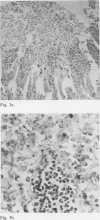
Abstract
Incubation in vitro of the intestine of the hamster and guinea pig with 5 mM sodium cholate and with 2 mM sodium deoxycholate and sodium chenodeoxycholate resulted in significant morphological changes compared with control incubations. Generally, no major differences were observed between proximal and distal small intestine or between the species used. Only when guinea pig intestine was incubated with 5 mM cholate was less damage found proximally than distally.
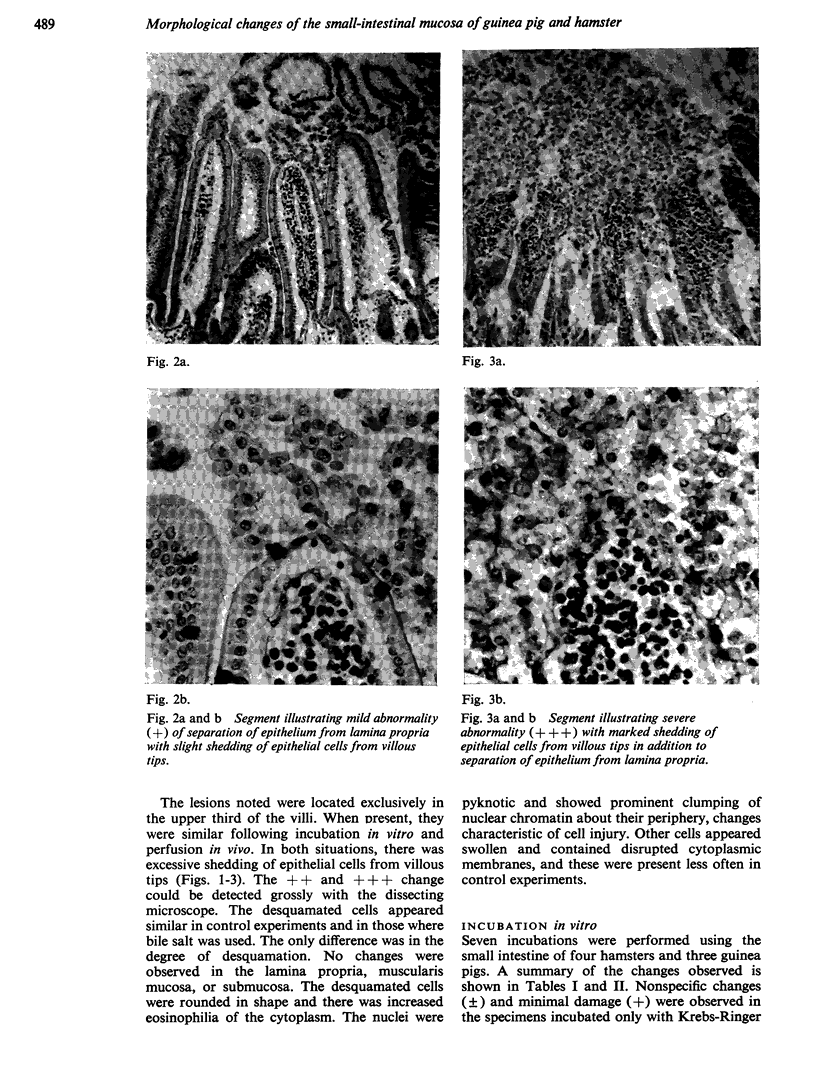
Perfusion in vivo of the intestine of the hamster and guinea pig with Krebs-Ringer phosphate results in separation of the epithelium from the lamina propria without excessive shedding of epithelial cells from villous tips. This change was also seen in specimens taken before perfusion and probably represents unavoidable trauma during handling of the intestine.
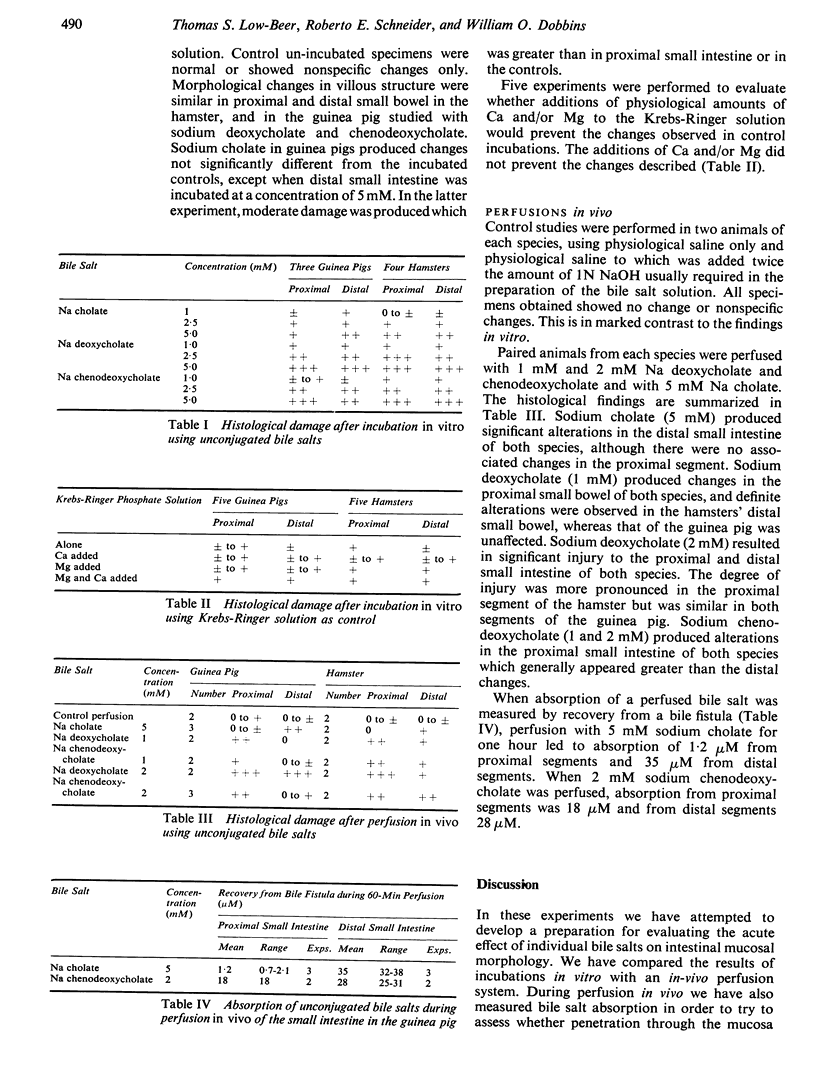
In contrast to studies in vitro, regional differences are readily demonstrable with perfusion of bile salts in vivo. Dihydroxy bile salts produce more marked alterations of both proximal and distal small intestine than the trihydroxy bile salt, sodium cholate. Dihydroxy bile salts result in significantly greater alterations in proximal than in distal mucosa.
When 5 mM cholate at pH 6·8 is perfused in the guinea pig, absorption occurs approximately 30 times more rapidly from distal than from proximal segments, while in proximal segments 2 mM chenodeoxycholate is absorbed approximately 15 times more rapidly than 5 mM cholate. A correlation is suggested between the morphological alteration produced in the region of the small intestine by a bile acid and the amount of bile salt passing through the cell. Furthermore, it is proposed that the ileal cells may be damaged to a lesser extent by bile acids normally found in that particular species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWSON A. M., ISSELBACHER K. J. Studies on lipid metabolism in the small intestine with observations on the role of bile salts. J Clin Invest. 1960 May;39:730–740. doi: 10.1172/JCI104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M. Effects of bile salts on intermediate metabolism of the intestinal mucosa. Fed Proc. 1967 Nov-Dec;26(6):1589–1598. [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. M., Jr Studies on the pathogenesis of steatorrhea in the blind loop syndrome. J Clin Invest. 1965 Nov;44(11):1815–1825. doi: 10.1172/JCI105289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRY R. J., STAFFELDT E. EFFECT OF A DIET CONTAINING SODIUM DEOXYCHOLATE ON THE INTESTINAL MUCOSA OF THE MOUSE. Nature. 1964 Sep 26;203:1396–1398. doi: 10.1038/2031396a0. [DOI] [PubMed] [Google Scholar]
- Forth W., Rummel W., Glasner H. Zur resorptionshemmenden Wirkung von Gallensäuren. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(4):364–380. [PubMed] [Google Scholar]
- HASLEWOOD G. A. THE BIOLOGICAL SIGNIFICANCE OF CHEMICAL DIFFERENCES IN BILE SALTS. Biol Rev Camb Philos Soc. 1964 Nov;39:537–574. doi: 10.1111/j.1469-185x.1964.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Heaton K. W., Lack L. Ileal bile salt transport: mutual inhibition in an in vivo system. Am J Physiol. 1968 Mar;214(3):585–590. doi: 10.1152/ajplegacy.1968.214.3.585. [DOI] [PubMed] [Google Scholar]
- Holt P. R. Competitive inhibition of intestinal bile salt absorption in the rat. Am J Physiol. 1966 Mar;210(3):635–639. doi: 10.1152/ajplegacy.1966.210.3.635. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Spritz N., Blum M., Terz J., Sherlock P. The role of altered bile acid metabolism in the steatorrhea of experimental blind loop. J Clin Invest. 1966 Jun;45(6):956–962. doi: 10.1172/JCI105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK L., WEINER I. M. In vitro absorption of bile salts by small intestine of rats and guinea pigs. Am J Physiol. 1961 Feb;200:313–317. doi: 10.1152/ajplegacy.1961.200.2.313. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Tyor M. P., Lack L. Effects of sulfation of taurolithocholic and glycolithocholic acids on their intestinal transport. Gastroenterology. 1969 Apr;56(4):721–726. [PubMed] [Google Scholar]
- NORMAN A., SJOVALL J. On the transformation and enterohepatic circulation of cholic acid in the rat: bile acids and steroids 68. J Biol Chem. 1958 Oct;233(4):872–885. [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Liver microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Mar 25;2(2):171–200. doi: 10.1083/jcb.2.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRANGE I., CHRISTENSEN F., DAM H. Alimentary production of gallstones in hamsters. 11. Relation between diet and composition of the bladder bile 1. Z Ernahrungswiss. 1962 Oct;3:59–78. doi: 10.1007/BF02021341. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. H., Hardison W. G., Bull D. M. Abnormal bile-salt patterns and intestinal bacterial overgrowth associated with malabsorption. N Engl J Med. 1967 Jun 22;276(25):1391–1397. doi: 10.1056/NEJM196706222762501. [DOI] [PubMed] [Google Scholar]
- TIDBALL C. S. INTESTINAL AND HEPATIC TRANSPORT OF CHOLATE AND ORGANIC DYES. Am J Physiol. 1964 Jan;206:239–242. doi: 10.1152/ajplegacy.1964.206.1.239. [DOI] [PubMed] [Google Scholar]
- Tabaqchali S., Booth C. C. Jejunal bacteriology and bile-salt metabolism in patients with intestinal malabsorption. Lancet. 1966 Jul 2;2(7453):12–15. doi: 10.1016/s0140-6736(66)91744-2. [DOI] [PubMed] [Google Scholar]
- WEINER I. M., LACK L. Absorption of bile salts from the small intestine in vivo. Am J Physiol. 1962 Jan;202:155–157. doi: 10.1152/ajplegacy.1962.202.1.155. [DOI] [PubMed] [Google Scholar]
- WEISSMANN G. LYSOSOMES. Blood. 1964 Nov;24:594–606. [PubMed] [Google Scholar]
- WHITEHOUSE M. W., STAPLE E. Regulation of cholesterol oxidation by liver in vitro. Proc Soc Exp Biol Med. 1959 Jul;101(3):439–441. doi: 10.3181/00379727-101-24971. [DOI] [PubMed] [Google Scholar]