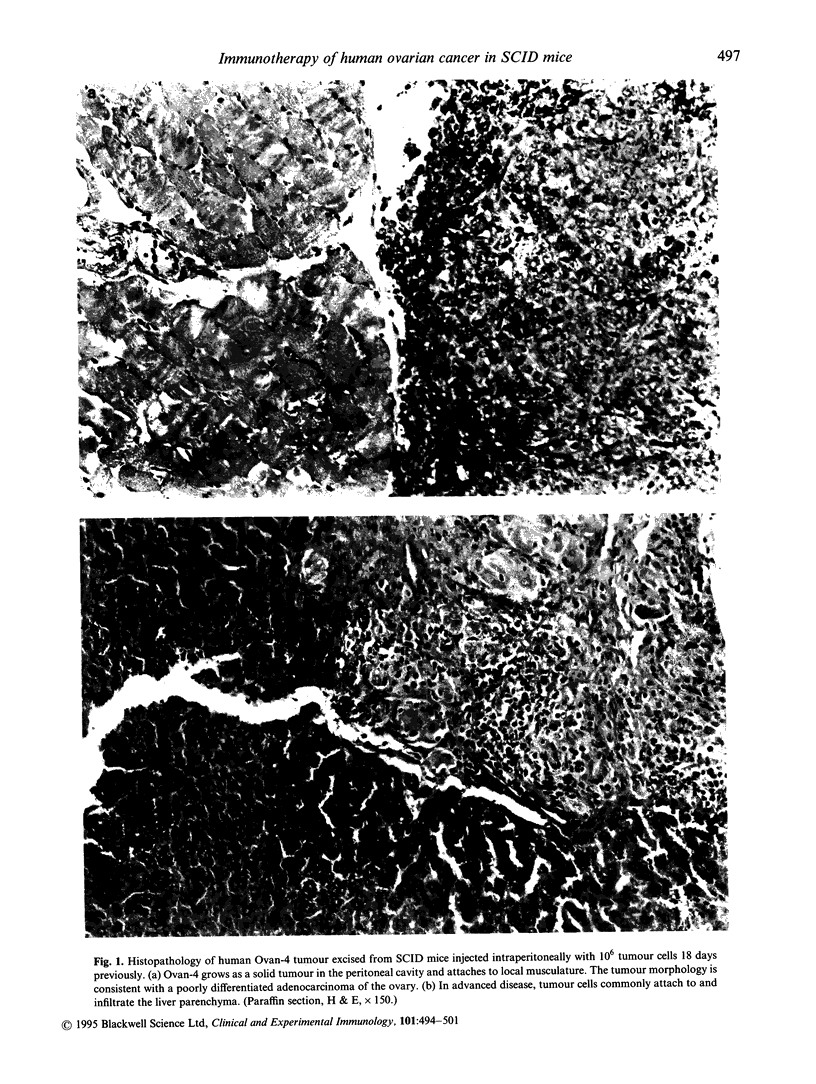
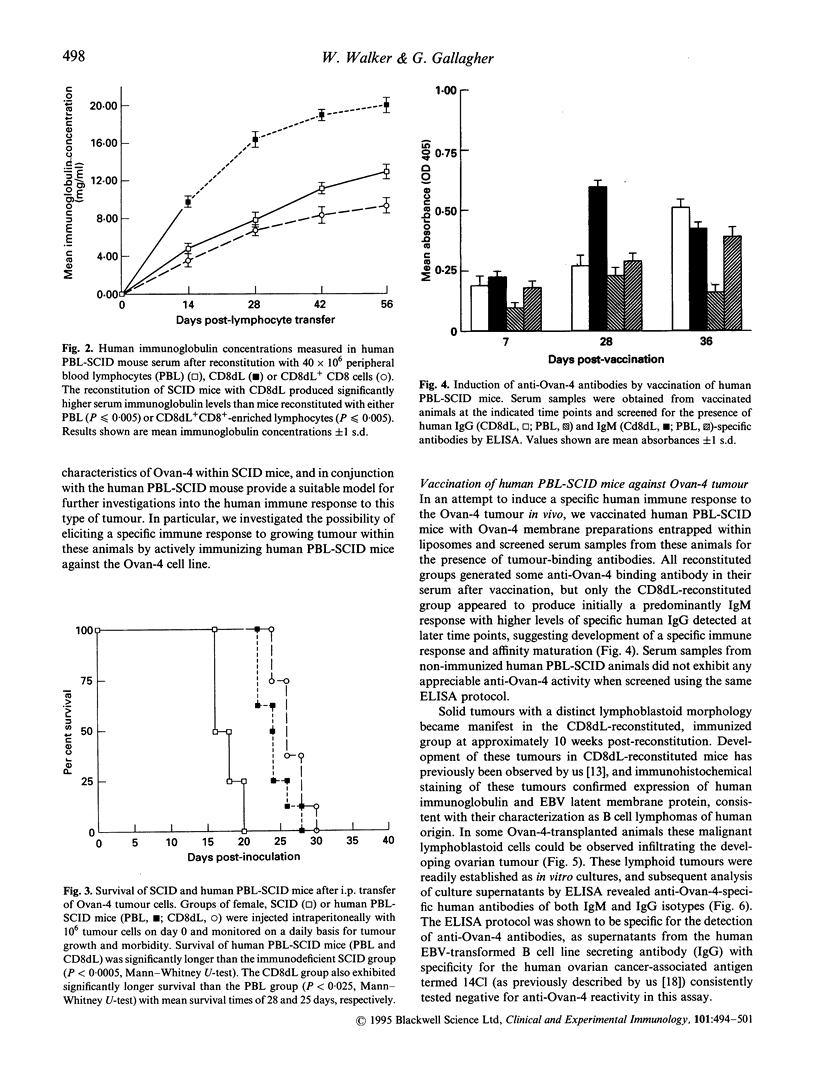
Abstract
The reported ability of SCID mice to accept xenografts of both human tumors and peripheral blood lymphocytes (PBL) provides the potential for the development of novel immunotherapy models in these animals. This study describes the development of a novel small animal model of human ovarian cancer. This was achieved by engrafting a human ovarian cancer cell line (Ovan-4) into the peritoneal cavity of immunodeficient SCID and immune reconstituted human PBL-SCID mice. When transplanted to SCID mice this cell line exhibited growth characteristics similar to the clinical disease observed in patients with implantation of metastatic nodules onto the interior surface of the peritoneal wall. Reconstituted human PBL-SCID mice challenged with identical numbers of Ovan-4 cells exhibited a significant increase in survival time, suggesting a role for cells of the human immune system in preventing the development of this type of malignancy in vivo. Furthermore, vaccination of human PBL-SCID mice against Ovan-4 produced tumour-specific human antibodies in the serum of these animals. Animals reconstituted with CD8-depleted PBL exhibited increased serum immunoglobulin levels and produced enhanced anti-Ovan-4 activity after vaccination. Subsequent challenge of these animals with Ovan-4 revealed a further increase in survival time. These results suggest that human antibodies may have a role in immunity against ovarian cancer and could be of therapeutic value in this type of disease.
Full text
PDF


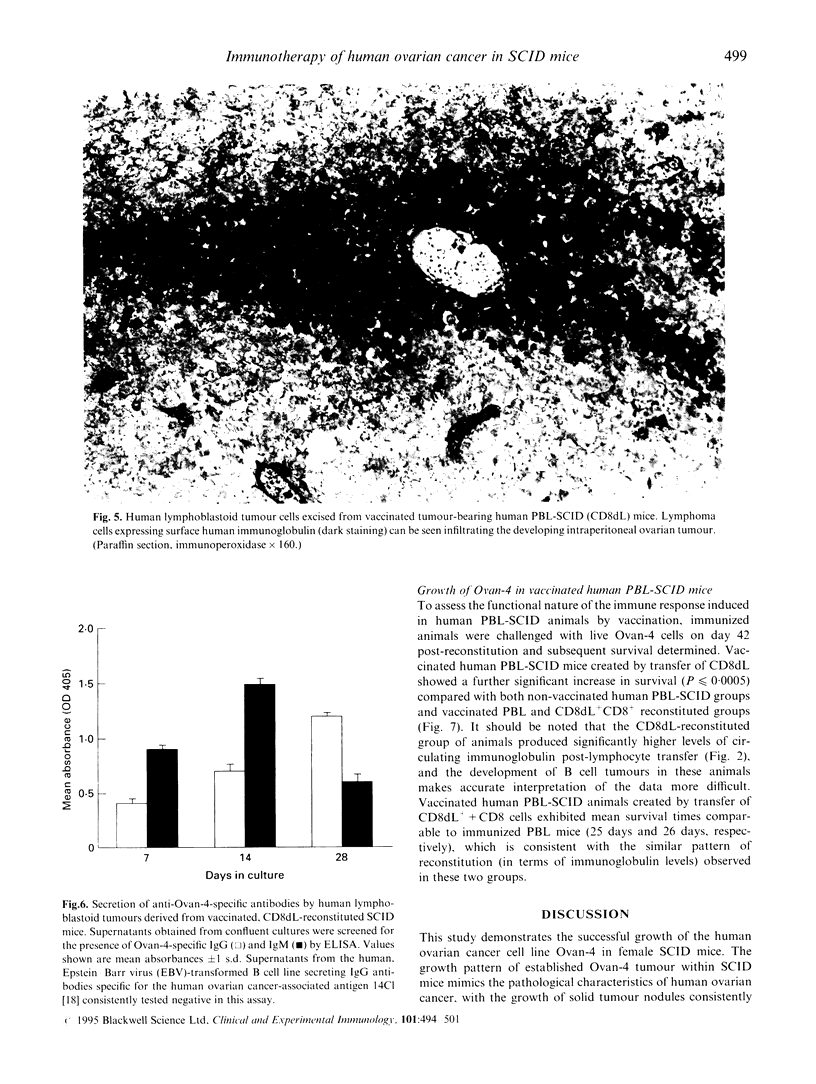
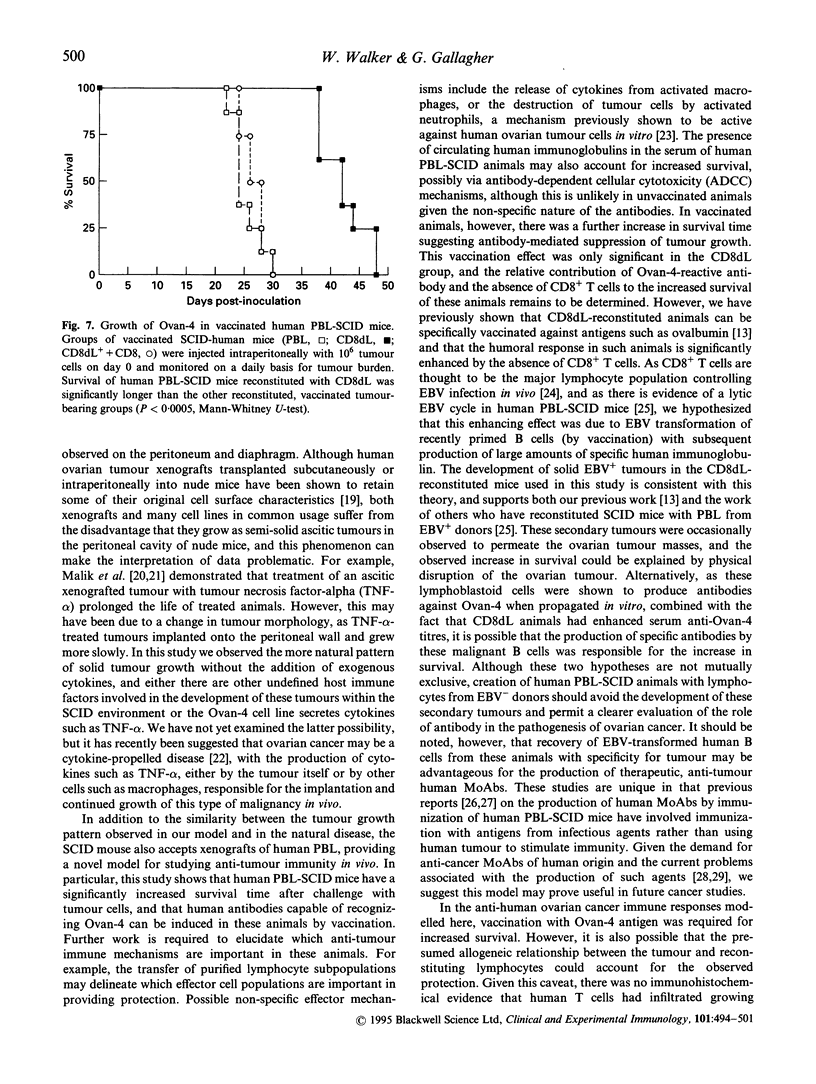

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Barber H. R. Ovarian cancer: diagnosis and management. Am J Obstet Gynecol. 1984 Dec 15;150(8):910–916. doi: 10.1016/0002-9378(84)90380-6. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Boven E., van der Vijgh W. J., Nauta M. M., Schlüper H. M., Pinedo H. M. Comparative activity and distribution studies of five platinum analogues in nude mice bearing human ovarian carcinoma xenografts. Cancer Res. 1985 Jan;45(1):86–90. [PubMed] [Google Scholar]
- Boyd J. E., James K. Human monoclonal antibodies: their potential, problems, and prospects. Adv Biotechnol Processes. 1989;11:1–43. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Mårtensson C., Kalliomäki S., Ohlin M., Borrebaeck C. A. Human peripheral blood lymphocytes transplanted into SCID mice constitute an in vivo culture system exhibiting several parameters found in a normal humoral immune response and are a source of immunocytes for the production of human monoclonal antibodies. J Immunol. 1992 Feb 15;148(4):1065–1071. [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Duchosal M. A., Eming S. A., Fischer P., Leturcq D., Barbas C. F., 3rd, McConahey P. J., Caothien R. H., Thornton G. B., Dixon F. J., Burton D. R. Immunization of hu-PBL-SCID mice and the rescue of human monoclonal Fab fragments through combinatorial libraries. Nature. 1992 Jan 16;355(6357):258–262. doi: 10.1038/355258a0. [DOI] [PubMed] [Google Scholar]
- Gallagher G., al-Azzawi F., Walsh L. P., Wilson G. 14C1, an antigen associated with human ovarian cancer, defined using a human IgG monoclonal antibody. Clin Exp Immunol. 1991 Jan;83(1):92–95. doi: 10.1111/j.1365-2249.1991.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Whelan R. D., Gibby E. M., Sheer D., Hosking L. K., Shellard S. A., Rupniak H. T. Establishment and characterisation of three new human ovarian carcinoma cell lines and initial evaluation of their potential in experimental chemotherapy studies. Int J Cancer. 1987 Feb 15;39(2):219–225. doi: 10.1002/ijc.2910390216. [DOI] [PubMed] [Google Scholar]
- Klein G. Viral latency and transformation: the strategy of Epstein-Barr virus. Cell. 1989 Jul 14;58(1):5–8. doi: 10.1016/0092-8674(89)90394-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A., Seelig M., Berek J., Zighelboim J. Human neutrophil-mediated lysis of ovarian cancer cells. Blood. 1989 Aug 1;74(2):805–809. [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Malik S. T., Griffin D. B., Fiers W., Balkwill F. R. Paradoxical effects of tumour necrosis factor in experimental ovarian cancer. Int J Cancer. 1989 Nov 15;44(5):918–925. doi: 10.1002/ijc.2910440529. [DOI] [PubMed] [Google Scholar]
- Malik S. T., Naylor M. S., East N., Oliff A., Balkwill F. R. Cells secreting tumour necrosis factor show enhanced metastasis in nude mice. Eur J Cancer. 1990;26(10):1031–1034. doi: 10.1016/0277-5379(90)90044-t. [DOI] [PubMed] [Google Scholar]
- Malik S., Balkwill F. Epithelial ovarian cancer: a cytokine propelled disease? Br J Cancer. 1991 Oct;64(4):617–620. doi: 10.1038/bjc.1991.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massazza G., Tomasoni A., Lucchini V., Allavena P., Erba E., Colombo N., Mantovani A., D'Incalci M., Mangioni C., Giavazzi R. Intraperitoneal and subcutaneous xenografts of human ovarian carcinoma in nude mice and their potential in experimental therapy. Int J Cancer. 1989 Sep 15;44(3):494–500. doi: 10.1002/ijc.2910440320. [DOI] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. Adoptive transfer of human lymphoid cells to severely immunodeficient mice: models for normal human immune function, autoimmunity, lymphomagenesis, and AIDS. Adv Immunol. 1991;50:303–325. doi: 10.1016/s0065-2776(08)60828-7. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Mueller B. M., Reisfeld R. A. Potential of the scid mouse as a host for human tumors. Cancer Metastasis Rev. 1991 Oct;10(3):193–200. doi: 10.1007/BF00050791. [DOI] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989 Nov 13;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Thorpe P. E., Uhr J. W. Immunotoxins: magic bullets or misguided missiles? Immunol Today. 1993 Jun;14(6):252–259. doi: 10.1016/0167-5699(93)90041-I. [DOI] [PubMed] [Google Scholar]
- Walker W., Gallagher G. The in vivo production of specific human antibodies by vaccination of human-PBL-SCID mice. Immunology. 1994 Oct;83(2):163–170. [PMC free article] [PubMed] [Google Scholar]
- Ward B. G., Wallace K. Localization of the monoclonal antibody HMFG2 after intravenous and intraperitoneal injection into nude mice bearing subcutaneous and intraperitoneal human ovarian cancer xenografts. Cancer Res. 1987 Sep 1;47(17):4714–4718. [PubMed] [Google Scholar]