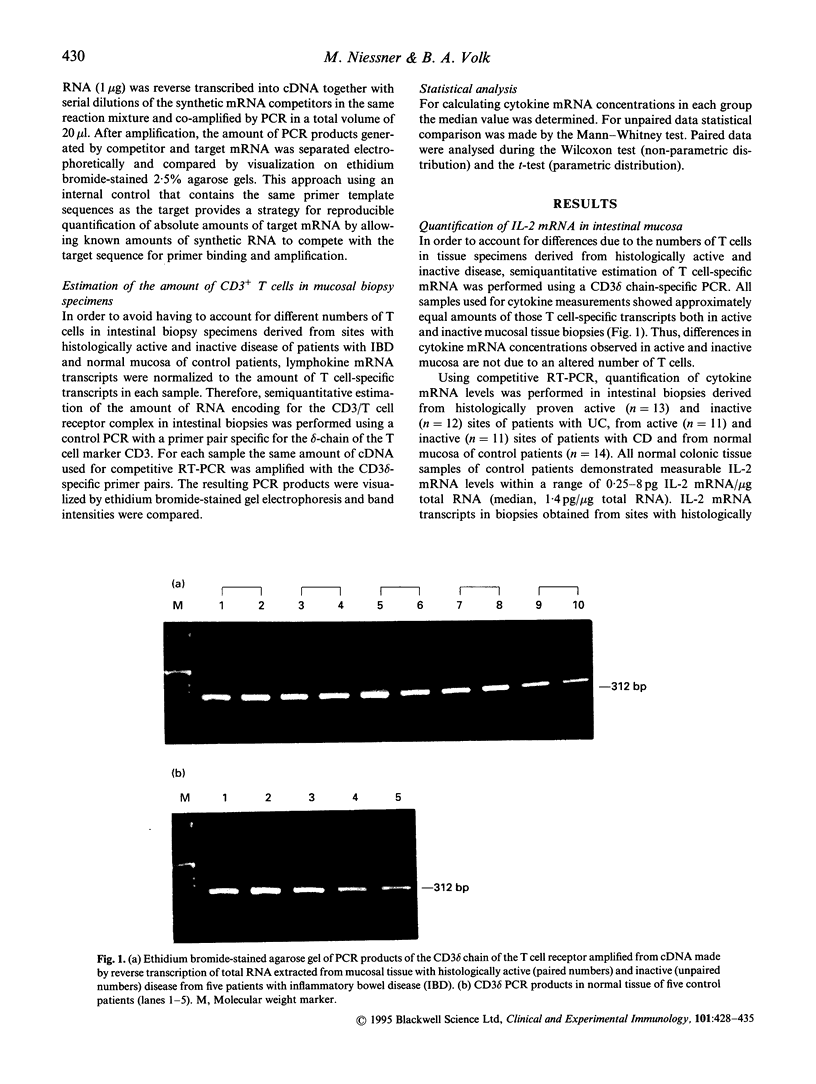
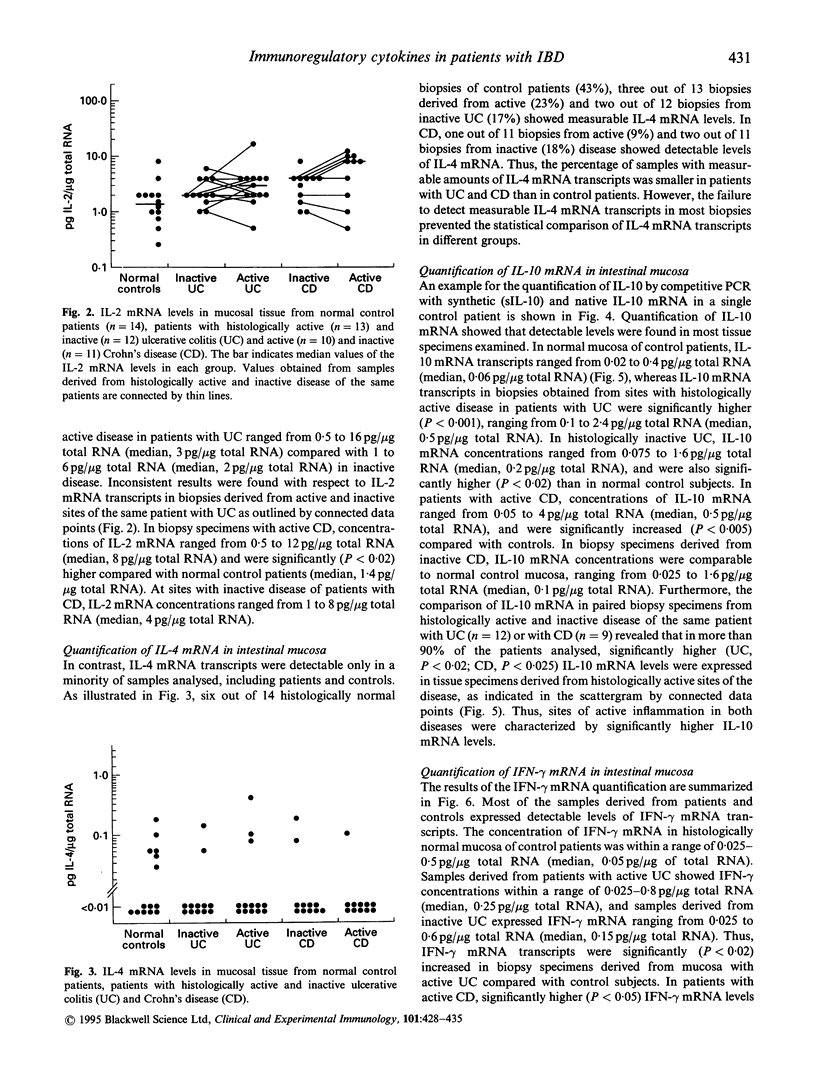
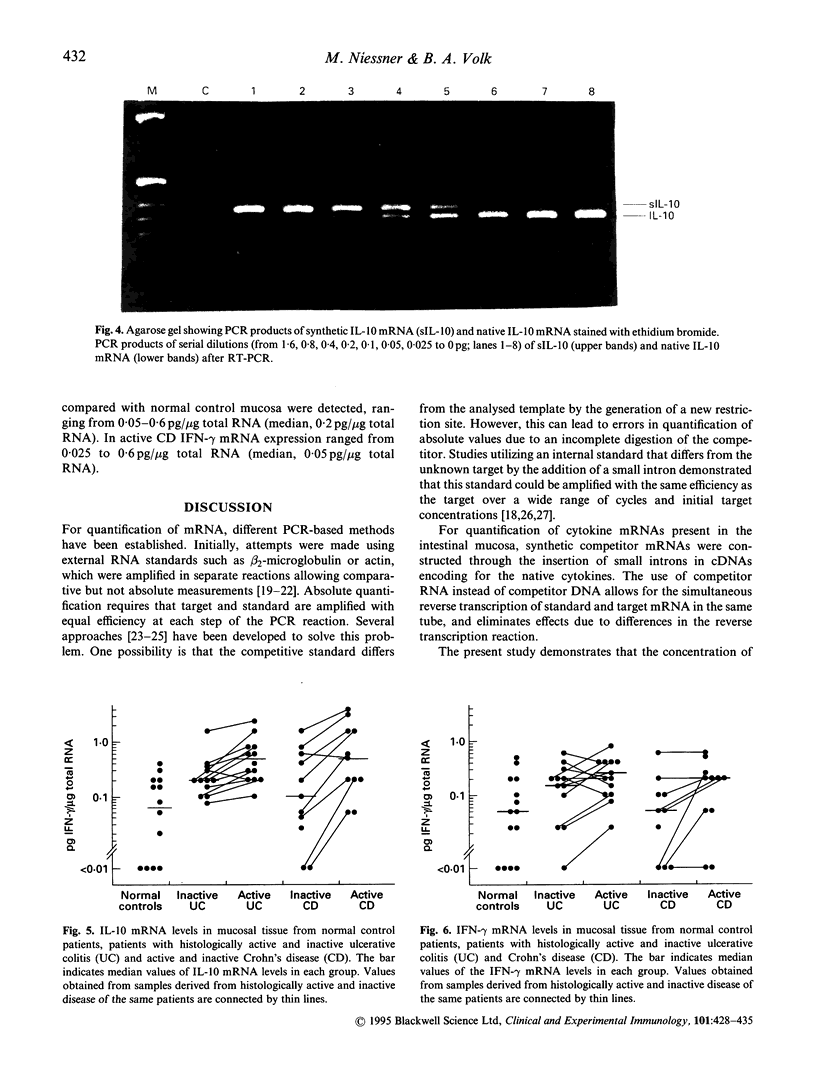
Abstract
Cytokines serve a central function as key factors in the regulation of the intestinal immune response and mediation of tissue damage in inflammatory bowel disease (IBD). Abnormalities in the expression of immunoregulatory cytokines such as IL-2, IL-4, IL-10 and interferon-gamma (IFN-gamma) may indicate a dysregulation of intestinal immunity probably associated with pathogenic events. Therefore, cytokine mRNA concentrations were determined in the mucosa of patients with IBD at sites of active (n = 13) and inactive (n = 12) ulcerative colitis (UC), active (n = 11) and inactive (n = 11) Crohn's disease (CD) and in control patients (n = 14) using quantitative RT-PCR. IL-10 mRNA concentrations were significantly increased in patients with both active UC (P < 0.001) and active CD (P < 0.005) compared with control patients. IFN-gamma mRNA concentrations were also significantly increased both in patients with active UC (P < 0.02) and active CD (P < 0.05) compared with control patients, whereas IL-2 mRNA levels were significantly (P < 0.02) increased only in active CD. IL-4 mRNA expression in the intestinal mucosa was frequently below the detection limit. Our results demonstrate that chronic intestinal inflammation in patients with CD is characterized by an increase of Th1-like cytokines. Furthermore, the increased IL-10 mRNA expression at sites of active IBD suggests that IL-10 is an important regulatory component involved in the control of the inflammatory response in inflammatory bowel disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beagley K. W., Elson C. O. Cells and cytokines in mucosal immunity and inflammation. Gastroenterol Clin North Am. 1992 Jun;21(2):347–366. [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M., Legoux P., Pességué B., Delpech B., Dumont X., Piechaczyk M., Casellas P., Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992 Oct 25;267(30):21830–21838. [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Brynskov J., Nielsen O. H., Ahnfelt-Rønne I., Bendtzen K. Cytokines in inflammatory bowel disease. Scand J Gastroenterol. 1992 Nov;27(11):897–906. doi: 10.3109/00365529209000160. [DOI] [PubMed] [Google Scholar]
- Cappello M., Keshav S., Prince C., Jewell D. P., Gordon S. Detection of mRNAs for macrophage products in inflammatory bowel disease by in situ hybridisation. Gut. 1992 Sep;33(9):1214–1219. doi: 10.1136/gut.33.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Chomarat P., Rissoan M. C., Banchereau J., Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med. 1993 Feb 1;177(2):523–527. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Fais S., Capobianchi M. R., Pallone F., Di Marco P., Boirivant M., Dianzani F., Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991 Apr;32(4):403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S., de la Barrera S., Minnucci F., Valdez R., Baliña L. M., Sasiain M. C. IFN-gamma, IL-6 and IL-4 modulate M. leprae- or PPD-specific cytotoxic T cells in leprosy patients. Scand J Immunol. 1993 Dec;38(6):551–558. doi: 10.1111/j.1365-3083.1993.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Gilberts E. C., Greenstein A. J., Katsel P., Harpaz N., Greenstein R. J. Molecular evidence for two forms of Crohn disease. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12721–12724. doi: 10.1073/pnas.91.26.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993 Feb 1;177(2):547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoof T., Riordan J. R., Tümmler B. Quantitation of mRNA by the kinetic polymerase chain reaction assay: a tool for monitoring P-glycoprotein gene expression. Anal Biochem. 1991 Jul;196(1):161–169. doi: 10.1016/0003-2697(91)90133-e. [DOI] [PubMed] [Google Scholar]
- Howard M., Muchamuel T., Andrade S., Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993 Apr 1;177(4):1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunnen R., Breese E. J., Walker-Smith J. A., MacDonald T. T. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol. 1994 Nov;47(11):1015–1018. doi: 10.1136/jcp.47.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikis P. D., Chu C. Q., Brennan F. M., Maini R. N., Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994 May 1;179(5):1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbor D., Hyjek E., Wiaderkiewicz R., Wang Z., Wang M., Loh E. Competitor mRNA fragments for quantitation of cytokine specific transcripts in cell lysates. Mol Immunol. 1993 Jan;30(1):1–7. doi: 10.1016/0161-5890(93)90420-g. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Martin R. J., Szefler S. J., Sher E. R., Ying S., Kay A. B., Hamid Q. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med. 1995 Jan 1;181(1):33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman B. Y., Fiocchi C., Youngman K. R., Sapatnekar W. K., Proffitt M. R. Interferon gamma production by human intestinal mucosal mononuclear cells. Decreased levels in inflammatory bowel disease. Dig Dis Sci. 1988 Oct;33(10):1297–1304. doi: 10.1007/BF01536683. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Marchant A., Devière J., Byl B., De Groote D., Vincent J. L., Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994 Mar 19;343(8899):707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- Matsuura T., West G. A., Youngman K. R., Klein J. S., Fiocchi C. Immune activation genes in inflammatory bowel disease. Gastroenterology. 1993 Feb;104(2):448–458. doi: 10.1016/0016-5085(93)90413-7. [DOI] [PubMed] [Google Scholar]
- McCabe R. P., Secrist H., Botney M., Egan M., Peters M. G. Cytokine mRNA expression in intestine from normal and inflammatory bowel disease patients. Clin Immunol Immunopathol. 1993 Jan;66(1):52–58. doi: 10.1006/clin.1993.1007. [DOI] [PubMed] [Google Scholar]
- Mohler K. M., Butler L. D. Quantitation of cytokine mRNA levels utilizing the reverse transcriptase-polymerase chain reaction following primary antigen-specific sensitization in vivo--I. Verification of linearity, reproducibility and specificity. Mol Immunol. 1991 Apr-May;28(4-5):437–447. doi: 10.1016/0161-5890(91)90157-f. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R. Regulation of immune responses by T cells with different cytokine secretion phenotypes: role of a new cytokine, cytokine synthesis inhibitory factor (IL10). Int Arch Allergy Appl Immunol. 1991;94(1-4):110–115. doi: 10.1159/000235340. [DOI] [PubMed] [Google Scholar]
- Mullin G. E., Lazenby A. J., Harris M. L., Bayless T. M., James S. P. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not ulcerative colitis. Gastroenterology. 1992 May;102(5):1620–1627. doi: 10.1016/0016-5085(92)91722-g. [DOI] [PubMed] [Google Scholar]
- Murphy L. D., Herzog C. E., Rudick J. B., Fojo A. T., Bates S. E. Use of the polymerase chain reaction in the quantitation of mdr-1 gene expression. Biochemistry. 1990 Nov 13;29(45):10351–10356. doi: 10.1021/bi00497a009. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Saito H., Kasanuki J., Tamura Y., Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992 Jul;33(7):933–937. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron F., Burdin N., Ringwald P., Vuillez J. P., Rousset F., Banchereau J. High levels of circulating IL-10 in human malaria. Clin Exp Immunol. 1994 Feb;95(2):300–303. doi: 10.1111/j.1365-2249.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Salomon P., Pizzimenti A., Panja A., Reisman A., Mayer L. The expression and regulation of class II antigens in normal and inflammatory bowel disease peripheral blood monocytes and intestinal epithelium. Autoimmunity. 1991;9(2):141–149. doi: 10.3109/08916939109006750. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Hiwatashi N., Yamazaki H., Noguchi M., Toyota T. The role of interferon gamma in the pathogenesis of Crohn's disease. Gastroenterol Jpn. 1992 Feb;27(1):29–36. [PubMed] [Google Scholar]
- Schwarz E. M., Salgame P., Bloom B. R. Molecular regulation of human interleukin 2 and T-cell function by interleukin 4. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7734–7738. doi: 10.1073/pnas.90.16.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz M., Greene W. C., Peffer N. J., James S. P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988 Mar;94(3):647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]