Abstract
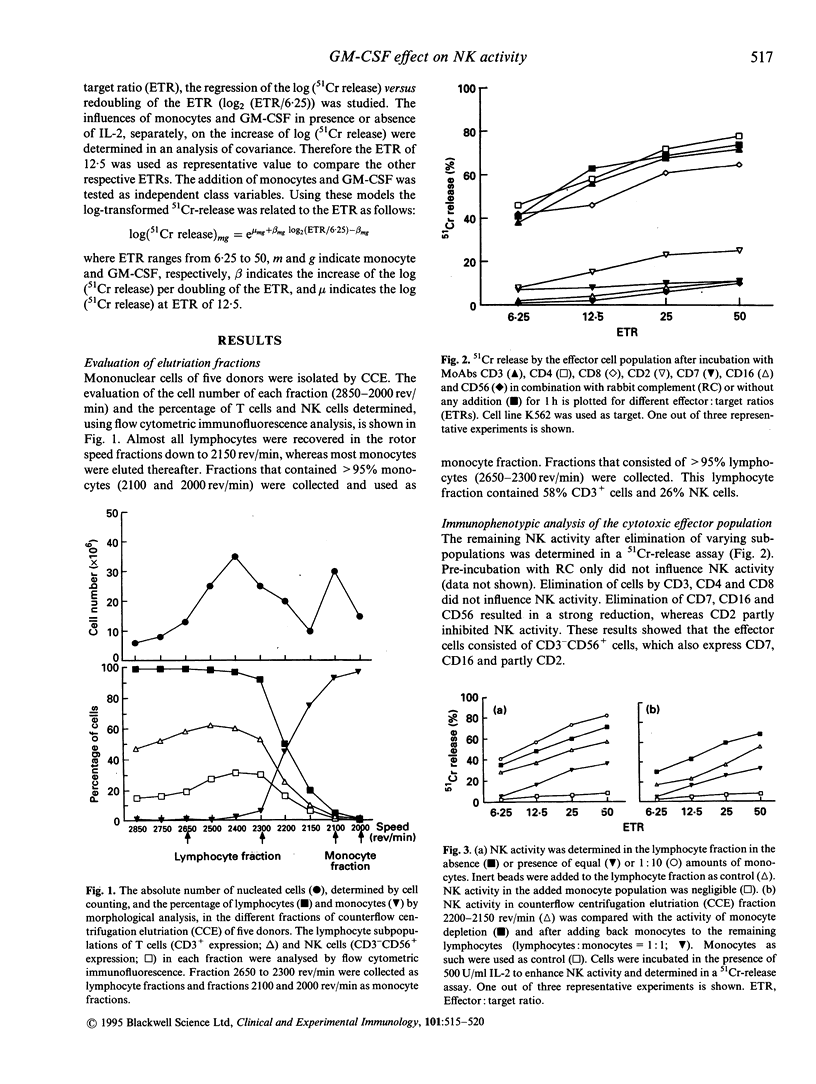
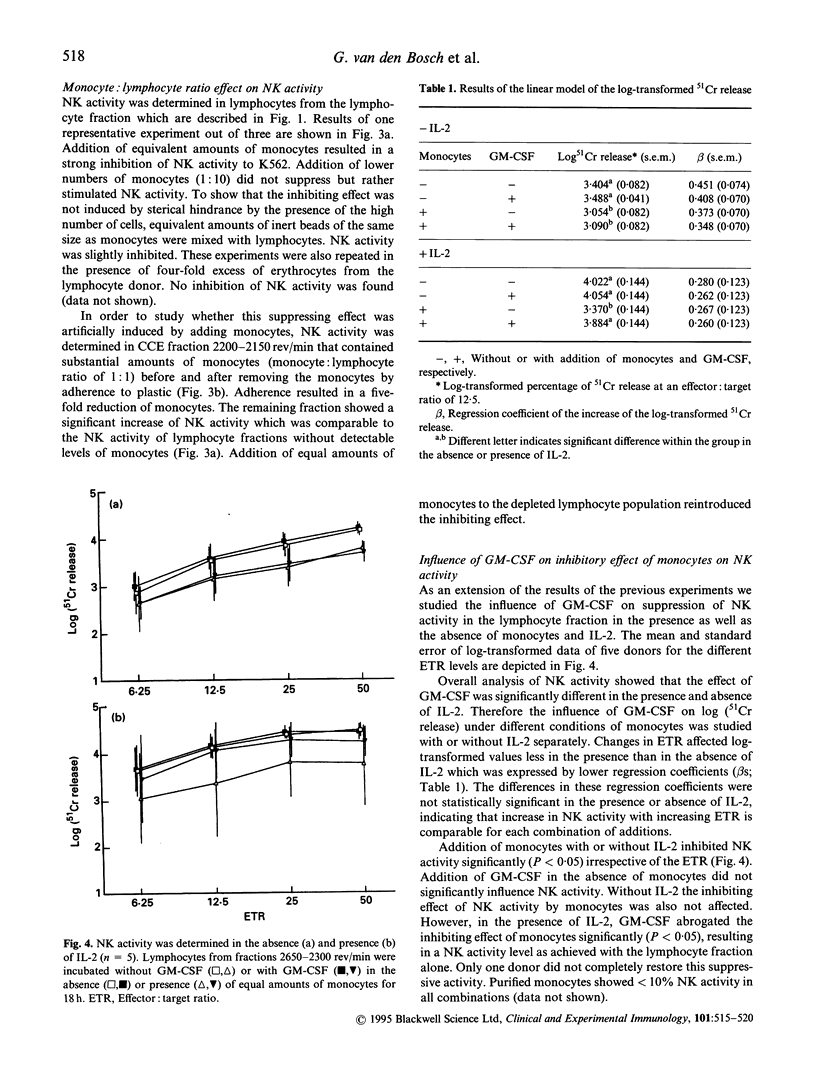
GM-CSF is known to accelerate haematopoietic recovery following allogeneic bone marrow transplantation (BMT). In addition, it may restore and enhance both granulocyte and monocyte functions. Stimulation of monocyte functions may induce a direct or an indirect anti-leukaemic activity due to an increase of cellular cytotoxicity and production of cytokines which may result in a reduction of the relapse rate after BMT. NK cells may play a crucial role in this activity. Therefore we studied the influence of monocytes on NK activity in combination with GM-CSF. Lymphocytes and monocytes were isolated from buffy coats of healthy individuals by counterflow centrifugation elutriation (CCE). NK activity was exerted by CD3-CD56+ cell populations and could be enhanced by IL-2 incubation overnight. Incubation of CD3-CD56+ cells with GM-CSF in the presence or absence of IL-2 hardly influenced NK activity of the lymphocyte population. Low amounts of monocytes enhanced NK activity. NK activity in lymphocyte population in the presence of equivalent numbers of monocytes with or without IL-2 was strongly decreased irrespective of the effector:target ratio (ETR). This appeared not to result from sterical hindrance effects of the present number of cells. However, addition of GM-CSF abrogated the inhibition of NK activity by monocytes in the presence of IL-2. In monocyte fractions neither IL-2 nor GM-CSF yielded NK activity. Our findings indicate that GM-CSF can affect NK activity by counteracting the suppressing effects of monocytes, and hence may improve the outcome after BMT.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxevanis C. N., Reclos G. J., Gritzapis A. D., Dedousis G. V., Missitzis I., Papamichail M. Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993 Jul 15;72(2):491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bloom E. T., Babbitt J. T. Prostaglandin E2, monocyte adherence and interleukin-1 in the regulation of human natural killer cell activity by monocytes. Nat Immun Cell Growth Regul. 1990;9(1):36–48. [PubMed] [Google Scholar]
- Bortin M. M., Horowitz M. M., Rowlings P. A., Rimm A. A., Sobocinski K. A., Zhang M. J., Gale R. P. 1993 progress report from the International Bone Marrow Transplant Registry. Advisory Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1993 Aug;12(2):97–104. [PubMed] [Google Scholar]
- Brandt S. J., Peters W. P., Atwater S. K., Kurtzberg J., Borowitz M. J., Jones R. B., Shpall E. J., Bast R. C., Jr, Gilbert C. J., Oette D. H. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on hematopoietic reconstitution after high-dose chemotherapy and autologous bone marrow transplantation. N Engl J Med. 1988 Apr 7;318(14):869–876. doi: 10.1056/NEJM198804073181401. [DOI] [PubMed] [Google Scholar]
- Brooks B., Parry H., Lawry J., Rees R. Evidence that interleukin-4 suppression of lymphokine-activated killer cell induction is mediated through monocytes. Immunology. 1992 Feb;75(2):343–348. [PMC free article] [PubMed] [Google Scholar]
- Cannistra S. A., Vellenga E., Groshek P., Rambaldi A., Griffin J. D. Human granulocyte-monocyte colony-stimulating factor and interleukin 3 stimulate monocyte cytotoxicity through a tumor necrosis factor-dependent mechanism. Blood. 1988 Mar;71(3):672–676. [PubMed] [Google Scholar]
- Chao N. J., Schmidt G. M., Niland J. C., Amylon M. D., Dagis A. C., Long G. D., Nademanee A. P., Negrin R. S., O'Donnell M. R., Parker P. M. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993 Oct 21;329(17):1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- Charak B. S., Agah R., Mazumder A. Granulocyte-macrophage colony-stimulating factor-induced antibody-dependent cellular cytotoxicity in bone marrow macrophages: application in bone marrow transplantation. Blood. 1993 Jun 15;81(12):3474–3479. [PubMed] [Google Scholar]
- De Mulder P. H., Wessels J. M., Rosenbrand D. A., Smeulders J. B., Wagener D. J., Haanen C. Monocyte purification with counterflow centrifugation monitored by continuous flow cytometry. J Immunol Methods. 1981;47(1):31–38. doi: 10.1016/0022-1759(81)90254-4. [DOI] [PubMed] [Google Scholar]
- De Witte T., Gratwohl A., Van Der Lely N., Bacigalupo A., Stern A. C., Speck B., Schattenberg A., Nissen C., Gluckman E., Fibbe W. E. Recombinant human granulocyte-macrophage colony-stimulating factor accelerates neutrophil and monocyte recovery after allogeneic T-cell-depleted bone marrow transplantation. Blood. 1992 Mar 1;79(5):1359–1365. [PubMed] [Google Scholar]
- Gasson J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991 Mar 15;77(6):1131–1145. [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Hellstrand K., Kjellson B., Hermodsson S. Monocyte-induced down-modulation of CD16 and CD56 antigens on human natural killer cells and its regulation by histamine H2-receptors. Cell Immunol. 1991 Nov;138(1):44–54. doi: 10.1016/0008-8749(91)90131-t. [DOI] [PubMed] [Google Scholar]
- Kupper T., Flood P., Coleman D., Horowitz M. Growth of an interleukin 2/interleukin 4-dependent T cell line induced by granulocyte-macrophage colony-stimulating factor (GM-CSF). J Immunol. 1987 Jun 15;138(12):4288–4292. [PubMed] [Google Scholar]
- Kushner B. H., Cheung N. K. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989 May 15;73(7):1936–1941. [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H. Natural killer cells. Curr Opin Immunol. 1992 Feb;4(1):38–42. doi: 10.1016/0952-7915(92)90121-t. [DOI] [PubMed] [Google Scholar]
- Linnemeyer P. A., Pollack S. B. Prostaglandin E2-induced changes in the phenotype, morphology, and lytic activity of IL-2-activated natural killer cells. J Immunol. 1993 May 1;150(9):3747–3754. [PubMed] [Google Scholar]
- Ljungman P., de Witte T., Verdonck L., Gahrton G., Freycon F., Gravett P., McCann S., Morgenstern H. G., Nikoskelainen J., Powles R. Bone marrow transplantation for acute myeloblastic leukaemia: an EBMT Leukaemia Working Party prospective analysis from HLA-typing. Br J Haematol. 1993 May;84(1):61–66. doi: 10.1111/j.1365-2141.1993.tb03025.x. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Pollock R. E., Fuchshuber P. Immunologic and clinical aspects of natural killer cells in human leukemia. Nat Immun Cell Growth Regul. 1990;9(3):173–181. [PubMed] [Google Scholar]
- Masucci G., Ragnhammar P., Wersäll P., Mellstedt H. Granulocyte-monocyte colony-stimulating-factor augments the interleukin-2-induced cytotoxic activity of human lymphocytes in the absence and presence of mouse or chimeric monoclonal antibodies (mAb 17-1A). Cancer Immunol Immunother. 1990;31(4):231–235. doi: 10.1007/BF01789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A. The clinical use of colony stimulating factors. Annu Rev Immunol. 1991;9:159–191. doi: 10.1146/annurev.iy.09.040191.001111. [DOI] [PubMed] [Google Scholar]
- Myśliwska J., Myśliwski A., Romanowski P., Bigda J., Sosnowska D., Foerster J. Monocytes are responsible for depressed natural killer (NK) activity in both young and elderly low NK responders. Gerontology. 1992;38(1-2):41–49. doi: 10.1159/000213305. [DOI] [PubMed] [Google Scholar]
- Nii A., Sone S., Utsugi T., Yanagawa H., Ogura T. Up- and down-regulation of human lymphokine (IL-2)-activated killer cell induction by monocytes, depending on their functional state. Int J Cancer. 1988 Jan 15;41(1):33–40. doi: 10.1002/ijc.2910410108. [DOI] [PubMed] [Google Scholar]
- Okamura S., Tanaka T., Yamaga S., Omori F., Niho Y. The effects of recombinant human granulocyte-macrophage colony-stimulating factor on the induction of lymphokine-activated killer cells in vitro. Int J Immunopharmacol. 1991;13(5):587–593. doi: 10.1016/0192-0561(91)90080-q. [DOI] [PubMed] [Google Scholar]
- Plas A., de Witte T., Wessels H., Haanen C. A new multichamber counterflow centrifugation rotor with high-separation capacity and versatile potentials. Exp Hematol. 1988 Jun;16(5):355–359. [PubMed] [Google Scholar]
- Richard C., Alsar M. J., Calavia J., Bello-Fernandez C., Baro J., Loyola I., Rios R., Cuadrado M. A., Gonzalez-Pardo C., Iriondo A. Recombinant human GM-CSF enhances T cell-mediated cytotoxic function after ABMT for hematological malignancies. Bone Marrow Transplant. 1993 Jun;11(6):473–478. [PubMed] [Google Scholar]
- Santoli D., Clark S. C., Kreider B. L., Maslin P. A., Rovera G. Amplification of IL-2-driven T cell proliferation by recombinant human IL-3 and granulocyte-macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):519–526. [PubMed] [Google Scholar]
- Schattenberg A., De Witte T., Preijers F., Raemaekers J., Muus P., Van der Lely N., Boezeman J., Wessels J., Van Dijk B., Hoogenhout J. Allogeneic bone marrow transplantation for leukemia with marrow grafts depleted of lymphocytes by counterflow centrifugation. Blood. 1990 Mar 15;75(6):1356–1363. [PubMed] [Google Scholar]
- Smith P. D., Lamerson C. L., Wong H. L., Wahl L. M., Wahl S. M. Granulocyte-macrophage colony-stimulating factor stimulates human monocyte accessory cell function. J Immunol. 1990 May 15;144(10):3829–3834. [PubMed] [Google Scholar]
- Sone S., Inamura N., Nii A., Ogura T. Heterogeneity of human lymphokine (IL-2)-activated killer (LAK) precursors and regulation of their LAK induction by blood monocytes. Int J Cancer. 1988 Sep 15;42(3):428–434. doi: 10.1002/ijc.2910420320. [DOI] [PubMed] [Google Scholar]
- Sone S., Kunishige E., Fawzy F., Yanagawa H., Nii A., Maeda K., Atagi S., Heike Y., Nishioka Y., Mizuno K. Interleukin-2-inducible killer activity and its regulation by blood monocytes from autologous lymphocytes of lung cancer patients. Jpn J Cancer Res. 1991 Jun;82(6):716–723. doi: 10.1111/j.1349-7006.1991.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Magee D. M., Whiteside T. L., Kaplan S. S., Shadduck R. K. Recombinant human granulocyte/macrophage colony-stimulating factor enhances monocyte cytotoxicity and secretion of tumor necrosis factor alpha and interferon in cancer patients. Blood. 1989 Feb 15;73(3):643–646. [PubMed] [Google Scholar]
- Young D. A., Lowe L. D., Clark S. C. Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J Immunol. 1990 Jul 15;145(2):607–615. [PubMed] [Google Scholar]
- van de Loosdrecht A. A., Beelen R. H., Ossenkoppele G. J., Broekhoven M. G., Langenhuijsen M. M. Cellular and cytokine dependent monocyte-mediated leukemic cell death: modulation by interferon-gamma and tumor necrosis factor-alpha. Exp Hematol. 1993 Mar;21(3):461–468. [PubMed] [Google Scholar]


