Abstract
Ataxia-telangiectasia mutated (ATM) is required for resistance to radiation-induced DNA breaks. Here we use chromatin immunoprecipitation to show that ATM also localizes to breaks associated with V(D)J recombination. ATM recruitment to the recombining locus correlates approximately with recruitment of the break-initiating factor RAG1 and precedes efficient break repair, consistent with localization of ATM to normal recombination intermediates. A product of ATM kinase activity, Ser 18-phosphorylated p53, was detected similarly at these breaks, arguing that ATM phosphorylates target proteins in situ. We suggest routine surveillance of intermediates in V(D)J recombination by ATM helps suppress potentially oncogenic translocations when repair fails.
Keywords: Checkpoint, DNA-PK, RAG1, p53, DNA repair, double-strand break
Ataxia-telangiectasia is characterized by cerebellar degeneration, immunodeficiency, and a high frequency of malignancy, usually lymphoid in origin (Morrell et al. 1986). The gene mutated in this disease (ataxia-telangiectasia mutated, or ATM) is required for resistance to DNA double-strand break (DSB) inducing agents such as ionizing radiation, and is an important trigger for the cellular response to DSBs (for review, see Rotman and Shiloh 1999). ATM may act as a primary sensor of DSBs, first binding directly to DSBs and consequently activating through phosphorylation several downstream effectors of the DNA damage response, including Nbs1, BRCA1, Chk2, and p53 (for review, see Durocher and Jackson 2001). For example, after treatment with ionizing radiation, phosphorylation of p53 at Ser 15 (Ser 18 in mice) in cells is largely ATM dependent (for review, see Giaccia and Kastan 1998) and is required for effective p53-dependent responses to this stimulus (Chao et al. 2000).
In contrast to its important role in mediating resistance to exogenous DSB-inducing agents, genetic evidence for a role for ATM in repair of DSB intermediates in V(D)J recombination is less clear. V(D)J recombination is an essential step in the generation of a diverse repertoire of immunoglobulins and T-cell receptors (for review, see Gellert 1997). RAG1 and RAG2 proteins initiate V(D)J recombination by introducing DSBs precisely adjacent to recombination-targeting signals that flank segments of immunoglobulin and T-cell receptor coding sequence. Efficient resolution of broken species requires factors implicated in end-joining DSB repair, including Ku, XRCC4, ligase IV, and the ATM-related kinase DNA-PKcs, but not ATM. Cells from patients with AT support normal levels of V(D)J recombination using an extra-chromosomal substrate assay (Hsieh et al. 1993), and mature antigen receptor-bearing lymphocytes are readily observed in ATM-deficient mice (Barlow et al. 1996; Elson et al. 1996; Xu et al. 1996).
Are V(D)J recombination intermediates recognized by ATM as DNA damage? The lack of requirement for ATM in V(D)J recombination might suggest that ATM is excluded from breaks associated with this pathway, possibly through masking of ends by RAG1 and RAG2, to avoid counter-productive apoptotic responses during this normal cellular process. However, both AT patients (for review, see Taylor et al. 1996) and ATM-deficient mice (Barlow et al. 1996; Liyanage et al. 2000; Xu et al. 1996) are prone to lymphoid malignancies that harbor translocations involving antigen receptor genes. Moreover, ATM-deficient mice no longer develop tumors with such translocations when V(D)J recombination is blocked, due to deficiency in RAG1 or RAG2 (Liao and Van Dyke 1999; Petiniot et al. 2000). Genetic evidence thus indicates that although ATM is normally not required for V(D)J recombination, this factor does play an important role in protection against tumors caused by aberrant V(D)J recombination.
However, the molecular basis for these latter observations is unknown. We therefore used chromatin immunoprecipitation (for review, see Orlando 2000) to show that both ATM and a product of ATM kinase activity, Ser 18-phosphorylated p53, are recruited to DSBs associated with V(D)J recombination. Our data further provide a biochemical basis for a model in which ATM monitors the repair of intermediates in V(D)J recombination, and subsequently helps protect against aberrant recombination when repair fails.
Results
ATM and Ser 18-phoshorylated p53 localize to V(D)J recombination-associated breaks
We initially used a temperature-sensitive Abelson Murine Leukemia virus transformed (ts-Ab-MuLV) cell line as a source of cells undergoing high levels of V(D)J recombination (Chen et al. 1994; Chang and Brown 1999). Culture of a ts-Ab-MuLV at the nonpermissive temperature induces high levels of RAG protein expression, which in turn mediates breakage of recombination signals at endogenous immunoglobulin κ loci (Igκ) (Chen et al. 1994; see also Fig. 3A,B, below). We analyzed breaks that retain the recombination signal (signal ends), as these breaks are longer lived than the ends of coding segments, but are nevertheless efficiently resolved into junctions and are thus normal recombination intermediates (Ramsden and Gellert 1995). Moreover, because the majority of functional V gene segments in the κ locus recombine by inversion (Thiebe et al. 1999) resolution of signal ends at this locus is typically required for maintenance of chromosomal integrity.
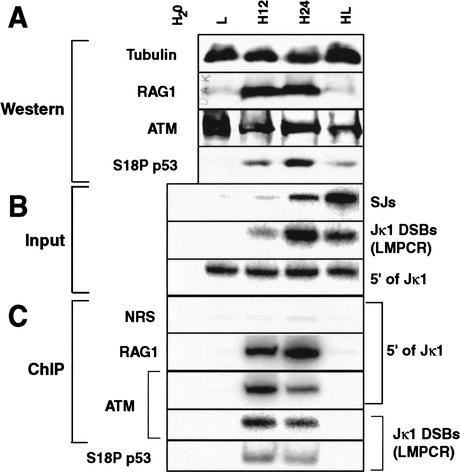
Figure 3.
Correlation of V(D)J recombination activity with factor localization. Cells were harvested before induction (L), after 12 h (H12) and 24 h (H24) of induction, and after 24 h of induction followed by 24 h without induction (HL). (H20) A PCR control with water substituted for template. (A) Western analysis of various proteins, as noted at left. (B) PCR analysis of different input DNA species, as noted at right. (SJs) VJκ1 signal junctions. Note that only a small subpopulation of total signal junctions are detected by this PCR as sequences 3′ of the ∼100 different Vκ segments are not conserved. (C) ChIP analysis of the localization of different proteins or the nonspecific NRS control, as noted at left, to different DNA species, as noted at right. PCR reactions in B and C were shown to be in the linear range by serial dilution of the H24 sample (E.J. Perkins and D.A. Ramsden, unpubl.).
Proteins that bind to these breaks were recovered by immunoprecipitation with appropriate antibodies, and the associated DNA was detected by PCR analysis. Antibodies to RAG1and DNA-PKcs efficiently recovered a 300-bp DNA fragment adjacent to Jκ1, a site of active V(D)J recombination in this cell line (Fig. 1A,B). A similar localization to Jκ1 was observed by use of antibodies specific to other factors implicated in the end-joining pathway (Ku, XRCC4; E.J. Perkins and D.A. Ramsden, unpubl.), but not control antibody preparations (IgG1, normal rabbit serum; all ChIP figures) or antibodies to other ubiquitous DNA-binding proteins (TFIID, Sp1; E.J. Perkins and D.A. Ramsden, unpubl.).
Figure 1.
ChIP analysis of Jκ1 breaks from ts-Ab-MuLV cells. (A) Diagram of κ locus, with recombination signals (triangles) and coding segments (rectangles) marked. The location of PCR primers (arrows) and PCR products (bars) at bottom. (B) ChIP analysis of extracts from ts-Ab-MuLV cells induced for 2 d. Comparison of PCR amplified, serial dilutions of input DNA template to DNA template immunoprecipitated (IP'd DNA) by antibodies to different proteins. (NRS) Normal rabbit serum control; (S18P p53) Ser 18-phosphorylated p53; (ATM) a pool of 9E3C8 and 2C1 antibodies. The same DNA samples were used as template to assess levels of three different DNA species; nonspecific locus (RAG1 gene), 5′ of Jκ1, and Jκ1 DSBs, as noted. (C) ChIP analysis with different ATM antibodies. The ability of different ATM monoclonal antibodies, used separately, to recover Jκ1 DSBs or a nonspecific locus (5′ of Jα50) was assessed.
Importantly, antibodies to ATM also recovered the Jκ1 species (Fig. 1B). Similar results were obtained with three different ATM monoclonal antibodies when used separately (Fig. 1C; more quantitative recovery of ATM-associated DNA was observed using a pool of two of these monoclonals). All antibody preparations precipitated negligible amounts of DNA species from several different loci not undergoing V(D)J recombination. This included a gene active in transcription but not V(D)J recombination (the RAG1 gene, Fig. 1B), an antigen receptor gene not active for recombination in this cell type (T-cell receptor Jα50; Fig. 1C), as well as a region near Cκ1 but 2.5 kb distal to a site of recombination; E.J. Perkins and D.A. Ramsden, unpubl.). We conclude RAG1, ATM, and DNA-PKcs specifically localize near actively recombining DNA.
We further characterized immunoprecipitated DNA by ligation-mediated PCR (LMPCR), which specifically detects blunt DSBs by ligation of a small double-stranded DNA linker to DNA ends (Fig. 1A). Results using LMPCR paralleled the previous analysis using the 5′ of Jκ1 product (Fig. 1B, cf. Jκ1 DSBs with 5′ of Jκ1). Localization of RAG1, DNA-PKcs, and ATM to this region thus occurs specifically at DSBs. The greater specificity of LMPCR for actively recombining DNA also allowed us to determine that Ser 18-phosphorylated p53 (S18P-p53) also localizes to DSBs at Jκ1 (Fig. 1B). Similar results were obtained by use of an antibody that recognizes p53 independent of phosphorylation status (E.J. Perkins and D.A. Ramsden, unpubl.). No p53 consensus binding sites can be found near these breaks, arguing localization is not sequence dependent. Instead, our data is consistent with prior observations indicating p53 associates with ATM (Khanna et al. 1998), and that phosphorylation of p53 on Ser 18 is largely ATM dependent in response to ionizing radiation (for review, see Giaccia and Kastan 1998).
Because localization of ATM to breaks associated with V(D)J recombination could be unique to our transformed cell line model, we assessed the ability of ATM to localize to DSBs at a T-cell receptor α locus recombination signal (Jα50) in thymocytes from 1-week-old mice . Antibodies to ATM recovered levels of Jα50 DSBs comparable with that recovered by the RAG1 antiserum, whereas an isotype-matched control monoclonal antibody did not recover this species (Jα50 DSBs, Fig. 2A). Again, neither RAG nor ATM antibodies recovered significant amounts of DNA from loci not active in V(D)J recombination, including the RAG1 gene as well as the 5′ of Jκ1 region (active only in B cells). In a parallel experiment performed using thymocytes from ATM-deficient littermates, Jα50 DSBs were still recovered by antibodies to RAG1 but not with antibodies to ATM, further confirming the specificity of the ATM antibodies in this assay (Fig. 2B). ATM therefore also localizes to V(D)J recombination-associated DSBs at the T-cell receptor α locus in primary thymocytes.
Figure 2.
ChIP analysis of Jα50 breaks from mouse thymocytes. (A) Comparison of input template to template recovered after immunoprecipitation (IP'd DNA) of thymocyte extract with antibodies to RAG1, ATM (pool IP used both 2C1 and ATXO8 IgG1 antibodies), and an isotype matched control (IgG1). The same DNA samples were used as template to assess levels of nonspecific loci (RAG1 gene, 5′ of Jκ1) and Jα50 DSBs. (B) ChIP analysis of ATM-deficient thymocyte extract with antibodies to RAG1 and ATM (using a pool of 2C1 and ATXO8). The same DNA samples were used as template to assess levels of a nonspecific locus (RAG1 gene) and Jα50 DSBs.
ATM localization is consistent with recruitment to normal intermediates
Unresolved signal ends accumulate in both ts-Ab-MuLV cells (Ramsden and Gellert 1995) and primary lymphocytes (Schlissel et al. 1993; Zhu and Roth 1995) upon induction of V(D)J recombination. However, analysis of signal ends in the ts-Ab-MuLV cell model has shown that these ends nevertheless resolve efficiently to signal junctions if cells are returned to pre-induction conditions, and are thus intermediates in the recombination pathway (Ramsden and Gellert 1995). We made use of the ability to control accumulation and resolution of signal ends to distinguish among the following possibilities: (1) ATM localization actually precedes chromosomal breakage, (2) ATM localizes only to breaks long after induction, suggestive of recruitment to a subpopulation of breaks whose resolution has been inhibited, or (3) ATM localization is approximately coincident with break induction and localization of the break-generating factor RAG1, consistent with a recruitment to normal DSB intermediates in V(D)J recombination.
ts-Ab-MuLV cells were harvested for ChIP and Western analysis prior to induction (L), at two different times after induction of recombination (H12 and H24) and after return to pre-induction conditions for a further 24 h (HL). Accumulation of Jκ1 breaks (Fig. 3B) correlates with increased localization of RAG1 to this region (Fig. 3C), as would be expected if RAG1 remains associated with broken ends after cleavage (Agrawal and Schatz 1997; Hiom and Gellert 1998). Partial resolution of accumulated Jκ1 DSBs to signal junctions is evident 24 h after return to pre-induction conditions (Fig. 3B), and localization of RAG1 to remaining breaks is no longer observed (Fig. 3C), consistent with reduced levels of RAG1 protein (Fig. 3A). Jκ1 DSBs were undetectable and levels of signal junctions further increased 48 h after return to pre-induction conditions, confirming the efficient resolution of breaks observed previously in this cell line model (E.J. Perkins and D.A. Ramsden, unpubl.; see also Chen et al. 1994; Ramsden and Gellert 1995).
Western analysis shows that total levels of ATM remain constant throughout the time course (Fig. 3A). Although levels of ATM do not change, this factor nevertheless clearly localizes to Jκ1 after 12 h of induction (Fig. 3C). Thus, there is no pronounced delay in localization of ATM to breaks, relative either to the appearance of breaks or the localization of the break-initiating factor RAG1. We observed a reduction in localization of ATM after 24 h relative to earlier time points, even though localization of RAG1 continues to rise in parallel with the accumulation of breaks. Localization of ATM to DSBs at Jκ1 is also no longer observed after return of these cells to pre-induction conditions for 24 h (Fig. 3C). ATM recruitment does not precede breakage, nor is ATM recruited only long after V(D)J recombination-induced breaks have been generated. Our ChIP analysis does not quantitatively recover ATM-associated DNA, thus we cannot exclude the possibility that ATM is localizing to a rare subpopulation of breaks that cannot be repaired. However, our results are most consistent with recruitment of ATM to normal intermediates in the pathway.
Western analysis showed that changes in levels of Ser 18-phosphorylated p53 paralleled the accumulation and resolution of signal ends over the entire time course (Fig. 3, cf. S18P p53 in A with Jκ1 DSBs in B). Localization of Ser 18 p53 to Jκ1 DSBs, as determined by ChIP analysis, roughly paralleled that of ATM, in that it was observed only during induction (Fig. 3C).
Phosphorylation of p53 at Ser 18 is enhanced in a scid cell line
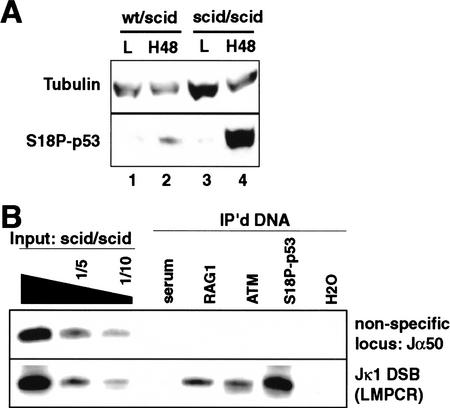
ATM and DNA-PKcs both localize to signal ends (Fig. 1B) and both phosphorylate p53 at Ser 18 in vitro (for review, see Giaccia and Kastan 1998), so either kinase could potentially mediate this phosphorylation event at V(D)J recombination intermediates. We therefore examined levels of Ser 18-phosphorylated p53 in ts-Ab-MuLV cells derived from a scid mouse, deficient in DNA-PKcs (Chang and Brown 1999), and compared these findings with our previous results from wild-type ts-Ab-MuLV cells. Upon induction of V(D)J recombination, levels of phosphorylation at Ser 18 of p53 were >10-fold higher in the scid cell line (Fig. 4A). Recruitment of Ser 18-phosphorylated p53 to breaks in scid cells was also much more readily detectable relative to previous experiments with wild-type cells (cf. levels of S18P p53 relative to ATM or RAG1 in Fig. 1B with Fig. 4B). DNA-PKcs is therefore not required for this event, as phosphorylation of p53 at Ser 18 instead appears to be much more efficient in the DNA-PKcs-deficient cell line. Similar results were obtained previously by comparing responses of wild-type and DNA-PKcs-deficient cells in response to ionizing radiation (Araki et al. 1999), arguing that the enhanced phosphorylation of p53 at Ser 18 in scid cells observed here is not unique to this cell line model.
Figure 4.
p53 phosphorylation in scid cells. (A) Samples from a wild-type/scid heterozygous ts-Ab-MuLV cell line (wt/scid) and a scid homozygous ts-Ab-MuLV cell line (scid/scid) both before (L) and after induction of V(D)J recombination for 2 d (H48) were compared by Western analysis. (S18P-p53) Ser 18-phosphorylated p53. (B) Extracts from scid ts-Ab-MuLV cells were analyzed by ChIP after 2 d of V(D)J recombination induction, as also performed for wild-type cells in Fig. 1C. The same DNA samples were used as template to assess levels of nonspecific loci (RAG1 gene, 5′ of Jα50, Cκ), and Jκ1 DSBs, as noted.
Discussion
We observe localization of RAG1 to signal ends generated at an endogenous locus active in V(D)J recombination. This supports previous work, using artificial recombination substrates, that shows RAG1/RAG2 complexes remain bound to signal ends after they complete signal-targeted cleavage (Agrawal and Schatz 1997; Hiom and Gellert 1998). DNA-PKcs, an end-joining factor primarily linked to resolution of coding end intermediates in V(D)J recombination, was also shown to localize to signal ends. However, localization of DNA-PKcs to signal ends is not completely unexpected, as deficiency in DNA-PKcs also results in a mild, but significant, impairment of signal end resolution (Lieber et al. 1988; Bogue et al. 1998; Fukumura et al. 2000).
Most importantly, we observed ATM at V(D)J recombination-associated breaks, both in the Igκ locus in a pre-B cell line, as well in the T-cell receptor α locus in primary mouse thymocytes. Recruitment of ATM to the recombining locus in the pre-B cell line was roughly coincident with both break induction and recruitment of RAG1, and preceded efficient resolution of breaks to repaired junctions. These results are consistent with recruitment of ATM to normal recombination intermediates.
We also observed Ser 18-phosphorylated p53 at breaks associated with V(D)J recombination. p53 physically associates with ATM (Khanna et al. 1998) and phosphorylation of p53 at Ser 18 is ATM dependent in response to other sources of double-strand breaks (for review, see Giaccia and Kastan 1998). Our data, taken together with these previous observations, argues that ATM is not only recruited to V(D)J recombination intermediates, but also responds to these breaks by phosphorylating associated molecules of p53. This is consistent with models in which ATM is a direct sensor of DNA damage (for review, see Durocher and Jackson 2001), and suggests that transduction of ATM-dependent signals may typically be initiated in situ, at the site of DNA damage.
Localization of an activated form of p53 at breaks associated with V(D)J recombination is consistent with genetic data. p53 deficiency increases the frequency of malignancy linked to aberrant V(D)J recombination in mice also deficient in genes required for end-joining (Vanasse et al. 1999; Difilippantonio et al. 2000; Frank et al. 2000). However, comparison of mice deficient in either p53 or ATM as well as mice deficient in both genes indicates the roles of these genes in suppressing tumorigenesis are overlapping, but not congruent (Westphal et al. 1997; Xu et al. 1998). Two other putative targets of ATM kinase activity, NBS1 and the histone H2AX, have been associated with chromatin actively undergoing V(D)J recombination (Chen et al. 2000). A significant role for these factors (or other ATM targets) in addition to p53 may be required for ATM's full protective effect.
How does ATM distinguish between breaks that are normal intermediates from breaks that are precursors to aberrant rearrangements? ATM may be recruited to all V(D)J recombination DSB intermediates, but ATM kinase activity (and consequent activation of its downstream effectors) is normally attenuated when these breaks use the end-joining pathway for repair (Fig. 5). However, the enhanced phosphorylation of p53 in DNA-PKcs-deficient cells (Fig. 4; Araki et al. 1999) suggests that if a complete end-joining complex is not assembled, the ATM kinase remains active, resulting in more effective triggering of downstream effectors (p53, NBS1, H2AX, etc.). This model can explain genetic data indicating that ATM is dispensable for normal V(D)J recombination, but is nevertheless required for effective suppression of malignancy caused by aberrant V(D)J recombination when repair fails.
Figure 5.
Model for role of ATM in V(D)J recombination.
Materials and methods
Antibodies
Three mouse IgG1 monoclonals recognizing ATM were used as follows: 9E3C8 (Ab-5, NeoMarker), ATX08 (Ab-8, NeoMarker), and 2C1 (GeneTex). A rabbit polyclonal antisera was used to detect Ser 18-phosphorylated p53 (Cell Signaling Technology) and the monoclonal Ab421 (Ab-1, Oncogene Research) was used to detect p53 independent of phosphorylation status. We verified the specificity of these antibodies in parallel immunoprecipitation experiments using extracts from the same cells, the same antibody formulations, and using the same conditions as used for ChIP analyis, except adapted for visualization of protein by Western analysis (E.J. Perkins and D.A. Ramsden, unpubl.). With respect to p53 antibodies, with this antibody, roughly equivalent amounts of p53 were recovered from an induced scid cell extract by both PAb421 and the antibody specific to Ser 18-phosphorylated p53, whereas only Ab421 recovered p53 from an extract sample treated with λ serine phosphatase (New England Biolabs; E.J. Perkins and D.A. Ramsden, unpubl.). A pool of monoclonals recognizing DNA-PKcs (Neomarkers), RAG-1 (PharMingen), and Ku (the gift of David Schatz, Yale University, New Haven, CT) were also used. Purified Mouse IgG1 (Pharmingen), normal rabbit serum (Sigma), antisera recognizing Sp1 (PEP2, Santa Cruz), a monoclonal recognizing TFIID (SI-1, Santa Cruz), and a monoclonal recognizing β tubulin (TUB 2.1, Sigma) were used as controls.
Cells and ChIP analysis
The wild-type/scid heterozygote SP9 and scid/scid homozygote S4 ts-Ab-MuLV cell lines were established, maintained, and induced as described previously (Chang and Brown 1999). Western analysis verified that levels of DNA-PKcs were >50-fold lower in the scid cell line compared with the wild-type cell line, whereas levels of ATM were equivalent (E.J. Perkins and D.A. Ramsden, unpubl.). Levels of DNA-PKcs recruited to Jκ1 breaks in scid cells as determined by ChIP analysis, although detectable, were also reduced >10-fold relative to wild-type cells. Thymocytes were harvested from 7-day-old mice, and single cell suspensions prepared by mincing and straining through a 100-μm mesh. Mice were either ATM+/− or ATM−/− littermates (genotype assessed by PCR; Liao and Van Dyke 1999) from a mating of ATM+/− mice (129/SvEv -C57Bl/6 background) (Barlow et al. 1996).
Cells were further processed for ChIP analysis as described previously (Chen et al. 1999). ChIP analysis was performed on extract equivalent to 4 × 106 cells for ts-Ab-MuLV cells or 8 × 106 cells for thymocytes, using 2 μg of purified antibody or 2 μL of antiserum.
PCR Analysis
Input DNA template equivalent to 1/100 of the amount used for immunoprecipitation, as well as 5-fold and 10-fold dilutions, were compared with 1/6 of the material recovered from each immunoprecipitation using a 27 cycle PCR. The nonspecific locus in Figure 1C (RAG1 gene) was amplified with DAR192 (5′-AGCAAGGAAGTCCTGAAGAAGATCT-3′) and DAR216 (5′-GATATCGGCAAGAGGGACAATAGCT-3′), an annealing step of 57°C, and detected using DAR193 (5′-GGAGATGGATT TCACAAAGTGTGCT-3′). The nonspecific locus in Figures 1D and 4A (5′ of Jα50) was amplified with DAR322 (5′-TCTCAGGGAAGATGGGC CTCTC-3′) and DAR323 (5′-CCCTGTCAGCTTGGTTCAAAGGC-3′), an annealing step of 58°C, and detected with DAR323. The 5′ of Jκ1 fragment was amplified with DAR11 (5′-AGTGCCACTAACTGCTGAGCCACCT-3′) and DAR6 (5′-GAGCAGTGGGTAGGCGAAAGCTTAACCCC-3′), an annealing step of 60°C, and detected with DAR10 (5′-CCACGCATGCTTGGAGAGGGGGTT-3′). A subset of Vκ-Jκ1 signal junctions (SJs) were amplified with DAR11 and DAR292 (5′-GGTT TTTGTTCCAGTCTGTATCACTG-3′), an annealing step of 65°C, and detected with DAR3 (5′-TGTACAGCCAGACAGTGGAGTACTACCA-3′). For LMPCR analysis of DSBs, a linker was prepared from FM11 (5′-CACTTCAGATC-3′) and FM25 (5′-GCGGTGACTCGGGAGATCTGA AGTG-3′), ligated to twice the amount of template DNA as was used for standard PCR analysis, and linker-ligated DNA recovered as described previously. In the first round of amplification, 1/4 of the ligated material was amplified for 20 cycles using FM25, DAR11, and a 60°C annealing step for Jκ1DSBs, or, for Jα50 DSBs, FM25, DAR321 (5′-GGTCCACGTCCAGATGCCAACT-3′), and a 58°C annealing step. A total of 1/25 of the first round PCR was further amplified for 25 cycles using the same annealing temperatures and FM25 and DAR289 (5′-GTTAAGCTTTCGCCTACCCACTGCTC-3′) for Jκ1 DSBs (detected with DAR3), or FM25 and DAR322 for Jα50 DSBs (detected with DAR323). PCR products were separated on 6% polyacrylamide gels, transferred to nylon membranes, and hybridized with 5′ 32P-labeled oligonucleotide probes (see above) by use of standard techniques.
Western analysis
Extract samples were prepared from cells using a buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.2% SDS, 10% glycerol, 5 mM Pyrophosphate, 1 mM beta glycerol phosphate, 5 mM EDTA, 1 mM EGTA, 1 mM DTT, and Complete protease inhibitors (Roche Biochemicals), sonicated and clarified by centrifugation. 250-μg extract samples were electrophoresed on a 6% SDS-polyacylamide (50:1 acrylamide:bis-acrylamide) gel for analysis of ATM and RAG1; for all other proteins, 50-μg samples were electrophoresed on a 10% (30:1 acrylamide:bis-acrylamide) SDS-polyacylamide gel. Gels were transferred to nitrocellulose membranes and probed with the noted antibodies (the 2C1 ATM antibody was used for Western analysis). Proteins were visualized by subsequent incubation with horseradish peroxidase conjugated anti-mouse or anti-rabbit immunoglobulin and lumiglo peroxidase substrate (New England Biolabs).
Acknowledgments
We thank M. Gellert, Y. Xiong, B. Weissman, A. Sancar, and M. Backlund for critical reading of this manuscript, as well as D. Schatz (Yale University) for the gift of antibodies that recognize mouse Ku. This work was supported by NIH grant CA 84442-01, ACS grant RPG-99-192-01-GMC, a Leukemia and Lymphoma Society fellowship to D.C. and a Searle Scholar grant to D.A.R.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL dale_ramsden@med.unc.edu; FAX (919) 966-3015.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.956902.
References
- Agrawal A, Schatz DG. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- Araki R, Fukumura R, Fujimori A, Taya Y, Shiloh Y, Kurimasa A, Burma S, Li GC, Chen DJ, Sato K, et al. Enhanced phosphorylation of p53 serine 18 following DNA damage in DNA-dependent protein kinase catalytic subunit-deficient cells. Cancer Res. 1999;59:3543–3546. [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Bogue MA, Jhappan C, Roth DB. Analysis of variable (diversity) joining recombination in DNA dependent protein kinase (DNA-PK)-deficient mice reveals DNA-PK-independent pathways for both signal and coding joint formation. Proc Natl Acad Sci. 1998;95:15559–15564. doi: 10.1073/pnas.95.26.15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Brown ML. Formation of coding joints in V(D)J recombination-inducible severe combined immune deficient pre-B cell lines. Proc Natl Acad Sci. 1999;96:191–196. doi: 10.1073/pnas.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C, Saito S, Anderson CW, Appella E, Xu Y. Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc Natl Acad Sci. 2000;97:11936–11941. doi: 10.1073/pnas.220252297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, Rogakou EP, Brotz TM, Bonner WM, Ried T, et al. Response to RAG-mediated VDJ cleavage by NBS1 and gamma-H2AX. Science. 2000;290:1962–1965. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Wang LC, Huang MS, Rosenberg N. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes & Dev. 1994;8:688–697. doi: 10.1101/gad.8.6.688. [DOI] [PubMed] [Google Scholar]
- Difilippantonio MJ, Zhu J, Chen HT, Meffre E, Nussenzweig MC, Max EE, Ried T, Nussenzweig A. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature. 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: Variations on a theme? Curr Opin Cell Biol. 2001;13:225–231. doi: 10.1016/s0955-0674(00)00201-5. [DOI] [PubMed] [Google Scholar]
- Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J, Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, Manis JP, Horner J, DePinho RA, Alt FW. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- Fukumura R, Araki R, Fujimori A, Tsutsumi Y, Kurimasa A, Li GC, Chen DJ, Tatsumi K, Abe M. Signal joint formation is also impaired in DNA-dependent protein kinase catalytic subunit knockout cells. J Immunol. 2000;165:3883–3889. doi: 10.4049/jimmunol.165.7.3883. [DOI] [PubMed] [Google Scholar]
- Gellert M. Recent advances in understanding V(D)J recombination. Adv Immunol. 1997;64:39–64. doi: 10.1016/s0065-2776(08)60886-x. [DOI] [PubMed] [Google Scholar]
- Giaccia AJ, Kastan MB. The complexity of p53 modulation: Emerging patterns from divergent signals. Genes & Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- Hiom K, Gellert M. Assembly of a 12/23 paired signal complex: A critical control point in V(D)J recombination. Mol Cell. 1998;1:1011–1019. doi: 10.1016/s1097-2765(00)80101-x. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Arlett CF, Lieber MR. V(D)J recombination in ataxia telangiectasia, Bloom's syndrome, and a DNA ligase I-associated immunodeficiency disorder. J Biol Chem. 1993;268:20105–20109. [PubMed] [Google Scholar]
- Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller SP, et al. ATM associates with and phosphorylates p53: Mapping the region of interaction. Nat Genet. 1998;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- Liao MJ, Van Dyke T. Critical role for Atm in suppressing V(D)J recombination-driven thymic lymphoma. Genes & Dev. 1999;13:1246–1250. doi: 10.1101/gad.13.10.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber MR, Hesse JE, Lewis S, Bosma GC, Rosenberg N, Mizuuchi K, Bosma MJ, Gellert M. The defect in murine severe combined immune deficiency: Joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Liyanage M, Weaver Z, Barlow C, Coleman A, Pankratz DG, Anderson S, Wynshaw-Boris A, Ried T. Abnormal rearrangement within the alpha/delta T-cell receptor locus in lymphomas from Atm-deficient mice. Blood. 2000;96:1940–1946. [PubMed] [Google Scholar]
- Morrell D, Cromartie E, Swift M. Mortality and cancer incidence in 263 patients with ataxia-telangiectasia. J Natl Cancer Inst. 1986;77:89–92. [PubMed] [Google Scholar]
- Orlando V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem Sci. 2000;25:99–104. doi: 10.1016/s0968-0004(99)01535-2. [DOI] [PubMed] [Google Scholar]
- Petiniot LK, Weaver Z, Barlow C, Shen R, Eckhaus M, Steinberg SM, Ried T, Wynshaw-Boris A, Hodes RJ. Recombinase-activating gene (RAG) 2-mediated V(D)J recombination is not essential for tumorigenesis in Atm-deficient mice. Proc Natl Acad Sci. 2000;97:6664–6669. doi: 10.1073/pnas.97.12.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden DA, Gellert M. Formation and resolution of double-strand break intermediates in V(D)J rearrangement. Genes & Dev. 1995;9:2409–2420. doi: 10.1101/gad.9.19.2409. [DOI] [PubMed] [Google Scholar]
- Rotman G, Shiloh Y. ATM: A mediator of multiple responses to genotoxic stress. Oncogene. 1999;18:6135–6144. doi: 10.1038/sj.onc.1203124. [DOI] [PubMed] [Google Scholar]
- Schlissel M, Constantinescu A, Morrow T, Baxter M, Peng A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5′-phosphorylated, RAG-dependent, and cell cycle regulated. Genes & Dev. 1993;7:2520–2532. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Metcalfe JA, Thick J, Mak YF. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996;87:423–438. [PubMed] [Google Scholar]
- Thiebe R, Schable KF, Bensch A, Brensing-Kuppers J, Heim V, Kirschbaum T, Mitlohner H, Ohnrich M, Pourrajabi S, Roschenthaler F, et al. The variable genes and gene families of the mouse immunoglobulin κ locus. Eur J Immunol. 1999;29:2072–2081. doi: 10.1002/(SICI)1521-4141(199907)29:07<2072::AID-IMMU2072>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Vanasse GJ, Halbrook J, Thomas S, Burgess A, Hoekstra MF, Disteche CM, Willerford DM. Genetic pathway to recurrent chromosome translocations in murine lymphoma involves V(D)J recombinase. J Clin Invest. 1999;103:1669–1675. doi: 10.1172/JCI6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal CH, Rowan S, Schmaltz C, Elson A, Fisher DE, Leder P. atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet. 1997;16:397–401. doi: 10.1038/ng0897-397. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yang EM, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol Cell Biol. 1998;18:4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Roth DB. Characterization of coding ends in thymocytes of scid mice: Implications for the mechanism of V(D)J recombination. Immunity. 1995;2:101–112. doi: 10.1016/1074-7613(95)90082-9. [DOI] [PubMed] [Google Scholar]