Abstract
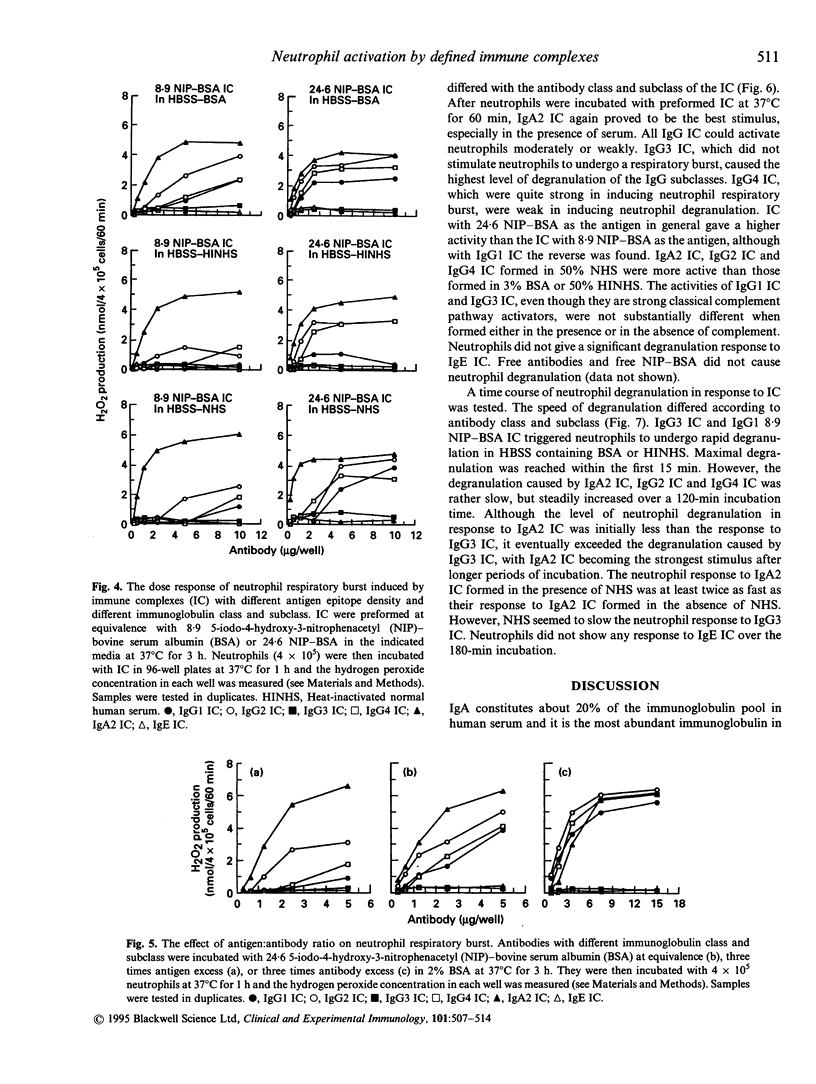
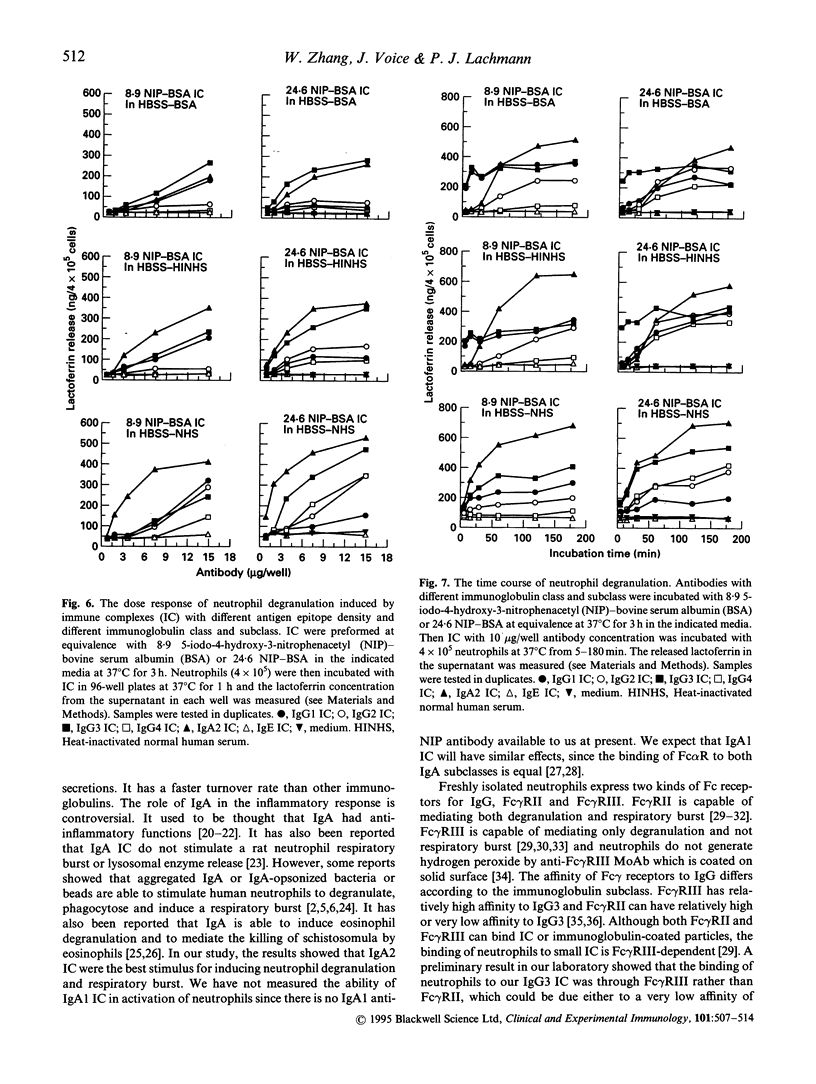
Defined immune complexes (IC) were used to compare the effect of antibodies of different classes and subclasses on neutrophil respiratory burst and degranulation. IC were made from 5-iodo-4-hydroxy-3-nitrophenacetyl (NIP) conjugated to bovine serum albumin (BSA) and chimaeric mouse-human anti-NIP monoclonal antibodies including IgA2, IgE and all four IgG subclasses. The activation of neutrophils by IC depended on antibody class and subclass, on antigen epitope density, on antigen: antibody ratio and on the medium used. The ability to generate the respiratory burst showed a different pattern to the ability to give rise to degranulation. Compared with other IC, IgA2 IC provided the strongest stimulus for neutrophil activation. IgG1 IC, IgG2 IC and IgG4 IC activated neutrophils moderately or weakly IgG3 IC were unable to stimulate the respiratory burst, but could cause strong degranulation. IgE IC could hardly cause any neutrophil response. Neutrophil degranulation in response to IgG3 IC in serum-free medium or heat-inactivated serum was fast, and it quickly reached maximum. Degranulation caused by IgA IC was relatively slow, but gradually increased during incubation. The activity of IgG1 IC, IgG2 IC and IgG4 IC generated a respiratory burst increased with antibody excess and decreased with antigen excess. The activity of IgA2 IC, however, was not affected by change of antigen and antibody ratio. A specific role of serum, possibly due to complement, was found in enhancing degranulation, both temporally and quantitatively, by IgA2 IC.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aase A., Michaelsen T. E. Opsonophagocytic activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand J Immunol. 1994 Jun;39(6):581–587. doi: 10.1111/j.1365-3083.1994.tb03416.x. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh R. I., Fujisawa T., Mestecky J., Kyle R. A., Gleich G. J. IgA-induced eosinophil degranulation. J Immunol. 1989 Apr 1;142(7):2393–2400. [PubMed] [Google Scholar]
- Albrechtsen M., Yeaman G. R., Kerr M. A. Characterization of the IgA receptor from human polymorphonuclear leucocytes. Immunology. 1988 Jun;64(2):201–205. [PMC free article] [PubMed] [Google Scholar]
- Brüggemann M., Williams G. T., Bindon C. I., Clark M. R., Walker M. R., Jefferis R., Waldmann H., Neuberger M. S. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987 Nov 1;166(5):1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham W. W., Blackburn W. D., Jr Fixation of C3 to IgG attenuates neutrophil HOCl generation and collagenase activation. J Immunol. 1993 Jul 15;151(2):949–958. [PubMed] [Google Scholar]
- Crockett-Torabi E., Fantone J. C. Soluble and insoluble immune complexes activate human neutrophil NADPH oxidase by distinct Fc gamma receptor-specific mechanisms. J Immunol. 1990 Nov 1;145(9):3026–3032. [PubMed] [Google Scholar]
- Czop J., Nussenzweig V. Studies on the mechanism of solubilization of immune precipitates by serum. J Exp Med. 1976 Mar 1;143(3):615–630. doi: 10.1084/jem.143.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne D. W., Richardson B. A., Jones F. M., Clark M., Thorne K. J., Butterworth A. E. The use of mouse/human chimaeric antibodies to investigate the roles of different antibody isotypes, including IgA2, in the killing of Schistosoma mansoni schistosomula by eosinophils. Parasite Immunol. 1993 Mar;15(3):181–185. doi: 10.1111/j.1365-3024.1993.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Gorter A., Hiemstra P. S., Leijh P. C., van der Sluys M. E., van den Barselaar M. T., van Es L. A., Daha M. R. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology. 1987 Jul;61(3):303–309. [PMC free article] [PubMed] [Google Scholar]
- Graham I. L., Lefkowith J. B., Anderson D. C., Brown E. J. Immune complex-stimulated neutrophil LTB4 production is dependent on beta 2 integrins. J Cell Biol. 1993 Mar;120(6):1509–1517. doi: 10.1083/jcb.120.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Hostoffer R. W., Krukovets I., Berger M. Increased Fc alpha R expression and IgA-mediated function on neutrophils induced by chemoattractants. J Immunol. 1993 May 15;150(10):4532–4540. [PubMed] [Google Scholar]
- Huizinga T. W., Dolman K. M., van der Linden N. J., Kleijer M., Nuijens J. H., von dem Borne A. E., Roos D. Phosphatidylinositol-linked FcRIII mediates exocytosis of neutrophil granule proteins, but does not mediate initiation of the respiratory burst. J Immunol. 1990 Feb 15;144(4):1432–1437. [PubMed] [Google Scholar]
- Huizinga T. W., Kerst M., Nuyens J. H., Vlug A., von dem Borne A. E., Roos D., Tetteroo P. A. Binding characteristics of dimeric IgG subclass complexes to human neutrophils. J Immunol. 1989 Apr 1;142(7):2359–2364. [PubMed] [Google Scholar]
- Huizinga T. W., Roos D., von dem Borne A. E. Neutrophil Fc-gamma receptors: a two-way bridge in the immune system. Blood. 1990 Mar 15;75(6):1211–1214. [PubMed] [Google Scholar]
- Huizinga T. W., van Kemenade F., Koenderman L., Dolman K. M., von dem Borne A. E., Tetteroo P. A., Roos D. The 40-kDa Fc gamma receptor (FcRII) on human neutrophils is essential for the IgG-induced respiratory burst and IgG-induced phagocytosis. J Immunol. 1989 Apr 1;142(7):2365–2369. [PubMed] [Google Scholar]
- Jack R. M., Fearon D. T. Altered surface distribution of both C3b receptors and Fc receptors on neutrophils induced by anti-C3b receptor or aggregated IgG. J Immunol. 1984 Jun;132(6):3028–3033. [PubMed] [Google Scholar]
- Johnson A., Harkin S., Steward M. W., Whaley K. The effects of immunoglobulin isotype and antibody affinity on complement-mediated inhibition of immune precipitation and solubilization. Mol Immunol. 1987 Nov;24(11):1211–1217. doi: 10.1016/0161-5890(87)90168-4. [DOI] [PubMed] [Google Scholar]
- Kerr M. A. The structure and function of human IgA. Biochem J. 1990 Oct 15;271(2):285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucisano Valim Y. M., Lachmann P. J. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991 Apr;84(1):1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucisano Y. M., Mantovani B. The role of complement in the stimulation of lysosomal enzyme release by polymorphonuclear leucocytes induced by immune complexes of IgG and of IgM. Immunology. 1988 Oct;65(2):171–175. [PMC free article] [PubMed] [Google Scholar]
- Mazengera R. L., Kerr M. A. The specificity of the IgA receptor purified from human neutrophils. Biochem J. 1990 Nov 15;272(1):159–165. doi: 10.1042/bj2720159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Russell M. W., Reinholdt J., Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989 Dec;19(12):2243–2249. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- Schifferli J. A., Bartolotti S. R., Peters D. K. Inhibition of immune precipitation by complement. Clin Exp Immunol. 1980 Nov;42(2):387–394. [PMC free article] [PubMed] [Google Scholar]
- Schifferli J. A., Taylor R. P. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989 Apr;35(4):993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- Sehgal G., Zhang K., Todd R. F., 3rd, Boxer L. A., Petty H. R. Lectin-like inhibition of immune complex receptor-mediated stimulation of neutrophils. Effects on cytosolic calcium release and superoxide production. J Immunol. 1993 May 15;150(10):4571–4580. [PubMed] [Google Scholar]
- Stengelin S., Stamenkovic I., Seed B. Isolation of cDNAs for two distinct human Fc receptors by ligand affinity cloning. EMBO J. 1988 Apr;7(4):1053–1059. doi: 10.1002/j.1460-2075.1988.tb02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W., Kerr M. A. The specificity of the human neutrophil IgA receptor (Fc alpha R) determined by measurement of chemiluminescence induced by serum or secretory IgA1 or IgA2. Immunology. 1990 Nov;71(3):328–334. [PMC free article] [PubMed] [Google Scholar]
- Tosi M. F., Berger M. Functional differences between the 40 kDa and 50 to 70 kDa IgG Fc receptors on human neutrophils revealed by elastase treatment and antireceptor antibodies. J Immunol. 1988 Sep 15;141(6):2097–2103. [PubMed] [Google Scholar]
- Truong M. J., Gruart V., Kusnierz J. P., Papin J. P., Loiseau S., Capron A., Capron M. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J Exp Med. 1993 Jan 1;177(1):243–248. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Williams R. C., Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976 Nov 2;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. A., Hagenlocker B. E., Stubbs E. B., Jr, Sandborg R. R., Agranoff B. W., Ward P. A. Signal transduction events and Fc gamma R engagement in human neutrophils stimulated with immune complexes. J Immunol. 1991 Jan 15;146(2):735–741. [PubMed] [Google Scholar]
- Warren J. S., Kunkel S. L., Johnson K. J., Ward P. A. In vitro activation of rat neutrophils and alveolar macrophages with IgA and IgG immune complexes. Implications for immune complex-induced lung injury. Am J Pathol. 1987 Dec;129(3):578–588. [PMC free article] [PubMed] [Google Scholar]
- Willis H. E., Browder B., Feister A. J., Mohanakumar T., Ruddy S. Monoclonal antibody to human IgG Fc receptors. Cross-linking of receptors induces lysosomal enzyme release and superoxide generation by neutrophils. J Immunol. 1988 Jan 1;140(1):234–239. [PubMed] [Google Scholar]
- Wright D. G. Human neutrophil degranulation. Methods Enzymol. 1988;162:538–551. doi: 10.1016/0076-6879(88)62102-1. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M., Eckmann C. M., Roos D. Characterization of the interaction of human eosinophils and neutrophils with opsonized particles. J Immunol. 1985 Aug;135(2):1378–1384. [PubMed] [Google Scholar]
- Yazdanbakhsh M., Eckmann C. M., Roos D. Characterization of the interaction of human eosinophils and neutrophils with opsonized particles. J Immunol. 1985 Aug;135(2):1378–1384. [PubMed] [Google Scholar]
- Zhang W., Lachmann P. J. Glycosylation of IgA is required for optimal activation of the alternative complement pathway by immune complexes. Immunology. 1994 Jan;81(1):137–141. [PMC free article] [PubMed] [Google Scholar]
- Zhou M. J., Brown E. J. CR3 (Mac-1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc gamma RIII and tyrosine phosphorylation. J Cell Biol. 1994 Jun;125(6):1407–1416. doi: 10.1083/jcb.125.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Todd R. F., 3rd, van de Winkel J. G., Petty H. R. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc gamma receptor III on human neutrophils. Possible role of lectin-like interactions. J Immunol. 1993 Apr 1;150(7):3030–3041. [PubMed] [Google Scholar]


