Abstract
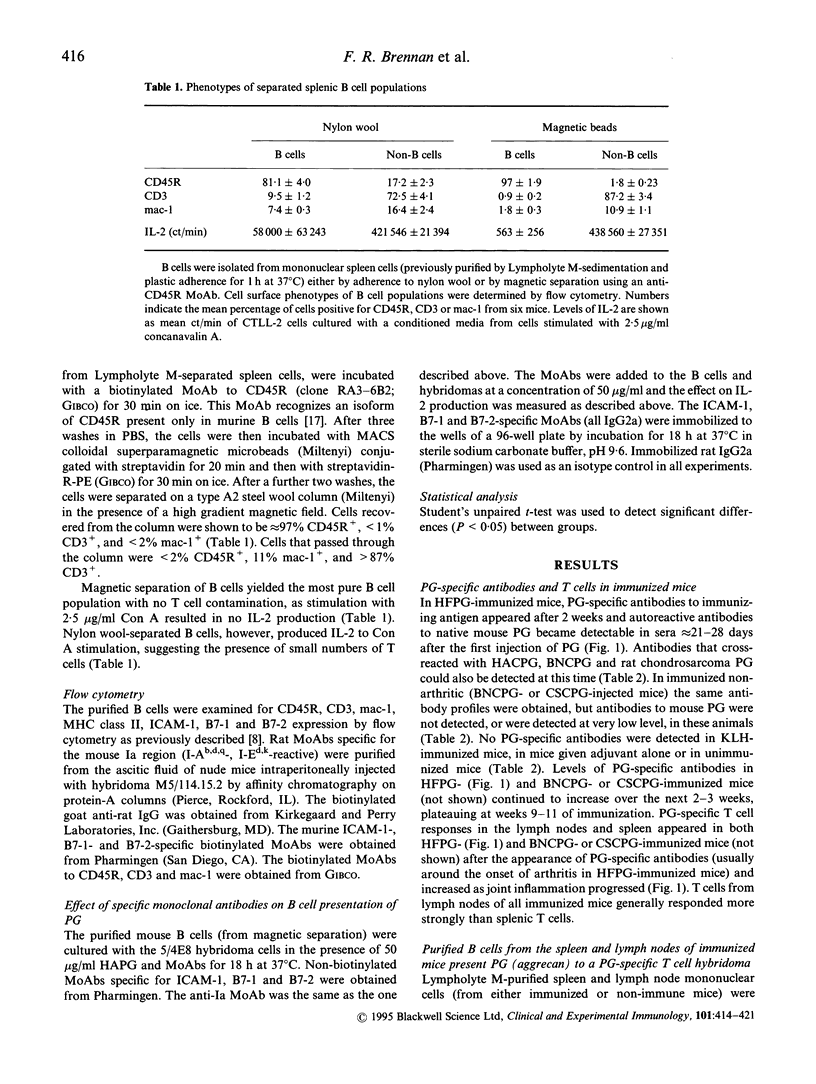
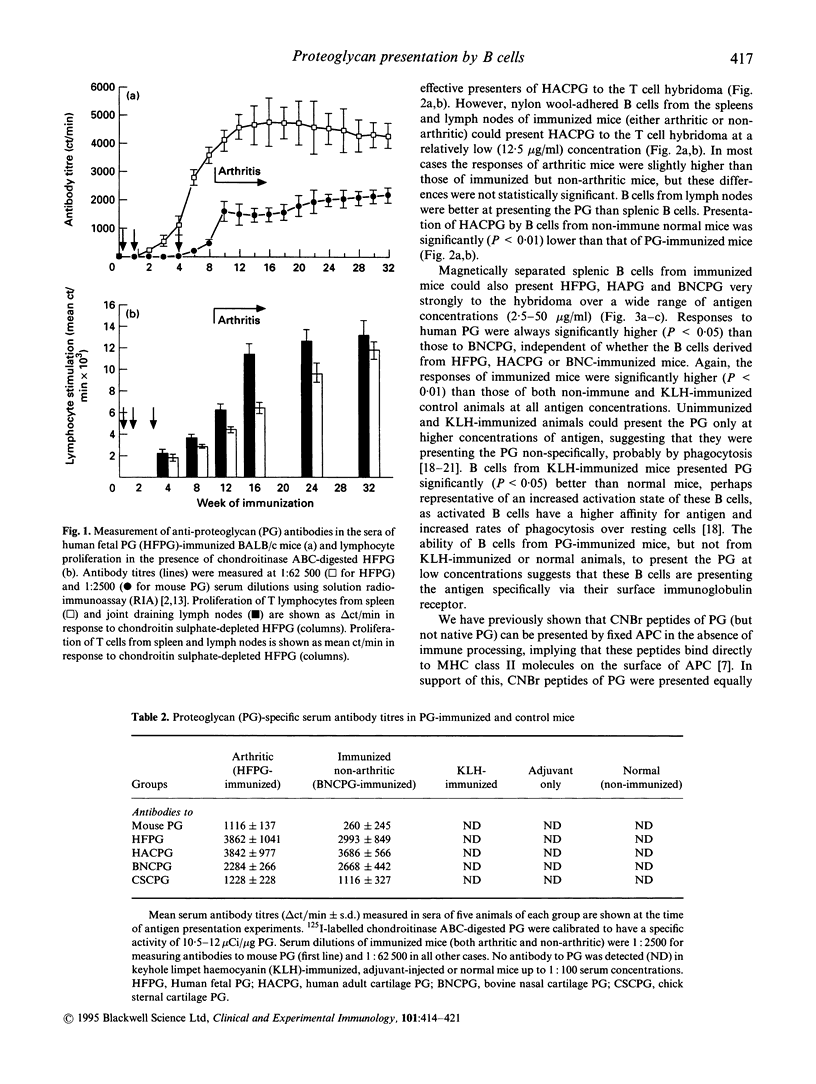
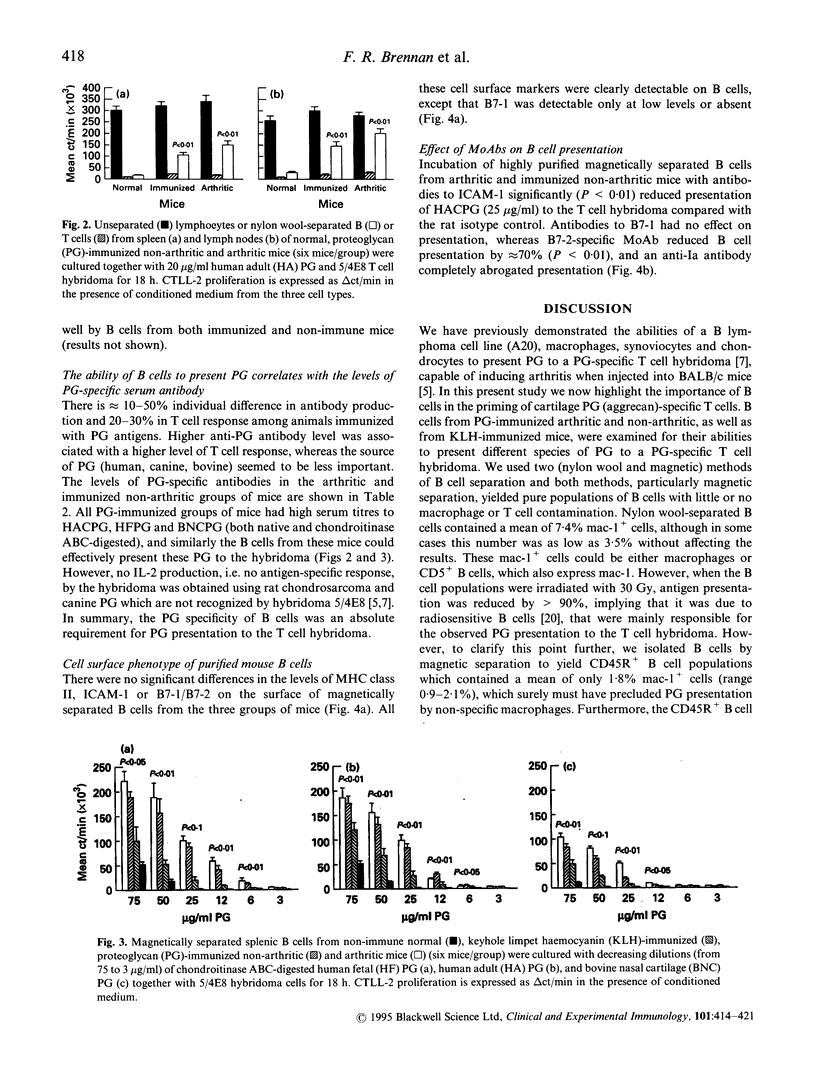
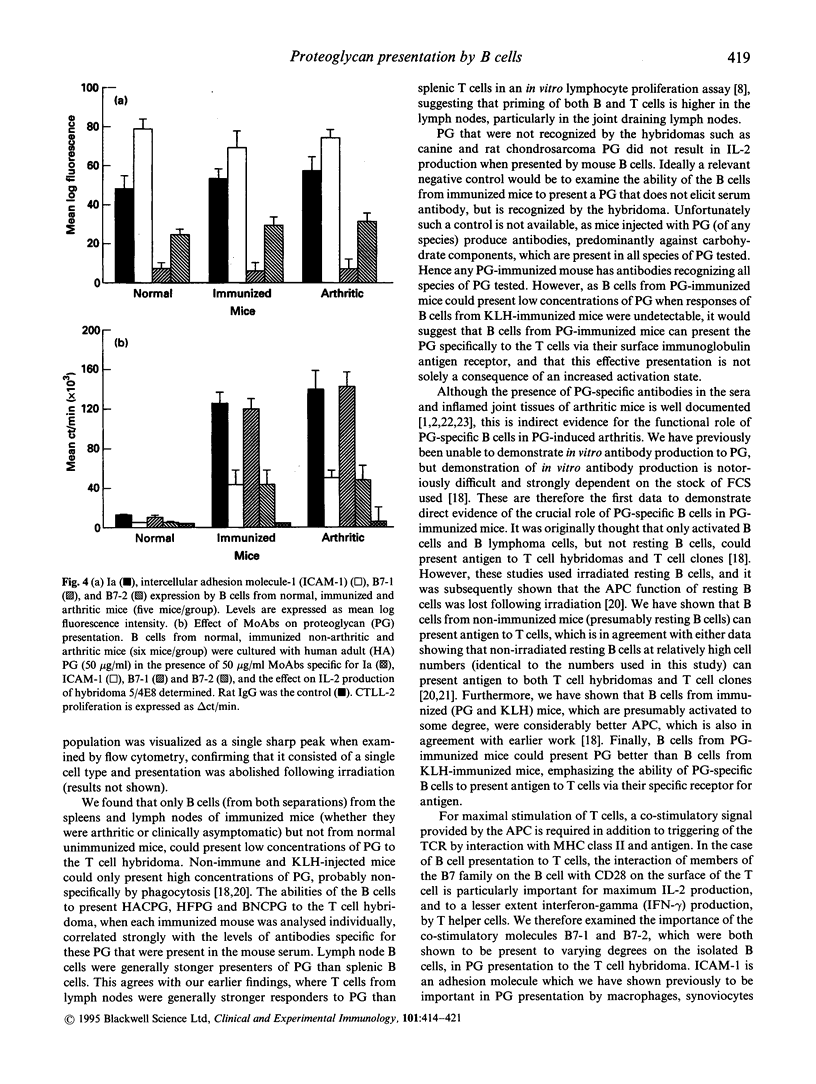
Cartilage proteoglycan (aggrecan)-induced polyarthritis in BALB/c mice is characterized by chronic inflammation and destruction of joint tissues similar to that observed in human rheumatoid arthritis. The immunization of mice with fetal human proteoglycan (PG) elicits specific antibodies to the immunizing antigen of which a population cross-reacts with native mouse PG. This (auto)antibody production is immediately followed by an explosive proliferation of autoreactive T cells, suggesting that PG-specific B cells may participate in antigen presentation of PG to autoreactive T cells. We therefore isolated B cells from the spleens and lymph nodes of PG-immunized mice and examined their ability to present PG to a PG-specific T cell hybridoma. The antigen-specific T cell responses elicited by B cells from PG-immunized mice (both arthritic and clinically asymptomatic) were markedly higher than those of non-immune mice and keyhole limpet haemocyanin (KLH)-immunized mice, and these B cells could present low PG concentrations. Levels of B cell presentation corresponded with the serum levels of PG-specific antibodies, implying that these B cells were presenting the PG specifically via their surface immunoglobulin. This B cell-T cell interaction was strongly dependent on MHC class II/T cell receptor (TCR), LFA-1/intercellular adhesion molecule-1 (ICAM-1) and CD28/B7 interactions, as antibodies to Ia, ICAM-1 and B7-2 (but not to B7-1) markedly reduced presentation. These data indicate that PG-specific B cells may play an essential role in governing the development of PG-induced arthritis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando D. G., Sercarz E. E., Hahn B. H. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J Immunol. 1987 May 15;138(10):3185–3190. [PubMed] [Google Scholar]
- Ashwell J. D., DeFranco A. L., Paul W. E., Schwartz R. H. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984 Mar 1;159(3):881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. R., Mikecz K., Buzás E. I., Glant T. T. Interferon-gamma but not granulocyte/macrophage colony-stimulating factor augments proteoglycan presentation by synovial cells and chondrocytes to an autopathogenic T cell hybridoma. Immunol Lett. 1995 Feb;45(1-2):87–91. doi: 10.1016/0165-2478(94)00249-q. [DOI] [PubMed] [Google Scholar]
- Brennan F. R., Negroiu G., Buzás E. I., Fülöp C., Holló K., Mikecz K., Glant T. T. Presentation of cartilage proteoglycan to a T cell hybridoma derived from a mouse with proteoglycan-induced arthritis. Clin Exp Immunol. 1995 Apr;100(1):104–110. doi: 10.1111/j.1365-2249.1995.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzás E. I., Mikecz K., Brennan F. R., Glant T. T. Mediators and autopathogenic effector cells in proteoglycan-induced arthritic and clinically asymptomatic BALB/c mice. Cell Immunol. 1994 Oct 15;158(2):292–304. doi: 10.1006/cimm.1994.1277. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Dayer E., Mathai L., Glant T. T., Mikecz K., Poole A. R. Cartilage proteoglycan-induced arthritis in BALB/c mice. Antibodies that recognize human and mouse cartilage proteoglycan and can cause depletion of cartilage proteoglycan with little or no synovitis. Arthritis Rheum. 1990 Sep;33(9):1394–1405. doi: 10.1002/art.1780330912. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Fülöp C., Mikecz K., Buzás E., Molnár G., Erhardt P. Proteoglycan-specific autoreactive antibodies and T-lymphocytes in experimental arthritis and human rheumatoid joint diseases. Biochem Soc Trans. 1990 Oct;18(5):796–799. doi: 10.1042/bst0180796. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Arzoumanian A., Poole A. R. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987 Feb;30(2):201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Poole A. R. Monoclonal antibodies to different protein-related epitopes of human articular cartilage proteoglycans. Biochem J. 1986 Feb 15;234(1):31–41. doi: 10.1042/bj2340031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Roughley P. J., Buzás E., Poole A. R. Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem J. 1986 May 15;236(1):71–75. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Kim K. J., Green I., Paul W. E. Ia antigen-bearing B cell tumor lines can present protein antigen and alloantigen in a major histocompatibility complex-restricted fashion to antigen-reactive T cells. J Exp Med. 1982 Feb 1;155(2):445–459. doi: 10.1084/jem.155.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresina T. F., Yoo J. U., Goldberg V. M. Evidence that a humoral immune response to autologous cartilage proteoglycan can participate in the induction of cartilage pathology. Arthritis Rheum. 1988 Feb;31(2):248–257. doi: 10.1002/art.1780310213. [DOI] [PubMed] [Google Scholar]
- Lenschow D. J., Su G. H., Zuckerman L. A., Nabavi N., Jellis C. L., Gray G. S., Miller J., Bluestone J. A. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male D. K., Pryce G., Cooke A., Hutchings P., Marshall-Clarke S., Roitt I. M. T and B cell connections in experimentally induced autoimmunity. Ann N Y Acad Sci. 1986;475:94–105. doi: 10.1111/j.1749-6632.1986.tb20859.x. [DOI] [PubMed] [Google Scholar]
- Mikecz K., Glant T. T., Buzás E., Poole A. R. Cartilage proteoglycans as potential autoantigens in humans and in experimental animals. Agents Actions. 1988 Feb;23(1-2):63–66. doi: 10.1007/BF01967190. [DOI] [PubMed] [Google Scholar]
- Mikecz K., Glant T. T. Migration and homing of lymphocytes to lymphoid and synovial tissues in proteoglycan-induced murine arthritis. Arthritis Rheum. 1994 Sep;37(9):1395–1403. doi: 10.1002/art.1780370919. [DOI] [PubMed] [Google Scholar]
- Mikecz K., Glant T. T., Poole A. R. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987 Mar;30(3):306–318. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Mondino A., Jenkins M. K. Surface proteins involved in T cell costimulation. J Leukoc Biol. 1994 Jun;55(6):805–815. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J., Wilbrink B., Helle M., van Roy J. L., den Otter W., Swaak A. J., Huber-Bruning O. Interleukin-1-induced interleukin-6 is required for the inhibition of proteoglycan synthesis by interleukin-1 in human articular cartilage. Arthritis Rheum. 1990 Nov;33(11):1695–1701. doi: 10.1002/art.1780331113. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmei M., Masuda K., Kikuchi T., Shimomura Y. Interleukin 1, tumor necrosis factor, and interleukin 6 as mediators of cartilage destruction. Semin Arthritis Rheum. 1989 Feb;18(3 Suppl 1):27–32. doi: 10.1016/0049-0172(89)90081-4. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Lin H., Brady W., Leiden J. M., Wei R. Q., Gibson M. L., Zheng X. G., Myrdal S., Gordon D. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]


