Abstract
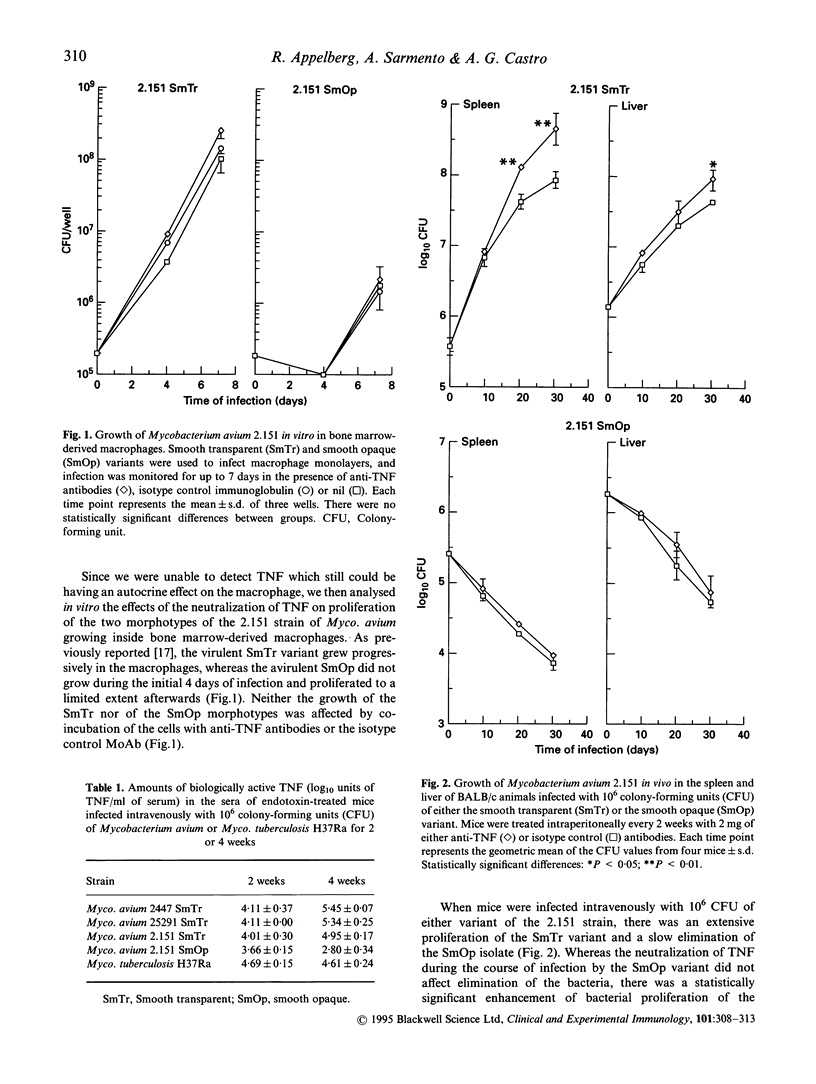
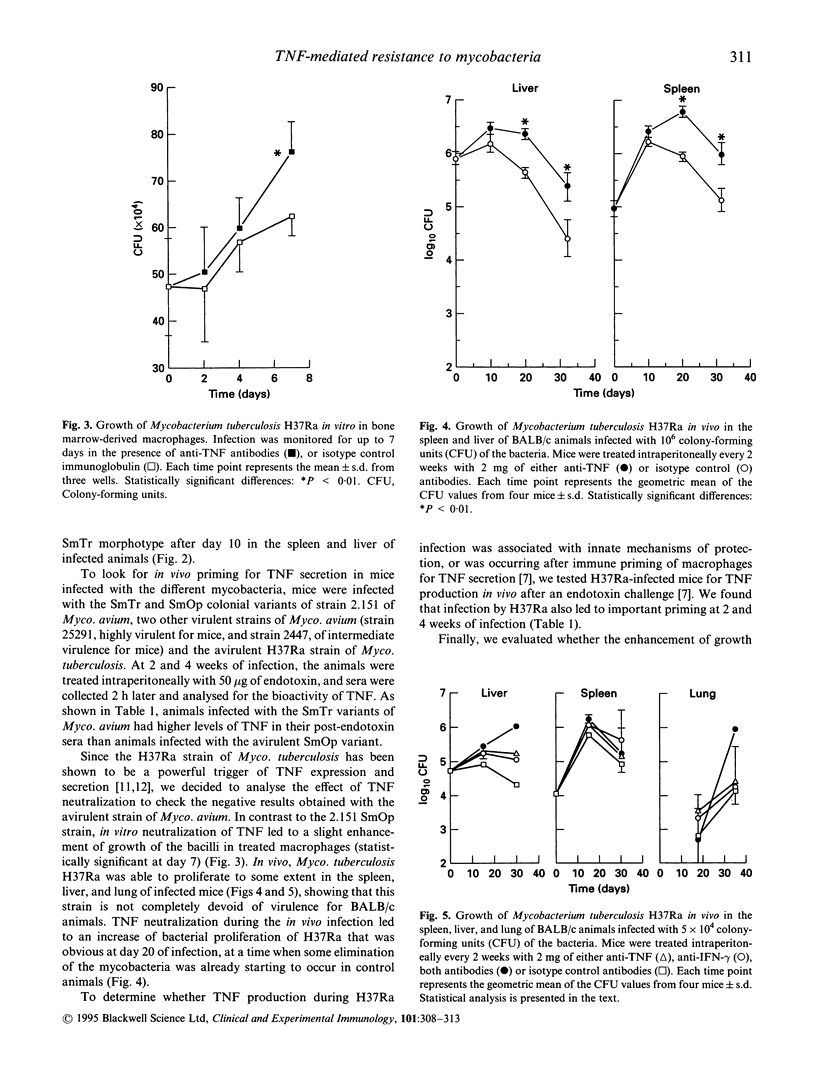
The relative virulence of different isolates of Mycobacterium avium has been linked to their capacity to trigger the secretion of TNF from the macrophages they infect. Smooth opaque (SmOp) variants of Myco. avium have been shown to trigger higher expression of TNF-alpha by macrophages in vitro than the smooth transparent (SmTr) variants. To analyse the role of TNF in resistance to infection by Myco. avium, we studied the infection by two different morphotypes of strain 2.151 of Myco. avium both in vitro and in vivo in the presence or absence of neutralizing antibodies to TNF. No effects were found in vitro regarding the growth of either isolate of Myco. avium. In vivo, only the virulent SmTr morphotype showed enhanced growth in the presence of the neutralizing antibodies. This enhancement occurred relatively late when priming for TNF secretion in vivo was evident. Among four isolates of Myco. avium, three virulent ones induced a marked priming for TNF release and one avirulent strain did not. Mycobacterium tuberculosis H37Ra, which is very active in inducing TNF release due to its lipoarabinomannan moiety, was used to compare with the previous results. The growth of H37Ra in macrophages was increased in vitro by the neutralization of TNF and neutralization of either TNF and/or interferon-gamma (IFN-gamma) enhanced the in vivo proliferation of this microbe in the spleen and liver of infected animals, whereas only the combination of both anti-TNF and anti-IFN-gamma enhanced bacterial proliferation in the lung. We conclude that resistance to the avirulent strains of Myco. avium did not involve TNF, but rather antimicrobial mechanisms expressed constitutively in the mononuclear phagocytes. In contrast, TNF plays an important role in the control of Myco. tuberculosis H37Ra infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Castro A. G., Pedrosa J., Silva R. A., Orme I. M., Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994 Sep;62(9):3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Orme I. M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993 Nov;80(3):352–359. [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin Exp Immunol. 1992 Mar;87(3):379–385. doi: 10.1111/j.1365-2249.1992.tb03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982 Apr;150(1):381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle J. T., Brennan P. J. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 1989 Jun;171(6):3465–3470. doi: 10.1128/jb.171.6.3465-3470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Oxidative and non-oxidative intracellular killing of Mycobacterium avium complex. Microb Pathog. 1989 Oct;7(4):289–298. doi: 10.1016/0882-4010(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. AIDS-related mycobacterial disease. Springer Semin Immunopathol. 1988;10(4):375–391. doi: 10.1007/BF02053847. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterium avium-complex infections and development of the acquired immunodeficiency syndrome: casual opportunist or causal cofactor? Int J Lepr Other Mycobact Dis. 1986 Sep;54(3):458–474. [PubMed] [Google Scholar]
- Furney S. K., Skinner P. S., Roberts A. D., Appelberg R., Orme I. M. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992 Oct;60(10):4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Inderlied C. B., Kemper C. A., Bermudez L. E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993 Jul;6(3):266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan P. R., Richman D. D., Kornbluth R. S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990 Aug;58(8):2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa J., Flórido M., Kunze Z. M., Castro A. G., Portaels F., McFadden J., Silva M. T., Appelberg R. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin Exp Immunol. 1994 Nov;98(2):210–216. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T. I., Barton C. H., Chatterjee D., Blackwell J. M. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-alpha. J Immunol. 1993 Mar 1;150(5):1886–1896. [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Saito H. Macrophage chemiluminescence induced by interaction with transparent and opaque colonial variants of Mycobacterium intracellulare. J Gen Microbiol. 1993 Dec;139(12):3011–3015. doi: 10.1099/00221287-139-12-3011. [DOI] [PubMed] [Google Scholar]


