Abstract
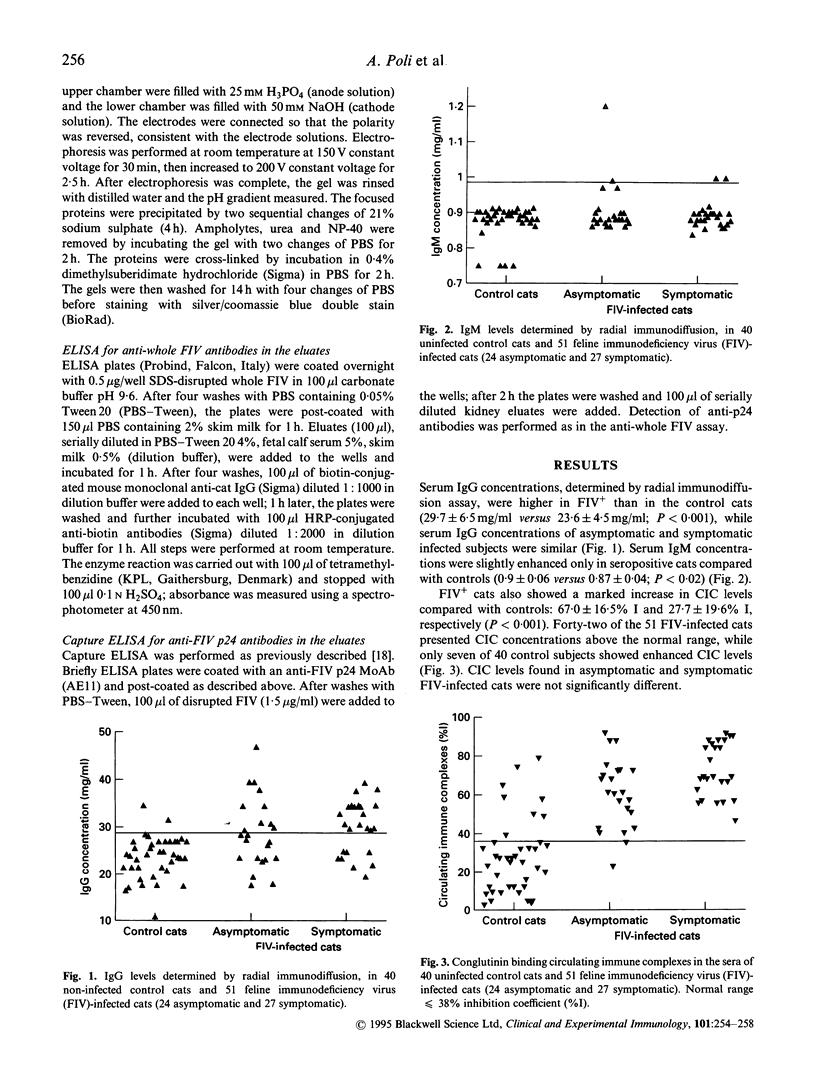
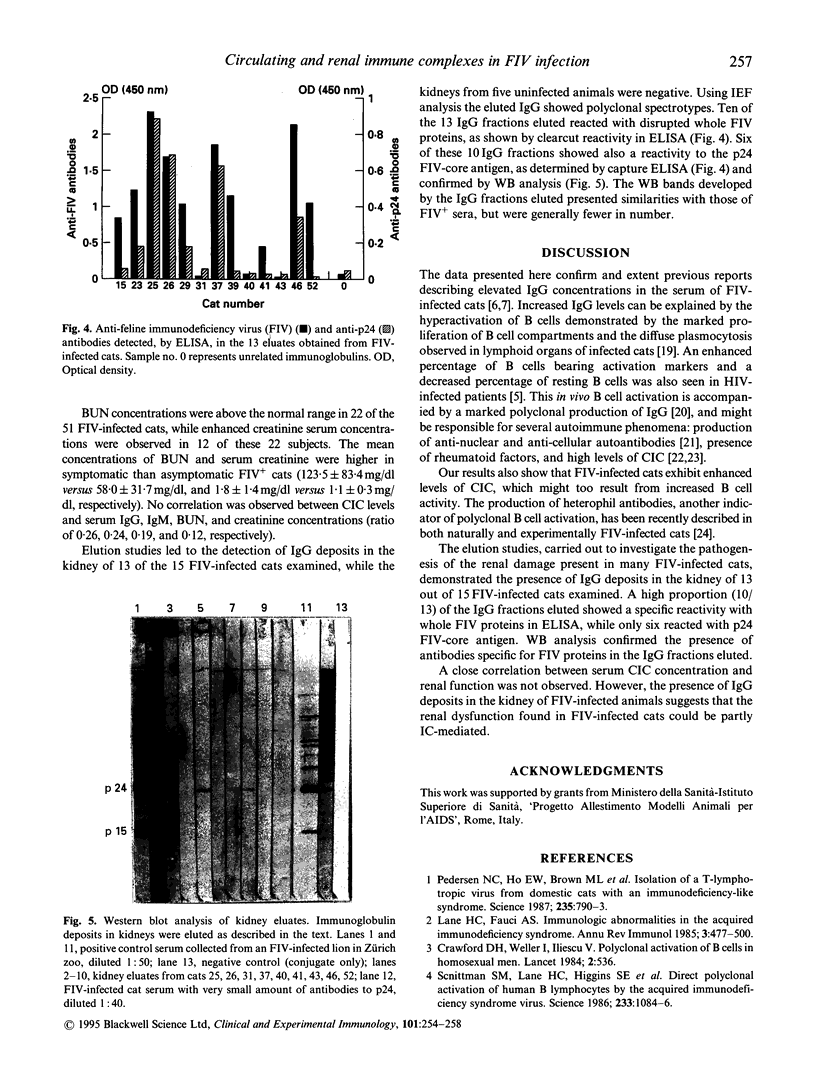
Total immunoglobulin content and concentration of immune complexes (IC) were determined in the sera of 51 cats infected with feline immunodeficiency virus (FIV) and of 40 controls. IgG and IgM were quantified by radial immunodiffusion and circulating IC (CIC) by the CIC-conglutinin assay. IgG fractions were obtained by acid elution from kidney tissues of 15 FIV-infected and five negative control cats to investigate the possible role of IC in the genesis of renal damage observed in infected animals. Mean concentrations of IgG and circulating IC were higher in FIV-infected cats than in controls (29.6 +/- 6.7 versus 23.0 +/- 1.9 mg/dl (mean +/- s.d.) P < 0.001; and 66.5 +/- 17.0 versus 27.4 +/- 19.9% I, P < 0.001, respectively), while IgM levels were only slightly increased (0.9 +/- 0.05 versus 0.87 +/- 0.04 mg/dl, P < 0.02). Immunoglobulin fractions were eluted from 10 of the 15 renal tissue samples from FIV-infected cats and were found to be polyclonal and at least partly specific for FIV antigens. These findings confirm the presence of a B cell activation in FIV-infected cats and demonstrate the presence of high levels of CIC in their sera. The presence of immune deposits in renal tissues suggests that IC might play a role in the pathogenesis of the renal damage observed in FIV-infected cats.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese L. H. The rheumatic manifestations of infection with the human immunodeficiency virus. Semin Arthritis Rheum. 1989 May;18(4):225–239. doi: 10.1016/0049-0172(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Flynn J. N., Cannon C. A., Lawrence C. E., Jarrett O. Polyclonal B-cell activation in cats infected with feline immunodeficiency virus. Immunology. 1994 Apr;81(4):626–630. [PMC free article] [PubMed] [Google Scholar]
- Katz I. R., Krown S. E., Safai B., Oettgen H. F., Hoffmann M. K. Antigen-specific and polyclonal B-cell responses in patients with acquired immunodeficiency disease syndrome. Clin Immunol Immunopathol. 1986 Jun;39(3):359–367. doi: 10.1016/0090-1229(86)90164-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Fauci A. S. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu Rev Immunol. 1985;3:477–500. doi: 10.1146/annurev.iy.03.040185.002401. [DOI] [PubMed] [Google Scholar]
- Lombardi S., Poli A., Massi C., Abramo F., Zaccaro L., Bazzichi A., Malvaldi G., Bendinelli M., Garzelli C. Detection of feline immunodeficiency virus p24 antigen and p24-specific antibodies by monoclonal antibody-based assays. J Virol Methods. 1994 Mar;46(3):287–301. doi: 10.1016/0166-0934(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Manca F., Migliorini P., Bombardieri S., Celada F. An enzymatically active antigen-antibody probe to measure circulating immune complexes by competition. I. Use of Escherichia coli beta-galactosidase in the probe and of bovine conglutinin as the complex-binding reagent. Clin Immunol Immunopathol. 1980 Jun;16(2):131–141. doi: 10.1016/0090-1229(80)90197-x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Martínez-Maza O., Crabb E., Mitsuyasu R. T., Fahey J. L., Giorgi J. V. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J Immunol. 1987 Jun 1;138(11):3720–3724. [PubMed] [Google Scholar]
- McDougal J. S., Hubbard M., Nicholson J. K., Jones B. M., Holman R. C., Roberts J., Fishbein D. B., Jaffe H. W., Kaplan J. E., Spira T. J. Immune complexes in the acquired immunodeficiency syndrome (AIDS): relationship to disease manifestation, risk group, and immunologic defect. J Clin Immunol. 1985 Mar;5(2):130–138. doi: 10.1007/BF00915011. [DOI] [PubMed] [Google Scholar]
- McHugh T. M., Stites D. P., Busch M. P., Krowka J. F., Stricker R. B., Hollander H. Relation of circulating levels of human immunodeficiency virus (HIV) antigen, antibody to p24, and HIV-containing immune complexes in HIV-infected patients. J Infect Dis. 1988 Nov;158(5):1088–1091. doi: 10.1093/infdis/158.5.1088. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Poli A., Abramo F., Taccini E., Guidi G., Barsotti P., Bendinelli M., Malvaldi G. Renal involvement in feline immunodeficiency virus infection: a clinicopathological study. Nephron. 1993;64(2):282–288. doi: 10.1159/000187327. [DOI] [PubMed] [Google Scholar]
- Poli A., Giannelli C., Pistello M., Zaccaro L., Pieracci D., Bendinelli M., Malvaldi G. Detection of salivary antibodies in cats infected with feline immunodeficiency virus. J Clin Microbiol. 1992 Aug;30(8):2038–2041. doi: 10.1128/jcm.30.8.2038-2041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccia S., Blasio R., Villa P., Lazzarin A., Bonacina C., Novati R., Bini T., Memoli M., Imondi N., Zanussi C. Rheumatoid factors and circulating immune complexes in HIV-infected individuals. AIDS. 1991 Dec;5(12):1441–1446. doi: 10.1097/00002030-199112000-00005. [DOI] [PubMed] [Google Scholar]
- Rideout B. A., Lowensteine L. J., Hutson C. A., Moore P. F., Pedersen N. C. Characterization of morphologic changes and lymphocyte subset distribution in lymph nodes from cats with naturally acquired feline immunodeficiency virus infection. Vet Pathol. 1992 Sep;29(5):391–399. doi: 10.1177/030098589202900504. [DOI] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Higgins S. E., Folks T., Fauci A. S. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986 Sep 5;233(4768):1084–1086. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Ackley C. D., Zochlinski H., Louie H., Pembroke E., Torten M., Hansen H., Munn R., Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32(6):361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]