Abstract
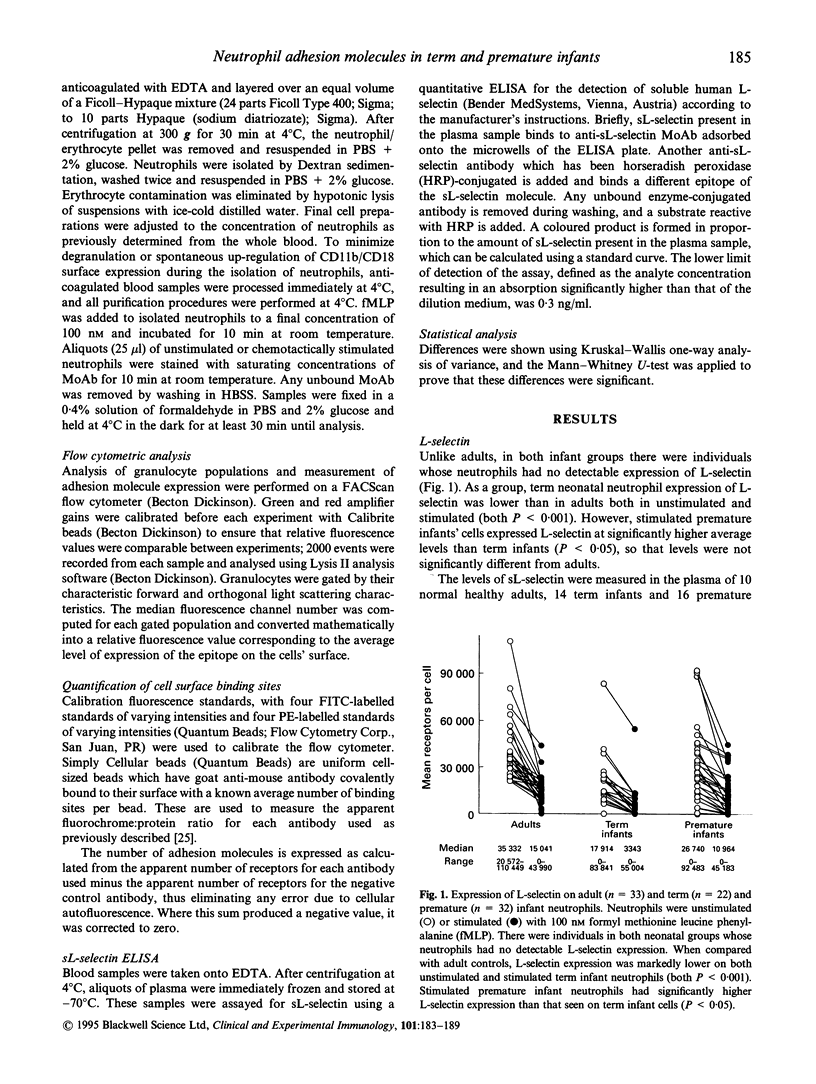
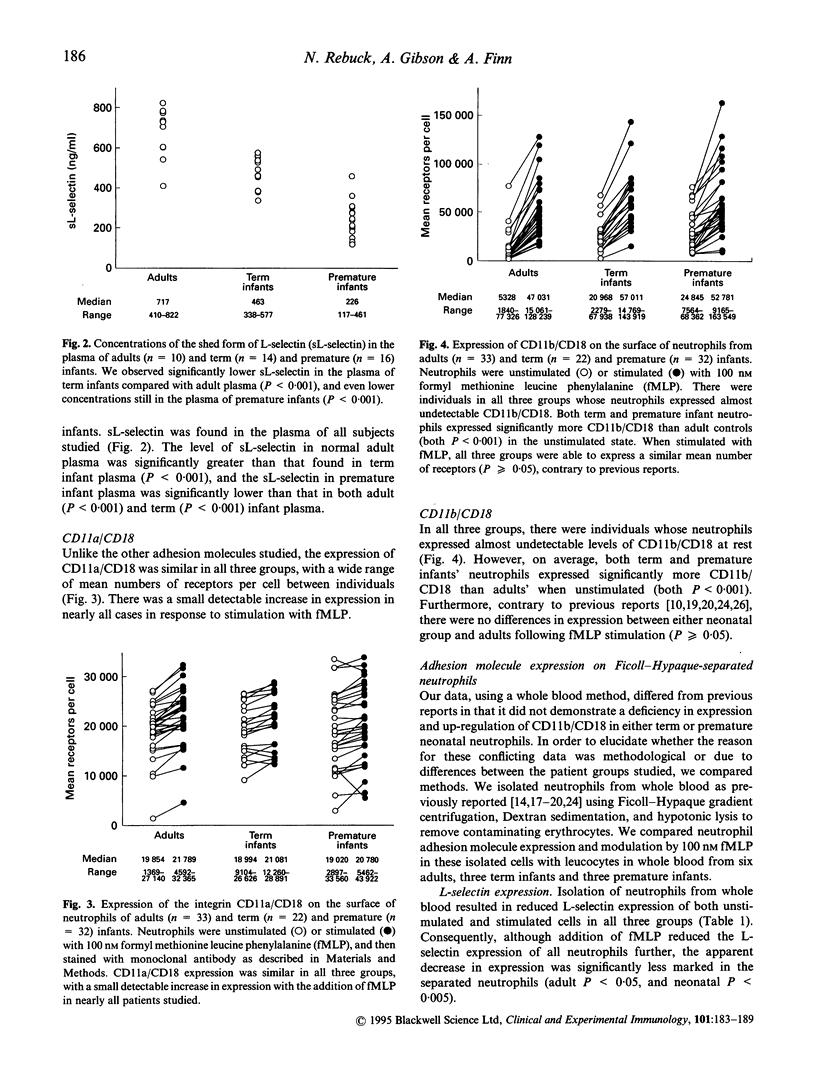
The functional deficits of neonatal neutrophils are well documented and are thought to contribute to the increased susceptibility of newborn infants to infection. We measured the adhesion molecules L-selectin, CD11a/CD18 and CD11b/CD18 on neutrophils from the cord blood of term (n = 22) and premature (n = 32) infants using a whole blood method with flow cytometry and quantitative bead standards to enumerate cell surface receptors. We also assayed plasma for the shed form of L-selectin (sL-selectin). Our results suggested that L-selectin expression on term infant neutrophils is lower than that on adult neutrophils (unstimulated and stimulated, both P < 0.001), but that stimulated premature infant cell express higher L-selectin than term infants (P < 0.05); it is possible that this deficiency is caused by physiological changes occurring around the normal time of parturition. We observed reduced sL-selectin in term infants (P < 0.001) compared with adults, and even lower concentrations in premature infants (P < 0.001). The sL-selectin concentrations in plasma may be a reflection of granulopoiesis, which may be reduced in premature infants. Our results showed increased resting neonatal neutrophil expression of CD11b/CD18 compared with adults, and the absence of any neonatal deficit of the ability to up-regulate CD11b/CD18 expression on stimulation. These findings are contrary to previous reports. Further studies suggested that the isolation procedures used in previous reports reduces the capability of the cells to respond to a formyl methionine leucine phenylalanine (fMLP) stimulus. This effect is more marked in neonatal neutrophils, suggesting that the previously reported deficiency is in fact due to the isolation techniques used rather than the cells' innate ability to up-regulate CD11b/CD18 expression. The results of our study lead us to propose that the adhesive function of neonatal neutrophils may be less defective than previously thought.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abughali N., Berger M., Tosi M. F. Deficient total cell content of CR3 (CD11b) in neonatal neutrophils. Blood. 1994 Feb 15;83(4):1086–1092. [PubMed] [Google Scholar]
- Anderson D. C., Abbassi O., Kishimoto T. K., Koenig J. M., McIntire L. V., Smith C. W. Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. J Immunol. 1991 May 15;146(10):3372–3379. [PubMed] [Google Scholar]
- Anderson D. C., Freeman K. L., Heerdt B., Hughes B. J., Jack R. M., Smith C. W. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface Mac-1 by chemotactic factors or secretagogues. Blood. 1987 Sep;70(3):740–750. [PubMed] [Google Scholar]
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Rothlein R., Marlin S. D., Krater S. S., Smith C. W. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood. 1990 Dec 15;76(12):2613–2621. [PubMed] [Google Scholar]
- Bruce M. C., Baley J. E., Medvik K. A., Berger M. Impaired surface membrane expression of C3bi but not C3b receptors on neonatal neutrophils. Pediatr Res. 1987 Mar;21(3):306–311. doi: 10.1203/00006450-198703000-00022. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Cairo M. S. Therapeutic implications of dysregulated colony-stimulating factor expression in neonates. Blood. 1993 Oct 15;82(8):2269–2272. [PubMed] [Google Scholar]
- Carr R., Pumford D., Davies J. M. Neutrophil chemotaxis and adhesion in preterm babies. Arch Dis Child. 1992 Jul;67(7 Spec No):813–817. doi: 10.1136/adc.67.7_spec_no.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenfeld L., Krause P. J., Herson V., Savidakis J., Bannon P., Maderazo E., Woronick C., Giuliano C., Banco L. Longitudinal study of neutrophil adherence and motility. J Pediatr. 1990 Dec;117(6):926–929. doi: 10.1016/s0022-3476(05)80139-8. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Schmalstieg F. C., Dempsey K., Krater S. S., Nannen D. D., Smith C. W., Anderson D. C. Subcellular distribution and mobilization of MAC-1 (CD11b/CD18) in neonatal neutrophils. Blood. 1990 Jan 15;75(2):488–498. [PubMed] [Google Scholar]
- Klein R. B., Fischer T. J., Gard S. E., Biberstein M., Rich K. C., Stiehm E. R. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977 Oct;60(4):467–472. [PubMed] [Google Scholar]
- Krause P. J., Herson V. C., Boutin-Lebowitz J., Eisenfeld L., Block C., LoBello T., Maderazo E. G. Polymorphonuclear leukocyte adherence and chemotaxis in stressed and healthy neonates. Pediatr Res. 1986 Apr;20(4):296–300. doi: 10.1203/00006450-198604000-00004. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Pahwa S. G., Pahwa R., Grimes E., Smithwick E. Cellular and humoral components of monocyte and neutrophil chemotaxis in cord blood. Pediatr Res. 1977 May;11(5):677–680. doi: 10.1203/00006450-197705000-00010. [DOI] [PubMed] [Google Scholar]
- Rebuck N., Finn A. Polymorphonuclear granulocyte expression of CD11a/CD18, CD11b/CD18 and L-selectin in normal individuals. FEMS Immunol Med Microbiol. 1994 Mar;8(3):189–195. doi: 10.1111/j.1574-695X.1994.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Schleiffenbaum B., Spertini O., Tedder T. F. Soluble L-selectin is present in human plasma at high levels and retains functional activity. J Cell Biol. 1992 Oct;119(1):229–238. doi: 10.1083/jcb.119.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Campbell D. E., Ludomirsky A., Polin R. A., Douglas S. D., Garty B. Z., Harris M. C. Expression of the complement receptors CR1 and CR3 and the type III Fc gamma receptor on neutrophils from newborn infants and from fetuses with Rh disease. Pediatr Res. 1990 Aug;28(2):120–126. doi: 10.1203/00006450-199008000-00009. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Tabsh K. M. Fetal neutrophils and eosinophils express normal levels of L-selectin. Pediatr Res. 1993 Sep;34(3):253–257. doi: 10.1203/00006450-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Spertini O., Freedman A. S., Belvin M. P., Penta A. C., Griffin J. D., Tedder T. F. Regulation of leukocyte adhesion molecule-1 (TQ1, Leu-8) expression and shedding by normal and malignant cells. Leukemia. 1991 Apr;5(4):300–308. [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Kansas G. S., Munro J. M., Griffin J. D., Gimbrone M. A., Jr, Tedder T. F. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991 Oct 15;147(8):2565–2573. [PubMed] [Google Scholar]
- Spertini O., Schleiffenbaum B., White-Owen C., Ruiz P., Jr, Tedder T. F. ELISA for quantitation of L-selectin shed from leukocytes in vivo. J Immunol Methods. 1992 Nov 25;156(1):115–123. doi: 10.1016/0022-1759(92)90017-n. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Isaacs C. M., Ernst T. J., Demetri G. D., Adler D. A., Disteche C. M. Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell surface molecule, LAM-1. Homology with the mouse lymphocyte homing receptor and other human adhesion proteins. J Exp Med. 1989 Jul 1;170(1):123–133. doi: 10.1084/jem.170.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török C., Lundahl J., Hed J., Lagercrantz H. Diversity in regulation of adhesion molecules (Mac-1 and L-selectin) in monocytes and neutrophils from neonates and adults. Arch Dis Child. 1993 May;68(5 Spec No):561–565. doi: 10.1136/adc.68.5_spec_no.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]


