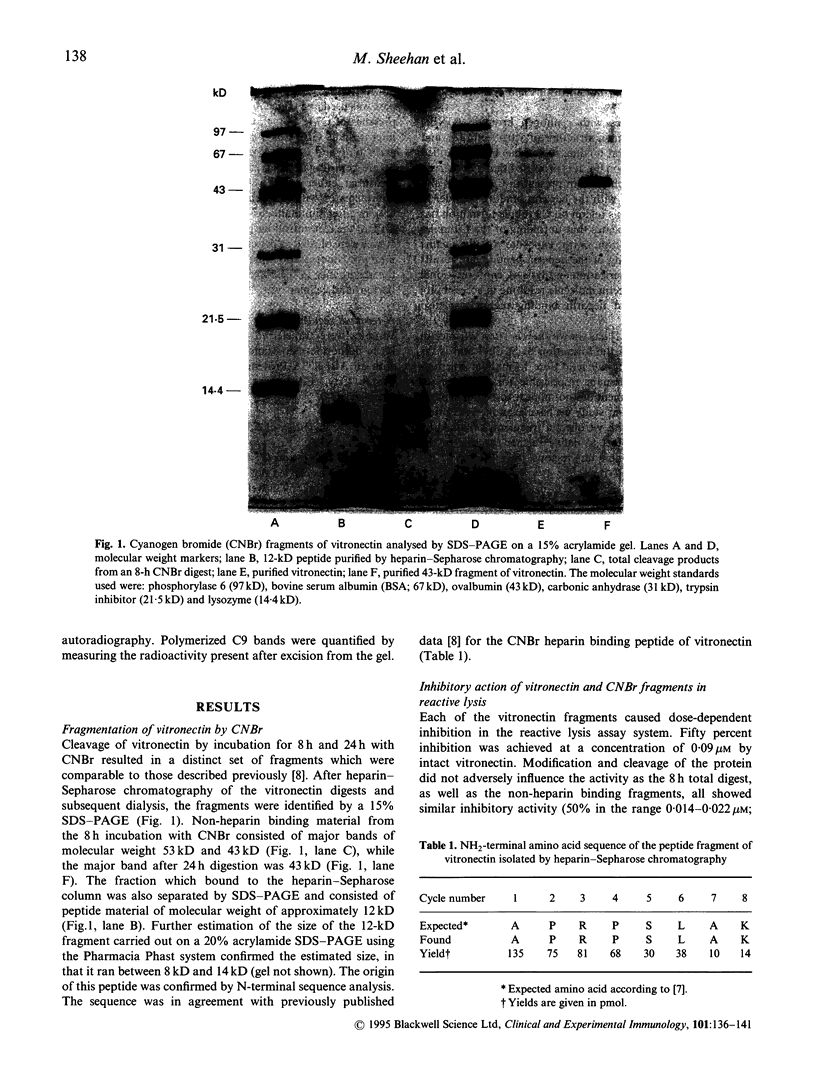
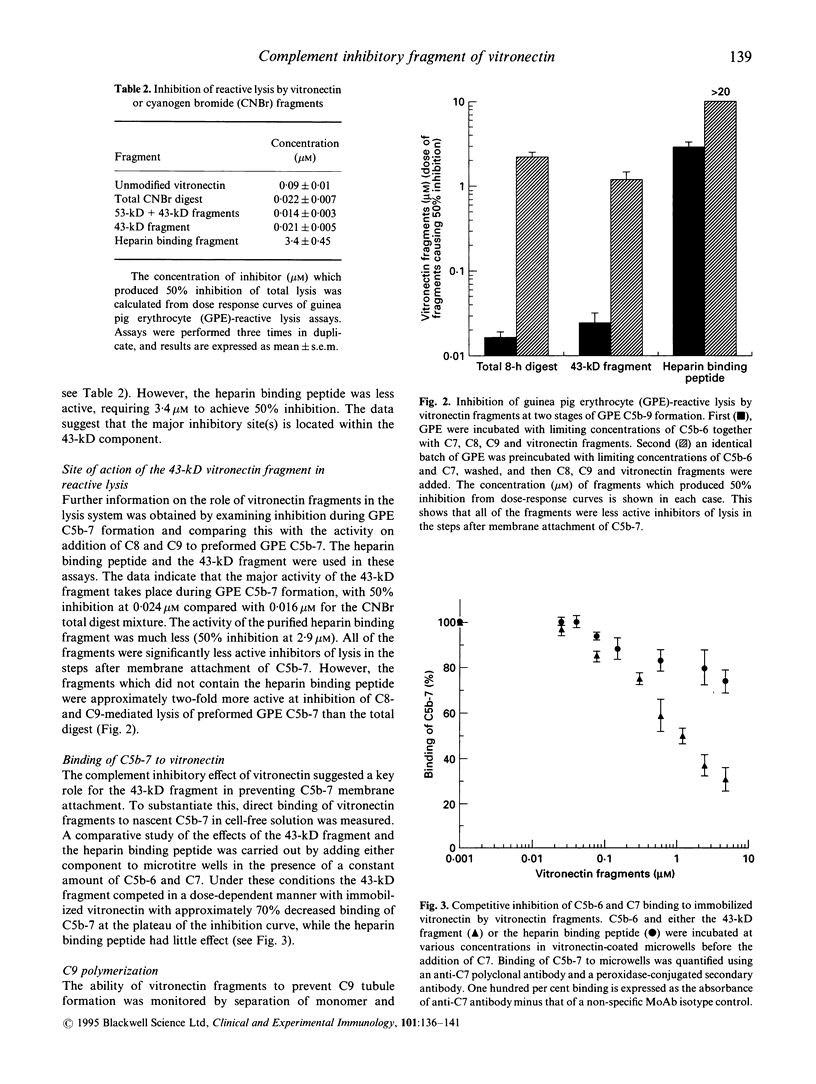
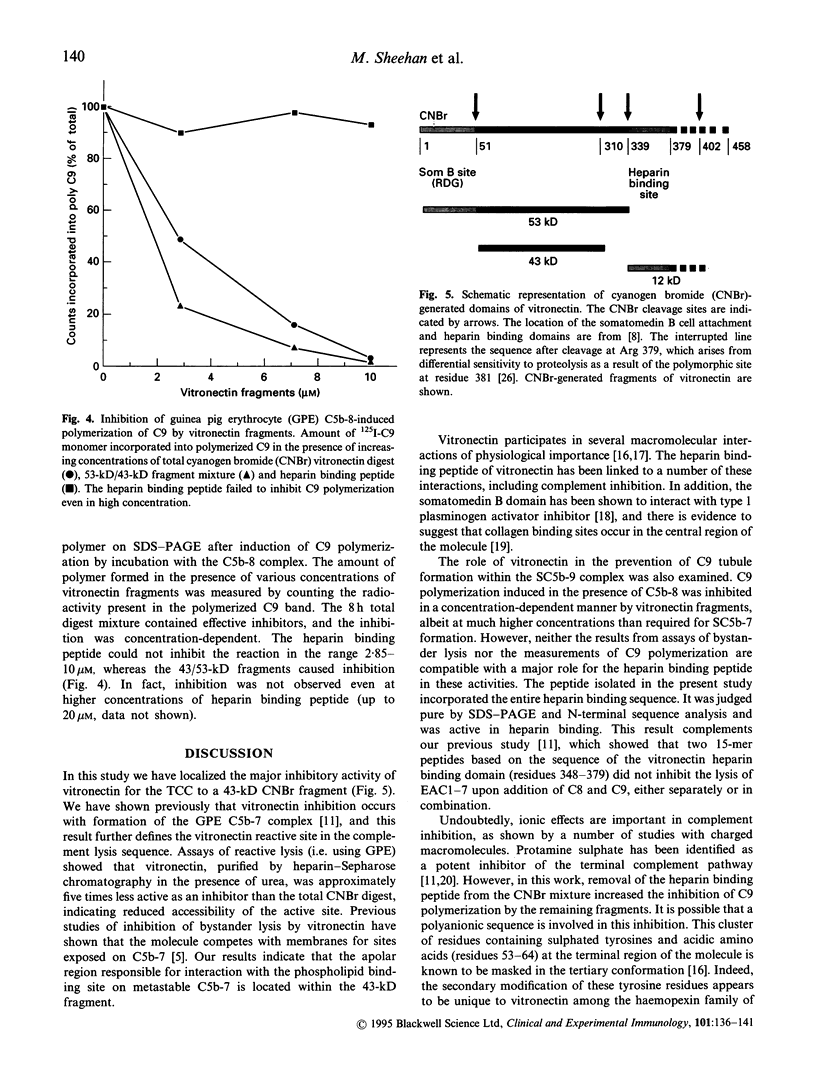
Abstract
Vitronectin (complement S-protein), a multifunctional glycoprotein, inhibits complement-mediated cytolysis at two identified stages of terminal complement complex (TCC) formation: blocking of C5b-7 membrane binding, and prevention of C9 polymerization. However, the functional domain(s) of vitronectin involved in these reactions remains incompletely defined. In order to identify the complement inhibition site, a 12-kD heparin binding fragment and two other internal fragments (53 kD and 43 kD) of vitronectin were isolated after cyanogen bromide (CNBr) treatment of the native molecule. Potent inhibition of guinea pig erythrocyte (GPE) reactive lysis was demonstrated with native vitronectin, total CNBr digest and the 53-kD and 43-kD fragments, but only very poorly by the heparin binding 12-kD peptide. Similarly, the 43-kD fragment blocked the binding of C5b-7 to immobilized vitronectin, whereas the 12-kD fragment had no effect. These data localize the C5b-7 binding site to a 43-kD internal region. Further characterization of the fragments was carried out in an assay which detected C9 polymerization in the presence of C5b-8. Polymerized material was separated by PAGE, detected by autoradiography and quantified after excision from the gels. Results showed that polymerization did not occur in the presence of the 53-kD and 43-kD fragments. However, the 12-kD heparin binding fragment had no effect. It is proposed that prevention of C5b-8-induced C9 polymerization resides at a site in an internal region of the vitronectin molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biesecker G. The complement SC5b-9 complex mediates cell adhesion through a vitronectin receptor. J Immunol. 1990 Jul 1;145(1):209–214. [PubMed] [Google Scholar]
- Charlesworth J. A., Peake P. W., Golding J., Mackie J. D., Pussell B. A., Timmermans V., Wakefield D. Hypercatabolism of C3 and C4 in active and inactive systemic lupus erythematosus. Ann Rheum Dis. 1989 Feb;48(2):153–159. doi: 10.1136/ard.48.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso A. P., Falk R. J., Raij L. The pathobiology of the terminal complement complexes. Complement Inflamm. 1989;6(1):36–48. doi: 10.1159/000463070. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Erdei A., Füst G., Gergely J. The role of C3 in the immune response. Immunol Today. 1991 Sep;12(9):332–337. doi: 10.1016/0167-5699(91)90011-H. [DOI] [PubMed] [Google Scholar]
- Hildebrand A. Identification of the beta-endorphin-binding subunit of the SC5b-9 complement complex: S protein exhibits specific beta-endorphin-binding sites upon complex formation with complement proteins. Biochem Biophys Res Commun. 1989 Mar 15;159(2):799–806. doi: 10.1016/0006-291x(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Høgåsen K., Mollnes T. E., Harboe M. Heparin-binding properties of vitronectin are linked to complex formation as illustrated by in vitro polymerization and binding to the terminal complement complex. J Biol Chem. 1992 Nov 15;267(32):23076–23082. [PubMed] [Google Scholar]
- Izumi M., Shimo-Oka T., Morishita N., Ii I., Hayashi M. Identification of the collagen-binding domain of vitronectin using monoclonal antibodies. Cell Struct Funct. 1988 Jun;13(3):217–225. doi: 10.1247/csf.13.217. [DOI] [PubMed] [Google Scholar]
- Jenne D., Stanley K. K. Nucleotide sequence and organization of the human S-protein gene: repeating peptide motifs in the "pexin" family and a model for their evolution. Biochemistry. 1987 Oct 20;26(21):6735–6742. doi: 10.1021/bi00395a024. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milis L., Morris C. A., Sheehan M. C., Charlesworth J. A., Pussell B. A. Vitronectin-mediated inhibition of complement: evidence for different binding sites for C5b-7 and C9. Clin Exp Immunol. 1993 Apr;92(1):114–119. doi: 10.1111/j.1365-2249.1993.tb05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977 Dec;119(6):2024–2029. [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. SC5b-9 complex of complement: formation of the dimeric membrane attack complex by removal of S-protein. J Immunol. 1980 Apr;124(4):1779–1783. [PubMed] [Google Scholar]
- Preissner K. T. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- Preissner K. T. The role of vitronectin as multifunctional regulator in the hemostatic and immune systems. Blut. 1989 Nov;59(5):419–431. doi: 10.1007/BF00349063. [DOI] [PubMed] [Google Scholar]
- Seiffert D., Loskutoff D. J. Evidence that type 1 plasminogen activator inhibitor binds to the somatomedin B domain of vitronectin. J Biol Chem. 1991 Feb 15;266(5):2824–2830. [PubMed] [Google Scholar]
- Suzuki S., Pierschbacher M. D., Hayman E. G., Nguyen K., Ohgren Y., Ruoslahti E. Domain structure of vitronectin. Alignment of active sites. J Biol Chem. 1984 Dec 25;259(24):15307–15314. [PubMed] [Google Scholar]
- Tollefsen D. M., Weigel C. J., Kabeer M. H. The presence of methionine or threonine at position 381 in vitronectin is correlated with proteolytic cleavage at arginine 379. J Biol Chem. 1990 Jun 15;265(17):9778–9781. [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. Vitronectin. Prog Hemost Thromb. 1991;10:269–305. [PubMed] [Google Scholar]
- Tschopp J., Masson D. Inhibition of the lytic activity of perforin (cytolysin) and of late complement components by proteoglycans. Mol Immunol. 1987 Sep;24(9):907–913. doi: 10.1016/0161-5890(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Masson D., Schäfer S., Peitsch M., Preissner K. T. The heparin binding domain of S-protein/vitronectin binds to complement components C7, C8, and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Biochemistry. 1988 May 31;27(11):4103–4109. doi: 10.1021/bi00411a029. [DOI] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]