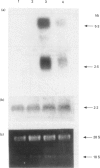
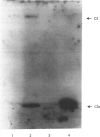
Abstract
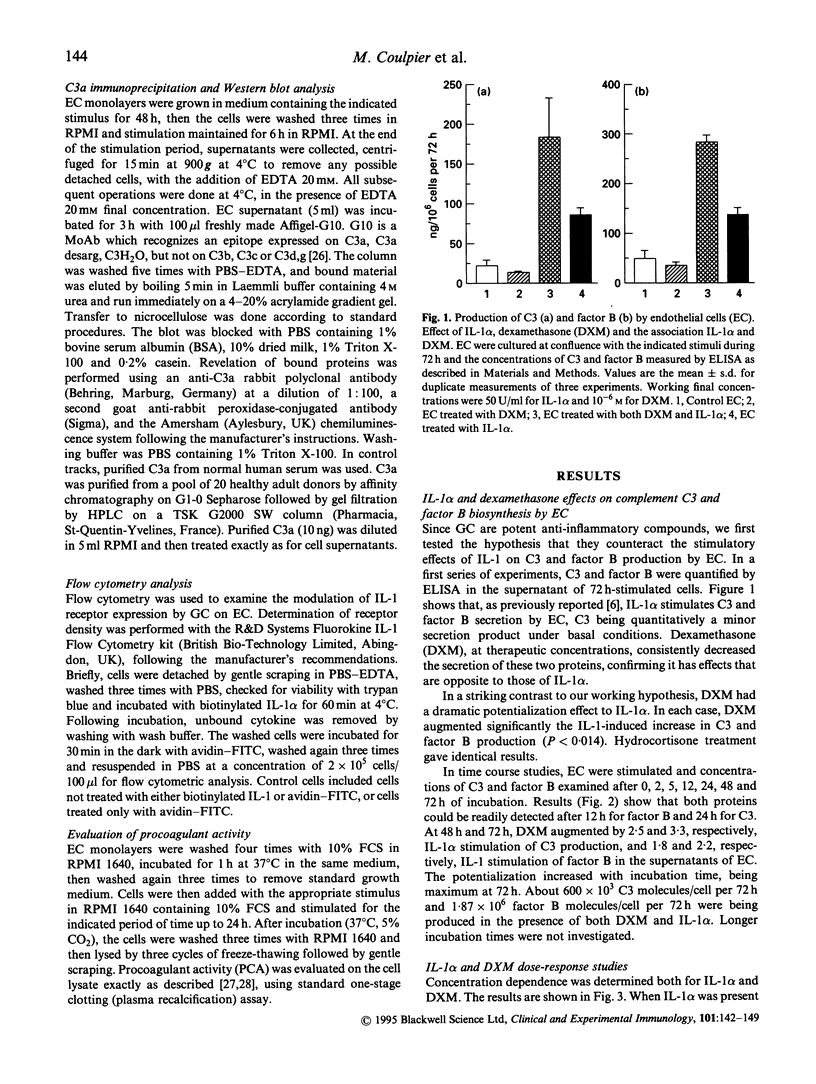
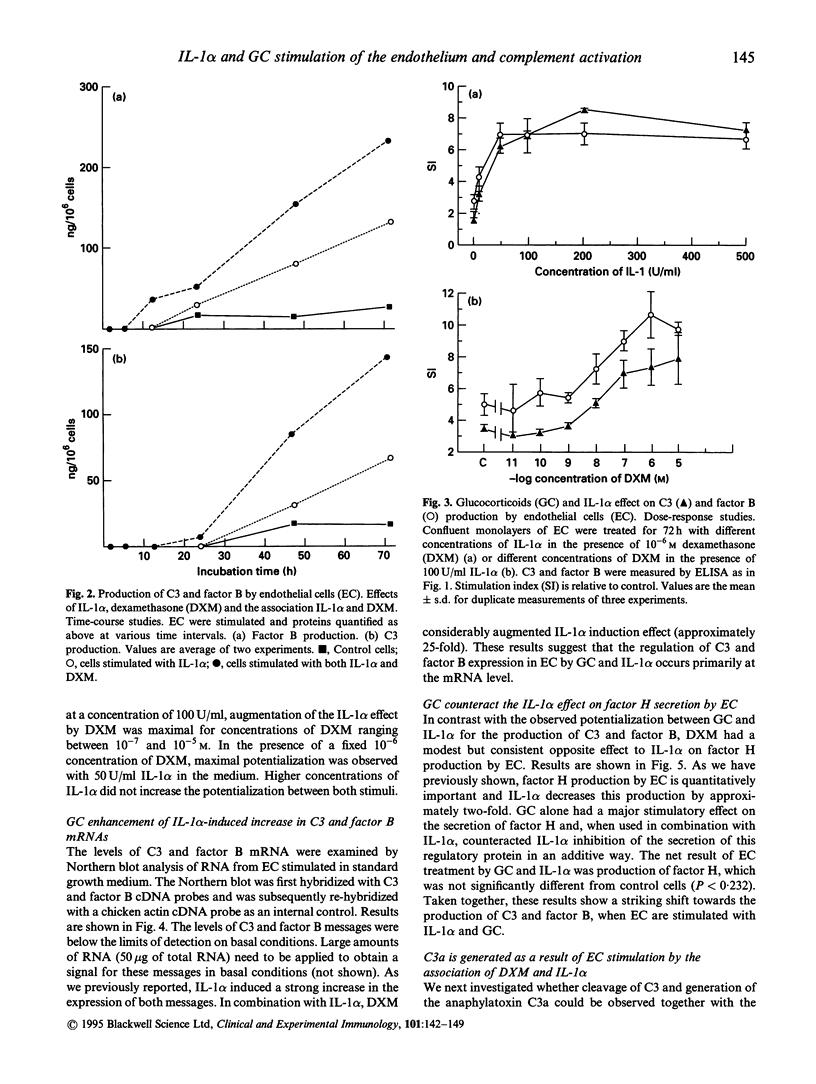
Constitutive secretion of complement C3 and factor B by the endothelial cell (EC) is lowered by therapeutic concentrations of glucocorticoids such as hydrocortisone or dexamethasone, whereas regulatory protein factor H production is increased by these hormones. In contrast, the proinflammatory cytokine IL-1 alpha has a stimulatory effect on C3 and factor B secretion by the endothelium and an inhibitory effect on factor H secretion. In this study, we examined the combined effect of IL-1 alpha and glucocorticoids on C3 and factor B expression by the endothelial cell. When dexamethasone or hydrocortisone were added to IL-1 alpha, significant potentialization of IL-1 alpha-induced stimulation of C3 and factor B production was observed, occurring at various concentrations of either stimuli. Dose-response experiments indicate that, in vitro, optimal concentrations are in the range of 10(-7) to 10(-5) M for dexamethasone and 50-200 U for IL-1 alpha. In contrast, dexamethasone counteracts, in an additive way, the inhibitory effect of IL-1 alpha on regulatory complement protein factor H production by EC. Such a potentialization between glucocorticoids and IL-1 alpha was not observed for another marker of endothelial activation, IL-1 alpha-induced stimulation of coagulation tissue factor expression. The association of glucocorticoids and IL-1 alpha therefore appears to be a specific and major stimulus for the secretion of complement C3 and factor B, two acute-phase proteins, by the endothelium. As a result of the in vitro endothelium stimulation by glucocorticoids and IL-1 alpha, C3a is generated in the vicinity of the endothelial cell. This study further suggests that complement activation, with its deleterious consequences, may result from the stimulation of endothelium in situations where high levels of IL-1 alpha and endogenous glucocorticoids coexist, such as in septic shock.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreev S. V., Ishchenko A. M., Pasechnik V. A., Ketlinskii S. A., Karamov E. V. Glubokoe rasshcheplenie C3-komponenta komplementa v syvorotkakh patsientov s sindromom priobretennogo immunodefitsita (SPID) i so SPID-podobnym kompleksom (SPK). Dokl Akad Nauk SSSR. 1987;296(5):1266–1268. [PubMed] [Google Scholar]
- Baumann H., Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990 Apr;7(2):147–159. [PubMed] [Google Scholar]
- Baumann H., Richards C., Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987 Dec 15;139(12):4122–4128. [PubMed] [Google Scholar]
- Berkenbosch F., van Oers J., del Rey A., Tilders F., Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238(4826):524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Beach J. E., Holaday J. W., Smallridge R. C., Fein H. G. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Brooimans R. A., Hiemstra P. S., van der Ark A. A., Sim R. B., van Es L. A., Daha M. R. Biosynthesis of complement factor H by human umbilical vein endothelial cells. Regulation by T cell growth factor and IFN-gamma. J Immunol. 1989 Mar 15;142(6):2024–2030. [PubMed] [Google Scholar]
- Brown S. L., Smith L. R., Blalock J. E. Interleukin 1 and interleukin 2 enhance proopiomelanocortin gene expression in pituitary cells. J Immunol. 1987 Nov 15;139(10):3181–3183. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choy L. N., Rosen B. S., Spiegelman B. M. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem. 1992 Jun 25;267(18):12736–12741. [PubMed] [Google Scholar]
- Dauchel H., Joly P., Delpech A., Thomine E., Sauger F., Le Loet X., Lauret P., Tron F., Fontaine M., Ripoche J. Local and systemic activation of the whole complement cascade in human leukocytoclastic cutaneous vasculitis; C3d,g and terminal complement complex as sensitive markers. Clin Exp Immunol. 1993 May;92(2):274–283. doi: 10.1111/j.1365-2249.1993.tb03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchel H., Julen N., Lemercier C., Daveau M., Ozanne D., Fontaine M., Ripoche J. Expression of complement alternative pathway proteins by endothelial cells. Differential regulation by interleukin 1 and glucocorticoids. Eur J Immunol. 1990 Aug;20(8):1669–1675. doi: 10.1002/eji.1830200808. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Emilie D., Crevon M. C., Auffredou M. T., Galanaud P. Glucocorticosteroid-dependent synergy between interleukin 1 and interleukin 6 for human B lymphocyte differentiation. Eur J Immunol. 1988 Dec;18(12):2043–2047. doi: 10.1002/eji.1830181226. [DOI] [PubMed] [Google Scholar]
- Ganapathi M. K., Rzewnicki D., Samols D., Jiang S. L., Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991 Aug 15;147(4):1261–1265. [PubMed] [Google Scholar]
- Girard M. T., Hjaltadottir S., Fejes-Toth A. N., Guyre P. M. Glucocorticoids enhance the gamma-interferon augmentation of human monocyte immunoglobulin G Fc receptor expression. J Immunol. 1987 May 15;138(10):3235–3241. [PubMed] [Google Scholar]
- Helin H., Edgington T. S. Allogeneic induction of the human T cell-instructed monocyte procoagulant response is rapid and is elicited by HLA-DR. J Exp Med. 1983 Sep 1;158(3):962–975. doi: 10.1084/jem.158.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hichens M., Hogans A. F. Radioimmunoassay for dexamethasone in plasma. Clin Chem. 1974 Feb;20(2):266–271. [PubMed] [Google Scholar]
- Hugli T. E. Biochemistry and biology of anaphylatoxins. Complement. 1986;3(3):111–127. doi: 10.1159/000467889. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julen N., Dauchel H., Lemercier C., Sim R. B., Fontaine M., Ripoche J. In vitro biosynthesis of complement factor I by human endothelial cells. Eur J Immunol. 1992 Jan;22(1):213–217. doi: 10.1002/eji.1830220131. [DOI] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987 Dec 15;139(12):4129–4134. [PubMed] [Google Scholar]
- Kunicka J. E., Talle M. A., Denhardt G. H., Brown M., Prince L. A., Goldstein G. Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell Immunol. 1993 Jun;149(1):39–49. doi: 10.1006/cimm.1993.1134. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemercier C., Julen N., Coulpier M., Dauchel H., Ozanne D., Fontaine M., Ripoche J. Differential modulation by glucocorticoids of alternative complement protein secretion in cells of the monocyte/macrophage lineage. Eur J Immunol. 1992 Apr;22(4):909–915. doi: 10.1002/eji.1830220405. [DOI] [PubMed] [Google Scholar]
- Lin R. Y., Astiz M. E., Saxon J. C., Saha D. C., Rackow E. C. Alterations in C3, C4, factor B, and related metabolites in septic shock. Clin Immunol Immunopathol. 1993 Nov;69(2):136–142. doi: 10.1006/clin.1993.1161. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K., Alpert A., Bressler L. In vivo administration of IL-1 induces thymic hypoplasia and increased levels of serum corticosterone. J Immunol. 1988 Sep 1;141(5):1456–1463. [PubMed] [Google Scholar]
- Neeck G., Federlin K., Graef V., Rusch D., Schmidt K. L. Adrenal secretion of cortisol in patients with rheumatoid arthritis. J Rheumatol. 1990 Jan;17(1):24–29. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche J., Mitchell J. A., Erdei A., Madin C., Moffatt B., Mokoena T., Gordon S., Sim R. B. Interferon gamma induces synthesis of complement alternative pathway proteins by human endothelial cells in culture. J Exp Med. 1988 Nov 1;168(5):1917–1922. doi: 10.1084/jem.168.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Stosić-Grujicić S., Simić M. M. Modulation of interleukin 1 production by activated macrophages: in vitro action of hydrocortisone, colchicine, and cytochalasin B. Cell Immunol. 1982 May 15;69(2):235–247. doi: 10.1016/0008-8749(82)90070-3. [DOI] [PubMed] [Google Scholar]
- Ueki A., Sai T., Oka H., Tabata M., Hosokawa K., Mochizuki Y. Biosynthesis and secretion of the third component of complement by human endothelial cells in vitro. Immunology. 1987 May;61(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Warren H. B., Pantazis P., Davies P. F. The third component of complement is transcribed and secreted by cultured human endothelial cells. Am J Pathol. 1987 Oct;129(1):9–13. [PMC free article] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989 Feb;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Fey G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci U S A. 1985 Feb;82(3):708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]