Abstract
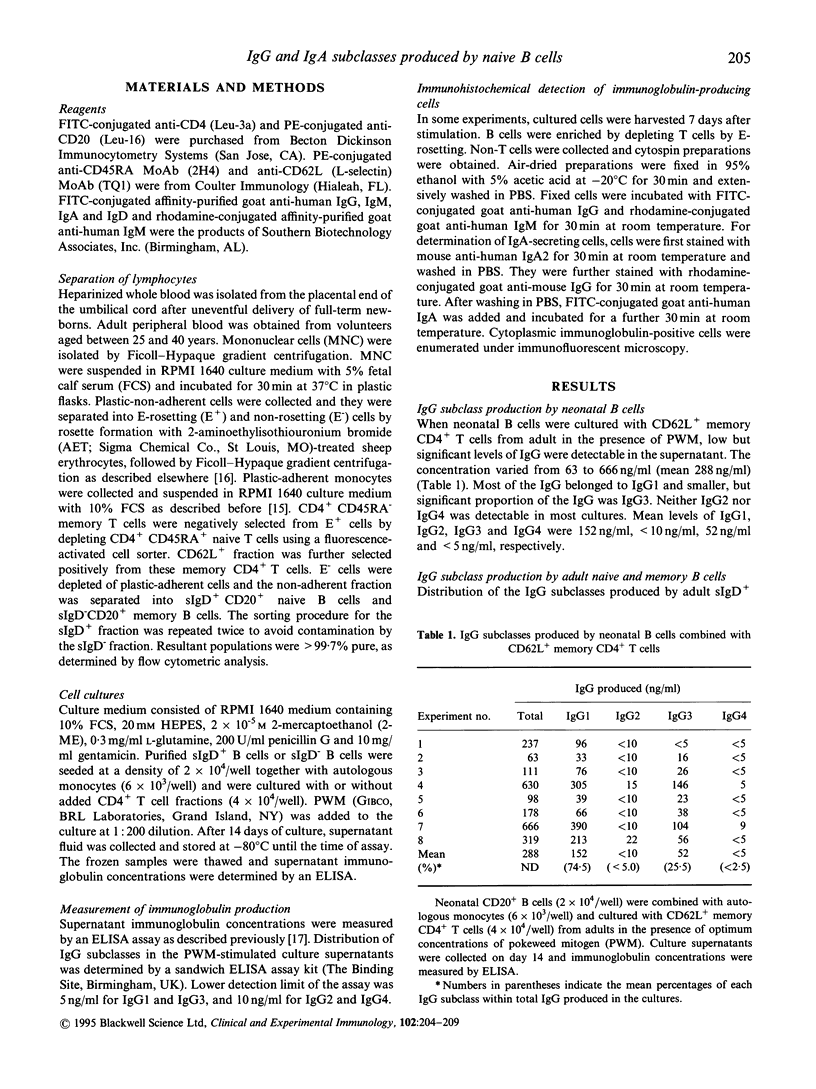
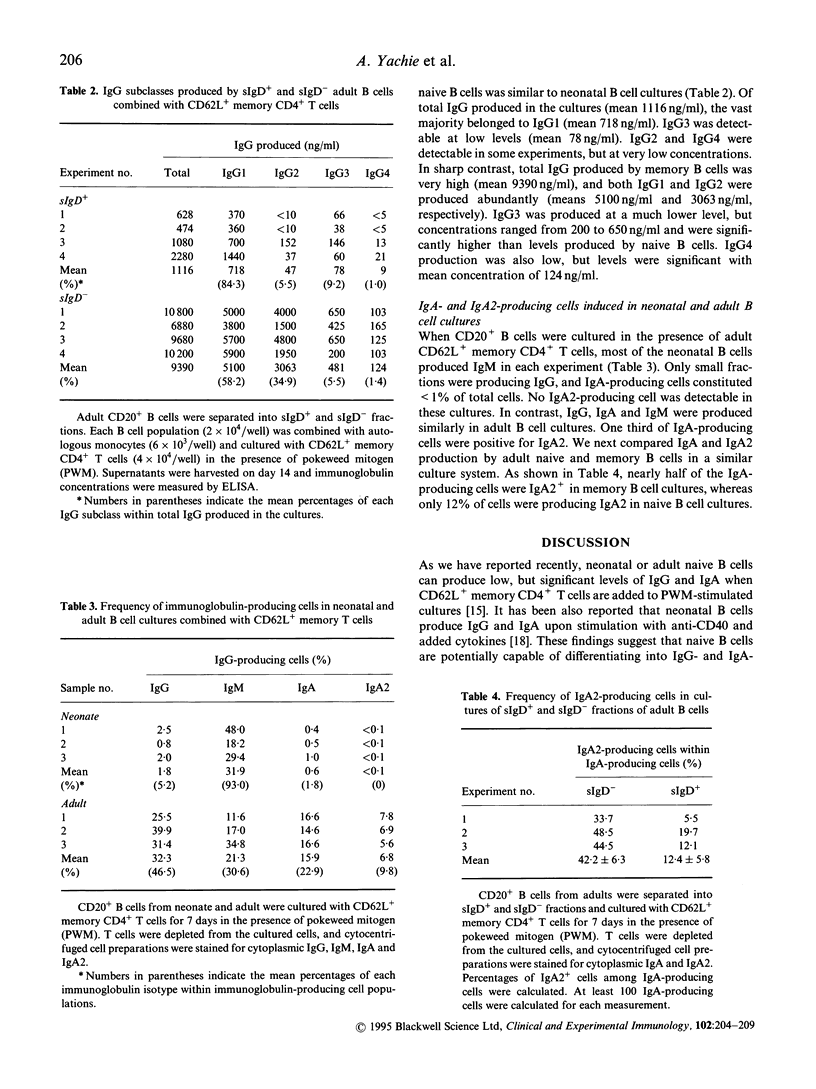
Neonatal B cells with the naive (sIgD+) phenotype are able to generate IgG- and IgA-producing cells as well as IgM production in the presence of memory CD4+ T cells expressing L-selectin (CD62L) in pokeweed mitogen-stimulated cultures. We used this system to examine comparatively the ability of naive B cells to produce IgG and IgA subclasses in newborn infants and adult individuals. Naive B cells were enriched from both donors on the basis of sIgD positivity, and memory (CD45RO+) CD4+ T cells with CD62L expression were isolated from adults. We here demonstrate some differences in profiles of IgG and IgA subclass production between neonatal and adult naive B cells. In neonatal B cells, IgG1 and IgG3 were predominantly produced, but IgG2 and IgG4 production was virtually absent. Similar to neonatal B cells, adult naive B cells produced mainly IgG1 and IgG3, although memory (sIgD-) B cells from adults secreted all of the IgG subclasses. It should be noted that low but detectable levels of IgG2 and IgG4 were found in adults' naive B cell cultures. Although IgA produced by neonatal B cells was exclusively IgA1, IgA2-secreting cells were identifiable in adult naive B cells. The results suggest that further class switch of naive B cells to IgG2, IgG4 and IgA2 in addition to IgG1 and IgG3 may be controlled by their own age-dependent maturation process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson U., Bird A. G., Britton B. S., Palacios R. Humoral and cellular immunity in humans studied at the cell level from birth to two years of age. Immunol Rev. 1981;57:1–38. doi: 10.1111/j.1600-065x.1981.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Rousset F. Human B lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol. 1992;52:125–262. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- Brière F., Servet-Delprat C., Bridon J. M., Saint-Remy J. M., Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994 Feb 1;179(2):757–762. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M. E., Arbeter A., Douglas S. D. Serum levels of IgA1 and IgA2 in children and in patients with IgA deficiency. Mol Immunol. 1983 Sep;20(9):977–981. doi: 10.1016/0161-5890(83)90038-x. [DOI] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Gathings W. E., Kubagawa H., Cooper M. D. A distinctive pattern of B cell immaturity in perinatal humans. Immunol Rev. 1981;57:107–126. doi: 10.1111/j.1600-065x.1981.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Ishii R., Yamasaki K., Kishimoto T., Hardy R. R. Isolation of high-affinity memory B cells: phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1379–1383. doi: 10.1073/pnas.84.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Black S. J., Tokuhisa T., Herzenberg L. A. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980 May 1;151(5):1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin P. D., Yamashita L. C., Coffman R. L., Kehry M. R. Separation of events mediating B cell proliferation and Ig production by using T cell membranes and lymphokines. J Immunol. 1990 Oct 1;145(7):2025–2034. [PubMed] [Google Scholar]
- Hodgkin P. D., Yamashita L. C., Seymour B., Coffman R. L., Kehry M. R. Membranes from both Th1 and Th2 T cell clones stimulate B cell proliferation and prepare B cells for lymphokine-induced differentiation to secrete Ig. J Immunol. 1991 Dec 1;147(11):3696–3702. [PubMed] [Google Scholar]
- Hofker M. H., Walter M. A., Cox D. W. Complete physical map of the human immunoglobulin heavy chain constant region gene complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5567–5571. doi: 10.1073/pnas.86.14.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R., Kumararatne D. S. Selective IgG subclass deficiency: quantification and clinical relevance. Clin Exp Immunol. 1990 Sep;81(3):357–367. doi: 10.1111/j.1365-2249.1990.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek D. F., Splawski J. B., Lipsky P. E. Human peripheral blood B lymphocyte subpopulations: functional and phenotypic analysis of surface IgD positive and negative subsets. J Immunol. 1986 Jan;136(1):83–92. [PubMed] [Google Scholar]
- Kanegane H., Miyawaki T., Kato K., Yokoi T., Uehara T., Yachie A., Taniguchi N. A novel subpopulation of CD45RA+ CD4+ T cells expressing IL-2 receptor alpha-chain (CD25) and having a functionally transitional nature into memory cells. Int Immunol. 1991 Dec;3(12):1349–1356. doi: 10.1093/intimm/3.12.1349. [DOI] [PubMed] [Google Scholar]
- Kitani A., Strober W. Regulation of C gamma subclass germ-line transcripts in human peripheral blood B cells. J Immunol. 1993 Oct 1;151(7):3478–3488. [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. II. Pokeweed mitogen-responsive B cells belong to a surface immunoglobulin D-negative subpopulation. J Exp Med. 1982 May 1;155(5):1561–1566. doi: 10.1084/jem.155.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. I., Heiner D. C., Wara D. Development of serum IgG subclass levels in children. Monogr Allergy. 1986;19:108–121. [PubMed] [Google Scholar]
- Mackay C. R. Immunological memory. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- Mestecky J., Lue C., Russell M. W. Selective transport of IgA. Cellular and molecular aspects. Gastroenterol Clin North Am. 1991 Sep;20(3):441–471. [PubMed] [Google Scholar]
- Miyawaki T., Kasahara Y., Taga K., Yachie A., Taniguchi N. Differential expression of CD45RO (UCHL1) and its functional relevance in two subpopulations of circulating TCR-gamma/delta+ lymphocytes. J Exp Med. 1990 May 1;171(5):1833–1838. doi: 10.1084/jem.171.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Kubagawa H., Butler J. L., Cooper M. D. Ig isotypes produced by EBV-transformed B cells as a function of age and tissue distribution. J Immunol. 1988 Jun 1;140(11):3887–3892. [PubMed] [Google Scholar]
- Miyawaki T., Moriya N., Nagaoki T., Taniguchi N. Maturation of B-cell differentiation ability and T-cell regulatory function in infancy and childhood. Immunol Rev. 1981;57:61–87. doi: 10.1111/j.1600-065x.1981.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Morell A., Van Loghem E., Nef M., Theilkaes L., Skvaril F. Determination of IgG subclasses and Gm allotypes in culture supernatants of pokeweed mitogen-stimulated human blood lymphocytes. J Immunol. 1981 Sep;127(3):1099–1102. [PubMed] [Google Scholar]
- Nagaoki T., Miyawaki T., Ciorbaru R., Yachie A., Uwadana N., Moriya N., Taniguchi N. Maturation of B cell differentiation ability and T cell regulatory function during child growth assessed in a Nocardia water soluble mitogen-driven system. J Immunol. 1981 May;126(5):2015–2019. [PubMed] [Google Scholar]
- Noelle R. J., Daum J., Bartlett W. C., McCann J., Shepherd D. M. Cognate interactions between helper T cells and B cells. V. Reconstitution of T helper cell function using purified plasma membranes from activated Th1 and Th2 T helper cells and lymphokines. J Immunol. 1991 Feb 15;146(4):1118–1124. [PubMed] [Google Scholar]
- Punnonen J., Aversa G. G., Vandekerckhove B., Roncarolo M. G., de Vries J. E. Induction of isotype switching and Ig production by CD5+ and CD10+ human fetal B cells. J Immunol. 1992 Jun 1;148(11):3398–3404. [PubMed] [Google Scholar]
- Ruuskanen O., Pittard W. B., 3rd, Miller K., Pierce G., Sorensen R. U., Polmar S. H. Staphylococcus aureus Cowan I-induced immunoglobulin production in human cord blood lymphocytes. J Immunol. 1980 Jul;125(1):411–413. [PubMed] [Google Scholar]
- Schittek B., Rajewsky K. Natural occurrence and origin of somatically mutated memory B cells in mice. J Exp Med. 1992 Aug 1;176(2):427–438. doi: 10.1084/jem.176.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinson E., Fernandez C., Stavnezer J. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins prior to switch recombination. Eur J Immunol. 1990 May;20(5):1079–1084. doi: 10.1002/eji.1830200520. [DOI] [PubMed] [Google Scholar]
- Sleasman J. W., Morimoto C., Schlossman S. F., Tedder T. F. The role of functionally distinct helper T lymphocyte subpopulations in the induction of human B cell differentiation. Eur J Immunol. 1990 Jun;20(6):1357–1366. doi: 10.1002/eji.1830200623. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Splawski J. B., Jelinek D. F., Lipsky P. E. Delineation of the functional capacity of human neonatal lymphocytes. J Clin Invest. 1991 Feb;87(2):545–553. doi: 10.1172/JCI115029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski J. B., Lipsky P. E. Cytokine regulation of immunoglobulin secretion by neonatal lymphocytes. J Clin Invest. 1991 Sep;88(3):967–977. doi: 10.1172/JCI115400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Nibu R., Iwai K., Kanegane H., Yachie A., Seki H., Miyawaki T., Taniguchi N. Efficient induction of immunoglobulin production in neonatal naive B cells by memory CD4+ T cell subset expressing homing receptor L-selectin. J Immunol. 1994 May 1;152(9):4417–4424. [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Nagaoki T., Yokoi T., Mukai M., Uwadana N., Taniguchi N. Regulation of B cell differentiation by T cell subsets defined with monoclonal OKT4 and OKT8 antibodies in human cord blood. J Immunol. 1981 Oct;127(4):1314–1317. [PubMed] [Google Scholar]
- Yarchoan R., Tosato G., Blaese R. M., Simon R. M., Nelson D. L. Limiting dilution analysis of Epstein-Barr virus-induced immunoglobulin production by human B cells. J Exp Med. 1983 Jan 1;157(1):1–14. doi: 10.1084/jem.157.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


