Abstract
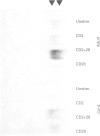
Accessory molecules play a crucial role in the development of the T cell response to antigenic challenge. We have examined the role of CD28 in modulating the 'naive' neonatal T cell response to anti-CD2-mediated activation. To compare the role of CD28, neonatal and adult T cells were stimulated with a pair of mitogenic anti-CD2 antibodies in the presence or absence of anti-CD28 MoAb. With anti-CD2 alone, neonatal T cells proliferated slightly but produced no detectable IL-2, whereas adult T cells proliferated vigorously, with significant IL-2 production. Costimulation with anti-CD28 MoAb greatly enhanced the proliferative response of neonatal T cells to levels equivalent to those of adult T cells, whereas adult T cells showed only slight increases. Although IL-2 secretion was increased in the presence of anti-CD28 MoAb, neonatal T cell IL-2 production remained lower than in adults. In contrast, enhancement of IL-2 mRNA expression in neonates was similar to adult levels. Anti-CD28 MoAb costimulation increased NF kappa B levels in neonates, albeit to levels lower than that of adults. The cellular mechanism governing the diminished proliferative response of neonatal T lymphocytes to anti-CD2 may therefore be due to decreased NF kappa B induction, reduced IL-2 mRNA expression and deficient IL-2 production. Although anti-CD28 MoAb costimulation enhances all of the above signals, NF kappa B and IL-2 levels remain lower than in adults, suggesting the need for further activation requirements in the neonate.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barve S. S., Cohen D. A., De Benedetti A., Rhoads R. E., Kaplan A. M. Mechanism of differential regulation of IL-2 in murine Th1 and Th2 T cell subsets. 1. Induction of IL-2 transcription in Th2 cells by up-regulation of transcription factors with the protein synthesis initiation factor 4E. J Immunol. 1994 Feb 1;152(3):1171–1181. [PubMed] [Google Scholar]
- Chilmonczyk B. A., Levin M. J., McDuffy R., Hayward A. R. Characterization of the human newborn response to herpesvirus antigen. J Immunol. 1985 Jun;134(6):4184–4188. [PubMed] [Google Scholar]
- Fairfax C. A., Borzy M. S. Interleukin 2 production, proliferative response, and receptor expression by cord blood mononuclear cells. J Clin Lab Immunol. 1988 Oct;27(2):63–67. [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Gauchat J. F., Gauchat D., De Weck A. L., Stadler B. M. Cytokine mRNA levels in antigen-stimulated peripheral blood mononuclear cells. Eur J Immunol. 1989 Jun;19(6):1079–1085. doi: 10.1002/eji.1830190618. [DOI] [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Crupi S., Arcangeli C., Marinelli I., Spinozzi F., Cernetti C., Angelella P., Rambotti P. Activation of cord T lymphocytes. I. Evidence for a defective T cell mitogenesis induced through the CD2 molecule. J Immunol. 1989 Apr 15;142(8):2583–2589. [PubMed] [Google Scholar]
- Gerli R., Rambotti P., Cernetti C., Velardi A., Spinozzi F. Evidence for phenotypic t precursor cells in human cord blood. Br J Haematol. 1983 Apr;53(4):685–686. doi: 10.1111/j.1365-2141.1983.tb07321.x. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Tan T. H., Rice N. R., Sica A., Young H. A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Chu S., Patterson J. A., Berger C. L., Edelson R. L., Chu A. C. Characterization of immature T cell subpopulations in neonatal blood. Blood. 1984 Jul;64(1):296–300. [PubMed] [Google Scholar]
- Hannet I., Erkeller-Yuksel F., Lydyard P., Deneys V., DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992 Jun;13(6):215–218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Interleukin-1 augments the diminished interleukin-2 mRNA expression and proliferative response of neonatal T lymphocytes to anti-CD2 antibodies. Scand J Immunol. 1994 Jun;39(6):597–601. doi: 10.1111/j.1365-3083.1994.tb03418.x. [DOI] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Neonatal CD4+ CD45RA+ T cells: precursors of adult CD4+ CD45RA+ T cells? Res Immunol. 1993 Feb;144(2):87–92. doi: 10.1016/0923-2494(93)80064-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Lindsten T., Thompson C. B. Evidence for the involvement of three distinct signals in the induction of IL-2 gene expression in human T lymphocytes. J Immunol. 1989 Jul 1;143(1):153–161. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh P. N., Williams D. C., O'Neill L. A. Interleukin-1 activates transcription factor NF kappa B in glial cells. Biochem J. 1993 Sep 1;294(Pt 2):343–347. doi: 10.1042/bj2940343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K. P., Kündig T. M., Kishihara K., Wakeham A., Kawai K., Ohashi P. S., Thompson C. B., Mak T. W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993 Jul 30;261(5121):609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Splawski J. B., Jelinek D. F., Lipsky P. E. Delineation of the functional capacity of human neonatal lymphocytes. J Clin Invest. 1991 Feb;87(2):545–553. doi: 10.1172/JCI115029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Geerts M., Aarden L. A. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem. 1991 Aug 5;266(22):14179–14182. [PubMed] [Google Scholar]
- Watson W., Oen K., Ramdahin R., Harman C. Immunoglobulin and cytokine production by neonatal lymphocytes. Clin Exp Immunol. 1991 Jan;83(1):169–174. doi: 10.1111/j.1365-2249.1991.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselborg S., Prüfer U., Wild M., Schraven B., Meuer S. C., Kabelitz D. Triggering via the alternative CD2 pathway induces apoptosis in activated human T lymphocytes. Eur J Immunol. 1993 Oct;23(10):2707–2710. doi: 10.1002/eji.1830231050. [DOI] [PubMed] [Google Scholar]
- Wilson C. B. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr. 1986 Jan;108(1):1–12. doi: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Westall J., Johnston L., Lewis D. B., Dower S. K., Alpert A. R. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986 Mar;77(3):860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Y., Denning S. M., Mizuno S., Dupont B., Haynes B. F. A novel activation pathway for mature thymocytes. Costimulation of CD2 (T,p50) and CD28 (T,p44) induces autocrine interleukin 2/interleukin 2 receptor-mediated cell proliferation. J Exp Med. 1988 Oct 1;168(4):1457–1468. doi: 10.1084/jem.168.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]