Abstract
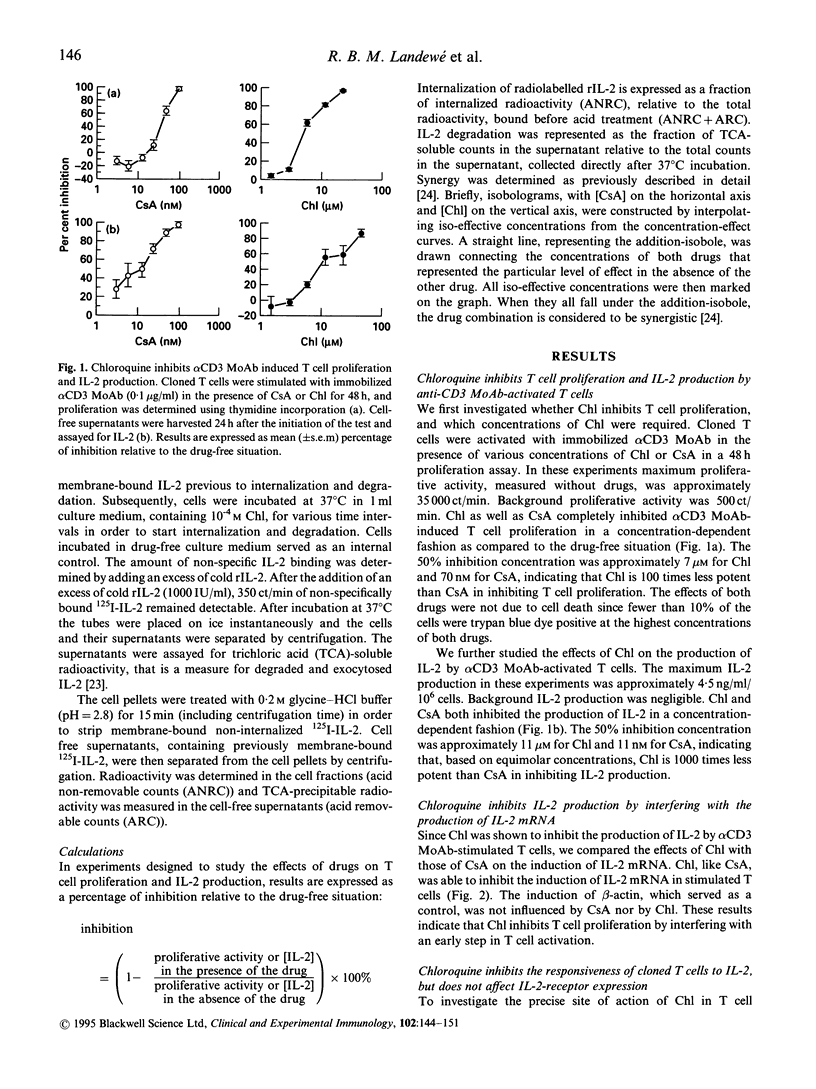
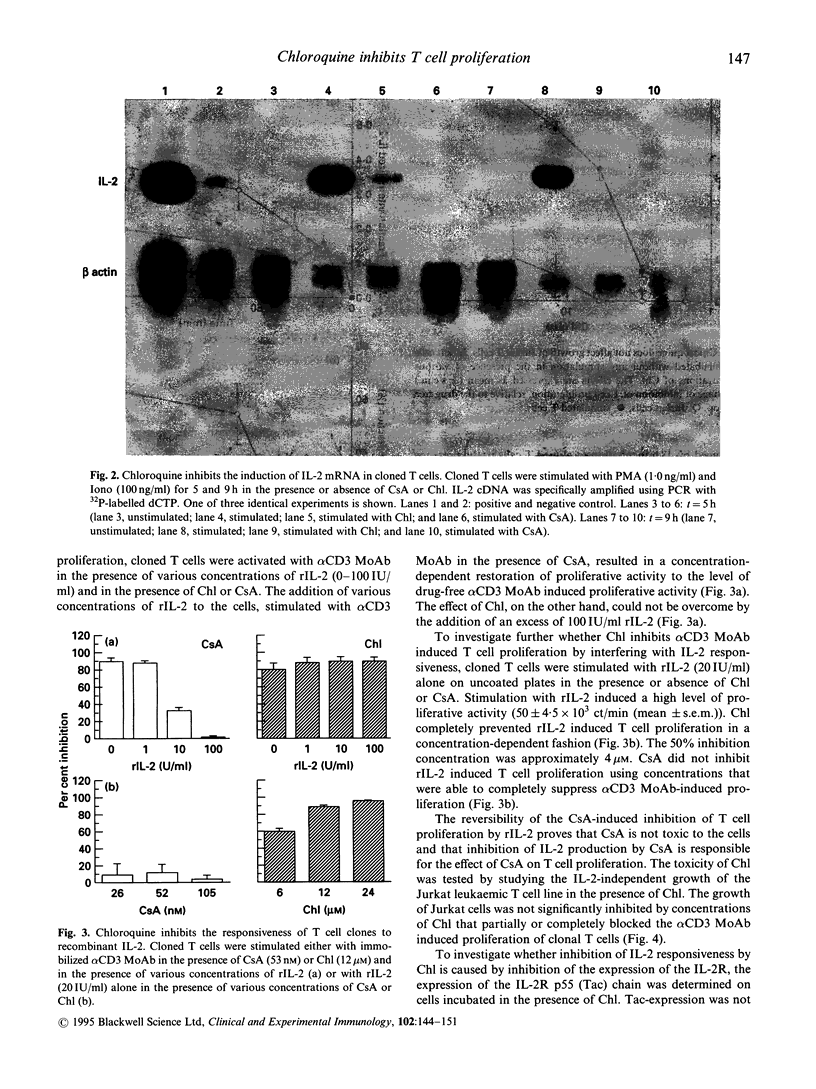
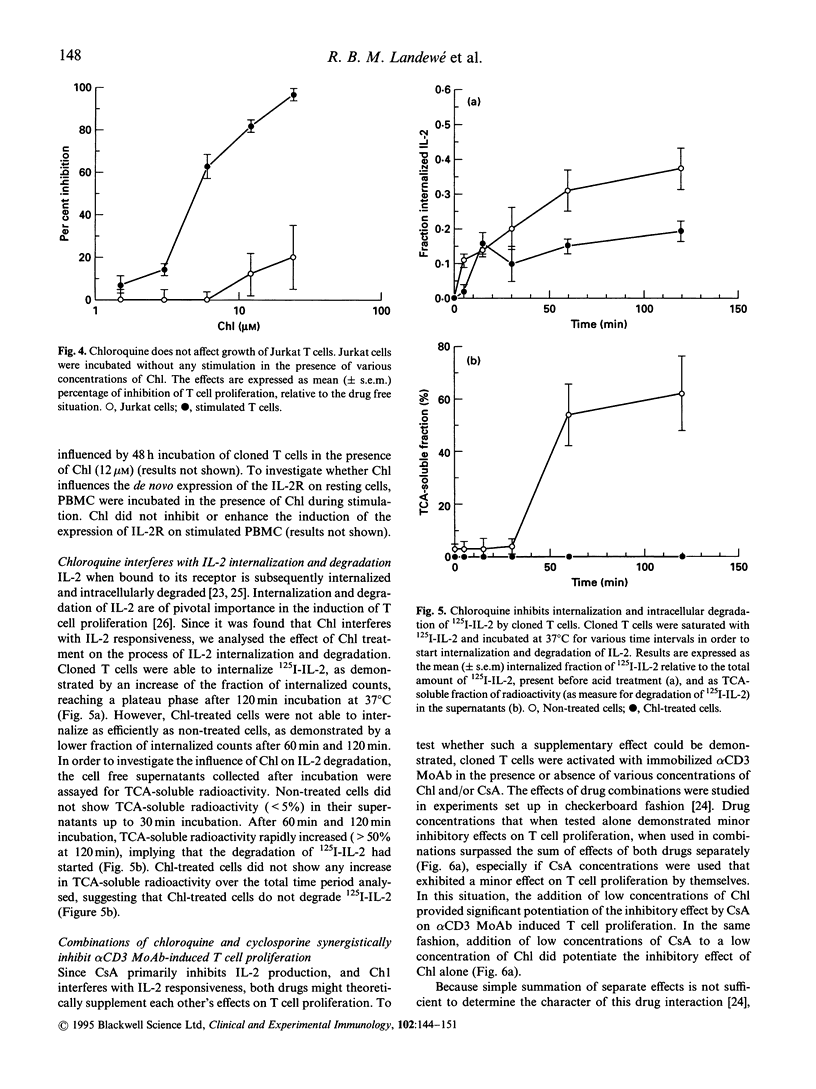
Chloroquine (Chl) is an anti-rheumatic drug that is widely used in the treatment of rheumatoid arthritis (RA). It seems that T cells are important in the pathogenesis of RA, but it is not known whether Chl acts via inhibition of T cell function. We here present evidence that Chl, just like cyclosporine A (CsA), inhibits T cell proliferation as induced with immobilized alpha CD3 MoAb in a concentration-dependent manner, at least partly through interfering with the production of IL-2 protein and the induction of IL-2 mRNA. Furthermore, Chl impedes the responsiveness of T cell clones to IL-2 since (1) the inhibition of alpha CD3 MoAb-induced proliferation by Chl could not be reversed by rIL-2 and (2) Chl directly blocks IL-2-driven proliferation of cloned T cells. Chl appeared to interfere with the internalization (50% inhibition) and degradation (total blockade) of rIL-2. Finally, the combination of Chl and CsA synergistically inhibited T cell proliferation. We conclude that Chl may inhibit functional properties of human T cells, although the drug is 100- to 1000-fold less potent than CsA in inhibiting T cell proliferation and IL-2 production, respectively. It is speculated that the in vitro effects of Chl might be relevant in explaining the anti-rheumatic effect of this drug in patients with RA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenbaum M. C. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin Exp Immunol. 1977 Apr;28(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E. A controlled study of chloroquine as an antirheumatic agent. Arthritis Rheum. 1958 Aug;1(4):297–312. doi: 10.1002/art.1780010403. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Collins M. K., Crumpton M. J. Autocrine regulation of T-lymphocyte proliferation: differential induction of IL-2 and IL-2 receptor. Immunology. 1988 Nov;65(3):343–349. [PMC free article] [PubMed] [Google Scholar]
- Clark P., Casas E., Tugwell P., Medina C., Gheno C., Tenorio G., Orozco J. A. Hydroxychloroquine compared with placebo in rheumatoid arthritis. A randomized controlled trial. Ann Intern Med. 1993 Dec 1;119(11):1067–1071. doi: 10.7326/0003-4819-119-11-199312010-00002. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Duprez V., Ferrer M., Cornet V., Olive D., Dautry-Varsat A. Modulation of interleukin 2 internalization and interleukin 2-dependent cell growth by antireceptor antibodies. J Biol Chem. 1991 Jan 25;266(3):1497–1501. [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- FREEDMAN A., STEINBERG V. L. Chloroquine in rheumatoid arthritis; a double blindfold trial of treatment for one year. Ann Rheum Dis. 1960 Sep;19:243–250. doi: 10.1136/ard.19.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Sugamura K., Sano K., Nakai M., Sugita K., Hinuma Y. High-affinity receptor-mediated internalization and degradation of interleukin 2 in human T cells. J Exp Med. 1986 Mar 1;163(3):550–562. doi: 10.1084/jem.163.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3. J Immunol. 1987 Mar 15;138(6):1660–1666. [PubMed] [Google Scholar]
- HURVITZ D., HIRSCHHORN K. SUPPRESSION OF IN VITRO LYMPHOCYTE RESPONSES BY CHLOROQUINE. N Engl J Med. 1965 Jul 1;273:23–26. doi: 10.1056/NEJM196507012730105. [DOI] [PubMed] [Google Scholar]
- Harder A., Kovatchev S., Debuch H. Interactions of chloroquine with different glycerophospholipids. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1847–1850. [PubMed] [Google Scholar]
- Landewé R. B., Goei Thè H. S., van Rijthoven A. W., Breedveld F. C., Dijkmans B. A. A randomized, double-blind, 24-week controlled study of low-dose cyclosporine versus chloroquine for early rheumatoid arthritis. Arthritis Rheum. 1994 May;37(5):637–643. doi: 10.1002/art.1780370506. [DOI] [PubMed] [Google Scholar]
- Landewé R. B., Miltenburg A. M., Breedveld F. C., Daha M. R., Dijkmans B. A. Cyclosporine and chloroquine synergistically inhibit the interferon-gamma production by CD4 positive and CD8 positive synovial T cell clones derived from a patient with rheumatoid arthritis. J Rheumatol. 1992 Sep;19(9):1353–1357. [PubMed] [Google Scholar]
- Lee T. P., Mookerjee B. K. Receptor-mediated endocytosis of IL-2 by PHA stimulated lymphocytes. Transplant Proc. 1989 Feb;21(1 Pt 1):100–102. [PubMed] [Google Scholar]
- Legrue S. J., Sheu T. L., Chernajovsky Y. The role of receptor-ligand endocytosis and degradation in interleukin-2 signaling and T-lymphocyte proliferation. Lymphokine Cytokine Res. 1991 Dec;10(6):431–436. [PubMed] [Google Scholar]
- Londei M., Grubeck-Loebenstein B., de Berardinis P., Greenall C., Feldmann M. Efficient propagation and cloning of human T cells in the absence of antigen by means of OKT3, interleukin 2, and antigen-presenting cells. Scand J Immunol. 1988 Jan;27(1):35–46. doi: 10.1111/j.1365-3083.1988.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie A. H. Pharmacologic actions of 4-aminoquinoline compounds. Am J Med. 1983 Jul 18;75(1A):5–10. doi: 10.1016/0002-9343(83)91264-0. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980 Jun 10;255(11):5190–5194. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenburg A. M., Meijer-Paape M. E., Daha M. R., Paul L. C. Endothelial cell lysis induced by lymphokine-activated human peripheral blood mononuclear cells. Eur J Immunol. 1987 Sep;17(9):1383–1386. doi: 10.1002/eji.1830170926. [DOI] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., de Kuiper R., Daha M. R., Breedveld F. C. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992 May;35(5):603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Nagarkatti P. S., Nagarkatti M., Jain V. C. In vivo and in vitro action of chloroquine on surface markers of human peripheral lymphocytes. Clin Exp Immunol. 1980 Jul;41(1):166–172. [PMC free article] [PubMed] [Google Scholar]
- POPERT A. J., MEIJERS K. A., SHARP J., BIER F. Chloroquine diphosphate in rheumatoid arthritis. A controlled trial. Ann Rheum Dis. 1961 Mar;20:18–35. doi: 10.1136/ard.20.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Picot S., Peyron F., Vuillez J. P., Polack B., Ambroise-Thomas P. Chloroquine inhibits tumor necrosis factor production by human macrophages in vitro. J Infect Dis. 1991 Oct;164(4):830–830. doi: 10.1093/infdis/164.4.830. [DOI] [PubMed] [Google Scholar]
- Poole S., Bristow A. F., Selkirk S., Rafferty B. Development and application of radioimmunoassays for interleukin-1 alpha and interleukin-1 beta. J Immunol Methods. 1989 Jan 17;116(2):259–264. doi: 10.1016/0022-1759(89)90212-3. [DOI] [PubMed] [Google Scholar]
- Salmeron G., Lipsky P. E. Immunosuppressive potential of antimalarials. Am J Med. 1983 Jul 18;75(1A):19–24. doi: 10.1016/0002-9343(83)91266-4. [DOI] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Talal N. Cyclosporine as an immunosuppressive agent for autoimmune disease: theoretical concepts and therapeutic strategies. Transplant Proc. 1988 Jun;20(3 Suppl 4):11–15. [PubMed] [Google Scholar]
- Telerman A., Amson R. B., Romasco F., Wybran J., Galand P., Mosselmans R. Internalization of human T lymphocyte receptors. Eur J Immunol. 1987 Jul;17(7):991–997. doi: 10.1002/eji.1830170715. [DOI] [PubMed] [Google Scholar]
- Tellides G., Dallman M. J., Morris P. J. Synergistic interaction of cyclosporine A with interleukin 2 receptor monoclonal antibody therapy. Transplant Proc. 1988 Apr;20(2 Suppl 2):202–206. [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: apparent inhibition of receptor recycling. Biochem Biophys Res Commun. 1980 Mar 13;93(1):1–8. doi: 10.1016/s0006-291x(80)80237-3. [DOI] [PubMed] [Google Scholar]
- Ueda H., Hancock W. W., Cheung Y. C., Diamantstein T., Tilney N. L., Kupiec-Weglinski J. W. The mechanism of synergistic interaction between anti-interleukin 2 receptor monoclonal antibody and cyclosporine therapy in rat recipients of organ allografts. Transplantation. 1990 Oct;50(4):545–550. doi: 10.1097/00007890-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Voss S. D., Leary T. P., Sondel P. M., Robb R. J. Identification of a direct interaction between interleukin 2 and the p64 interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2428–2432. doi: 10.1073/pnas.90.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]



