Abstract
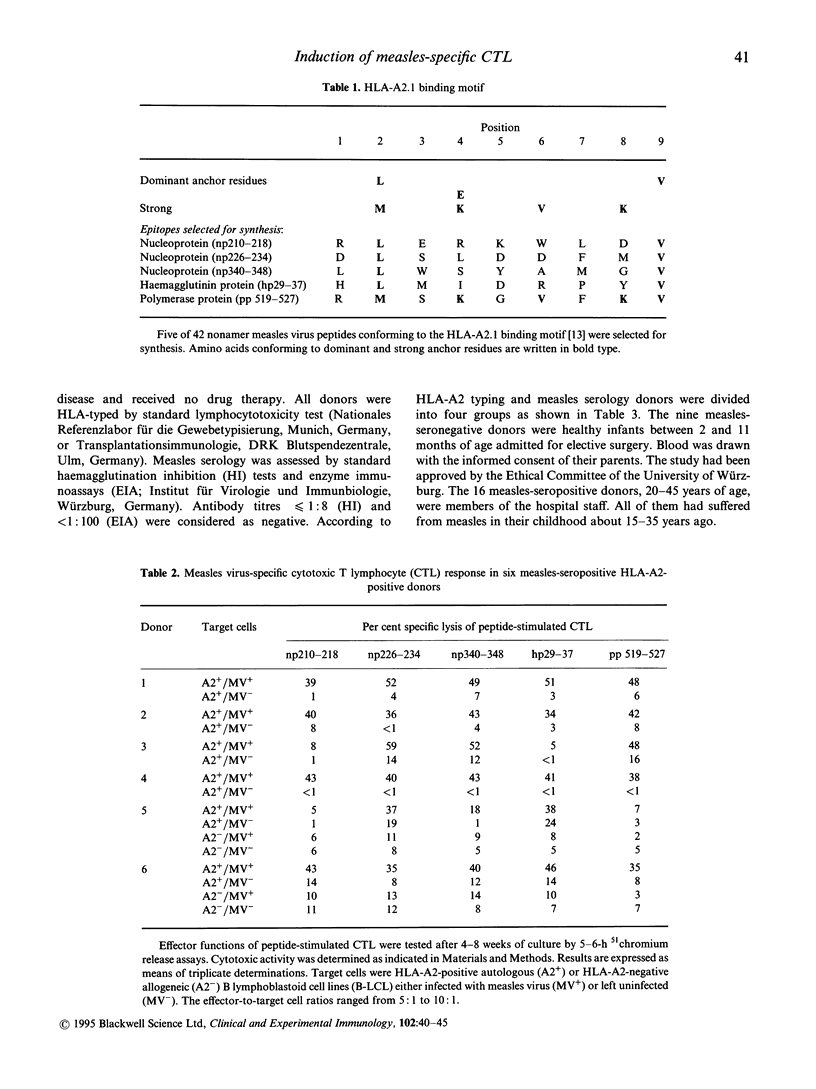
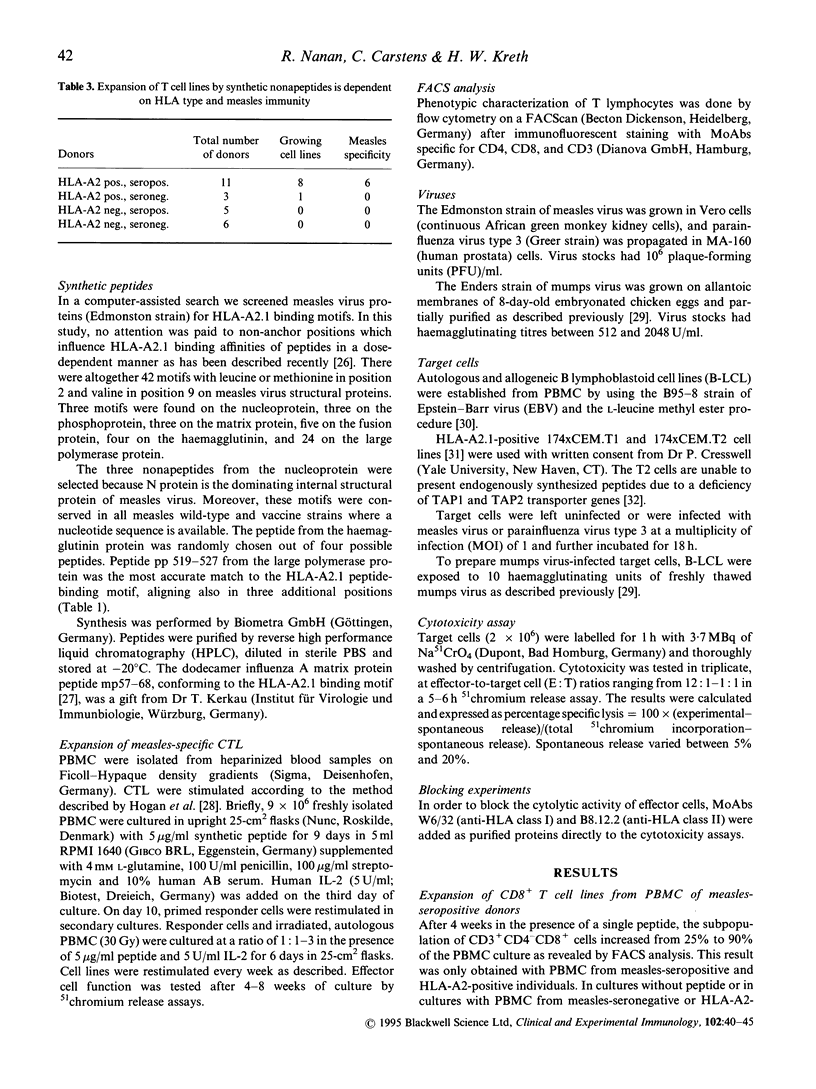
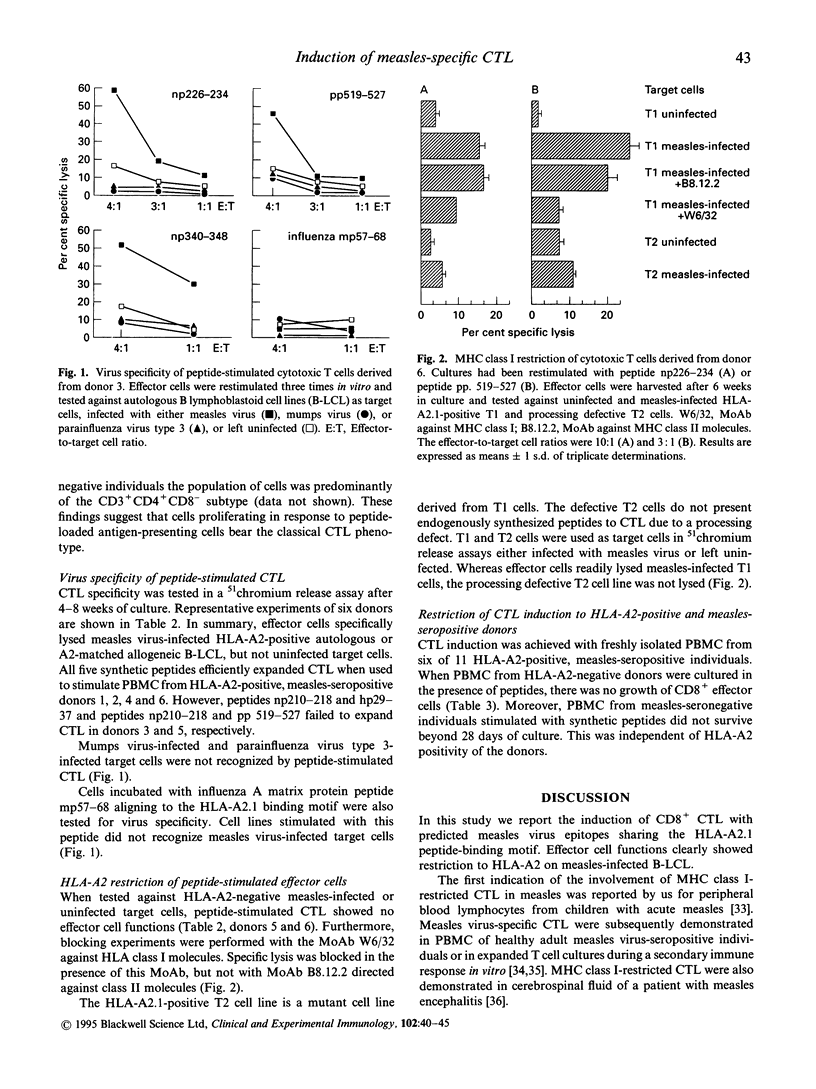
CD8+ cytotoxic T lymphocytes (CTL) in measles virus infection have been difficult to investigate due to the strong immunosuppressive effects exhibited by infectious measles virus in vitro. In order to circumvent immunosuppression we used predicted peptide epitopes to induce measles virus-specific CTL. This was done by screening the structural proteins of measles virus for HLA-A2.1 peptide-binding motifs with valine in position 2 and leucine in position 9. Synthetic peptides np210-218, np226-234, and np340-348 from the nucleoprotein, peptide hp29-37 from the haemagglutinin protein, and peptide pp 519-527 from the polymerase protein were synthesized and used to expand measles virus-specific CD8+ CTL in vitro. Induction of CTL with synthetic peptides was restricted to HLA-A2-positive peripheral blood mononuclear cells (PBMC) from measles-seropositive individuals. We conclude that this method is a useful tool to demonstrate memory CD8+ CTL in measles-seropositive adults and to evaluate the role of structural proteins in CTL responses against measles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bednarek M. A., Sauma S. Y., Gammon M. C., Porter G., Tamhankar S., Williamson A. R., Zweerink H. J. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J Immunol. 1991 Dec 15;147(12):4047–4053. [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Carbone F. R., Moore M. W., Sheil J. M., Bevan M. J. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988 Jun 1;167(6):1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins J., Gould K. G., Smith M., Driscoll P., Brownlee G. G. Precise prediction of a Kk-restricted cytotoxic T cell epitope in the NS1 protein of influenza virus using an MHC allele-specific motif. Virology. 1993 Mar;193(1):289–295. doi: 10.1006/viro.1993.1124. [DOI] [PubMed] [Google Scholar]
- DiBrino M., Parker K. C., Shiloach J., Knierman M., Lukszo J., Turner R. V., Biddison W. E., Coligan J. E. Endogenous peptides bound to HLA-A3 possess a specific combination of anchor residues that permit identification of potential antigenic peptides. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1508–1512. doi: 10.1073/pnas.90.4.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Kreth H. W. Clonal expansion and functional analysis of virus-specific T lymphocytes from cerebrospinal fluid in measles encephalitis. Hum Immunol. 1983 Aug;7(4):239–248. doi: 10.1016/0198-8859(83)90061-7. [DOI] [PubMed] [Google Scholar]
- Gotch F., McMichael A., Rothbard J. Recognition of influenza A matrix protein by HLA-A2-restricted cytotoxic T lymphocytes. Use of analogues to orientate the matrix peptide in the HLA-A2 binding site. J Exp Med. 1988 Dec 1;168(6):2045–2057. doi: 10.1084/jem.168.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991 Nov 1;174(5):969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. A., Michel H., Sakaguchi K., Shabanowitz J., Appella E., Hunt D. F., Engelhard V. H. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992 Mar 6;255(5049):1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Elvin J., Willis A. C., Aidoo M., Allsopp C. E., Gotch F. M., Gao X. M., Takiguchi M., Greenwood B. M., Townsend A. R. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992 Dec 3;360(6403):434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- Hogan K. T., Shimojo N., Walk S. F., Engelhard V. H., Maloy W. L., Coligan J. E., Biddison W. E. Mutations in the alpha 2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza virus matrix peptide. J Exp Med. 1988 Aug 1;168(2):725–736. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbiers J. G., Nijman H. W., van der Burg S. H., Drijfhout J. W., Kenemans P., van de Velde C. J., Brand A., Momburg F., Kast W. M., Melief C. J. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993 Sep;23(9):2072–2077. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Nepom G. T., Richert J. R., Biddison W. E., McFarland H. F. Identification of a specific HLA DR2 Ia molecule as a restriction element for measles virus-specific HLA class II-restricted cytotoxic T cell clones. J Exp Med. 1985 Jan 1;161(1):263–268. doi: 10.1084/jem.161.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Jacobson S., Rose J. W., Flerlage M. L., McFarlin D. E., McFarland H. F. Induction of measles virus-specific human cytotoxic T cells by purified measles virus nucleocapsid and hemagglutinin polypeptides. Viral Immunol. 1987;1(3):153–162. doi: 10.1089/vim.1987.1.153. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kreth H. W., Kress L., Kress H. G., Ott H. F., Eckert G. Demonstration of primary cytotoxic T cells in venous blood and cerebrospinal fluid of children with mumps meningitis. J Immunol. 1982 Jun;128(6):2411–2415. [PubMed] [Google Scholar]
- Kreth H. W., ter Meulen V., Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979 Jan 24;165(4):203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Biddison W. E., Nelson D. L., Shaw S. Killing of measles virus-infected cells by human cytotoxic T cells. Infect Immun. 1982 Oct;38(1):226–232. doi: 10.1128/iai.38.1.226-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Dongworth D. W., Clark A., Potter C. W. Declining T-cell immunity to influenza, 1977-82. Lancet. 1983 Oct 1;2(8353):762–764. doi: 10.1016/s0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H. J., Vitiello A., Chesnut R., Person J. L., Redeker A. G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993 May 15;150(10):4659–4671. [PubMed] [Google Scholar]
- Nijman H. W., Houbiers J. G., Vierboom M. P., van der Burg S. H., Drijfhout J. W., D'Amaro J., Kenemans P., Melief C. J., Kast W. M. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993 Jun;23(6):1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- Pamer E. G., Harty J. T., Bevan M. J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991 Oct 31;353(6347):852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert J. R., McFarland H. F., McFarlin D. E., Bellini W. J., Lake P. Cloned measles virus-specific T lymphocytes from a twin with multiple sclerosis. Proc Natl Acad Sci U S A. 1983 Jan;80(2):555–559. doi: 10.1073/pnas.80.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert J. R., Rose J. W., Reuben-Burnside C., Kearns M. C., Jacobson S., Mingioli E. S., Hartzman R. J., McFarland H. F., McFarlin D. E. Polypeptide specificities of measles virus-reactive T cell lines and clones derived from a patient with multiple sclerosis. J Immunol. 1986 Oct 1;137(7):2190–2194. [PubMed] [Google Scholar]
- Ruppert J., Sidney J., Celis E., Kubo R. T., Grey H. M., Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993 Sep 10;74(5):929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K. Naturally-occurring peptide antigens derived from the MHC class-I-restricted processing pathway. Immunol Today. 1991 Dec;12(12):447–455. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Stevanović S., Jung G., Rammensee H. G. Peptide motifs of closely related HLA class I molecules encompass substantial differences. Eur J Immunol. 1992 Sep;22(9):2453–2456. doi: 10.1002/eji.1830220940. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 1986 May;5(5):943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Stroehmann I., Brandis H. Generation of cytolytic T-cell cultures displaying measles virus specificity and human histocompatibility leukocyte antigen restriction. Infect Immun. 1982 May;36(2):657–661. doi: 10.1128/iai.36.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhrbier A., Schmidt C., Fernan A. Prediction of an HLA B8-restricted influenza epitope by motif. Immunology. 1993 May;79(1):171–173. [PMC free article] [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Modulation of human natural killer cell function by L-leucine methyl ester: monocyte-dependent depletion from human peripheral blood mononuclear cells. J Immunol. 1985 Feb;134(2):786–793. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Zhang Q. J., Gavioli R., Klein G., Masucci M. G. An HLA-A11-specific motif in nonamer peptides derived from viral and cellular proteins. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2217–2221. doi: 10.1073/pnas.90.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Binnendijk R. S., Poelen M. C., Kuijpers K. C., Osterhaus A. D., Uytdehaag F. G. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. Clonal analyses of human CD8+ class I MHC-restricted CTL. J Immunol. 1990 Mar 15;144(6):2394–2399. [PubMed] [Google Scholar]


