Abstract
The neural bHLH genes Mash1 and Ngn2 are expressed in complementary populations of neural progenitors in the central and peripheral nervous systems. Here, we have systematically compared the activities of the two genes during neural development by generating replacement mutations in mice in which the coding sequences of Mash1 and Ngn2 were swapped. Using this approach, we demonstrate that Mash1 has the capacity to respecify the identity of neuronal populations normally derived from Ngn2-expressing progenitors in the dorsal telencephalon and ventral spinal cord. In contrast, misexpression of Ngn2 in Mash1-expressing progenitors does not result in any overt change in neuronal phenotype. Taken together, these results demonstrate that Mash1 and Ngn2 have divergent functions in specification of neuronal subtype identity, with Mash1 having the characteristics of an instructive determinant whereas Ngn2 functions as a permissive factor that must act in combination with other factors to specify neuronal phenotypes. Moreover, the ectopic expression of Ngn2 can rescue the neurogenesis defects of Mash1 null mutants in the ventral telencephalon and sympathetic ganglia but not in the ventral spinal cord and the locus coeruleus, indicating that Mash1 contribution to the specification of neuronal fates varies greatly in different lineages, presumably depending on the presence of other determinants of neuronal identity.
Keywords: Knock-in mutations, bHLH factors, CNS, PNS, neuronal specification
The mechanisms underlying the generation by multipotent stem cells of the vast array of neuronal and glial subtypes that constitute the nervous system remain poorly characterized. Recently, evidence have been obtained that genes of the bHLH class are important regulators of several steps in neural lineage development (Brunet and Ghysen 1999; Cepko 1999; Guillemot 1999; Morrison 2001). In particular, a subset of neural bHLH genes, the proneural genes, has been shown to play a central role in the selection of neural progenitor cells. Two families of proneural genes have been identified in Drosophila, the achaete-scute genes (ac-sc) which control the generation of progenitors for the central nervous system (CNS) and external sense organs in the peripheral nervous system (PNS), and genes of the atonal family (ato) which control the generation of progenitors for photoreceptors, chordotonal organs, and olfactory receptors (Modolell 1997; Campos-Ortega 1998). In vertebrates, a large number of bHLH genes are expressed in the developing nervous system, but only a fraction of them appear to have a proneural function. Loss-of-function (LOF) analyses in mouse have demonstrated that the ac-sc homolog Mash1 and the ato-related genes Neurogenin (Ngn) 1 and Ngn2 are required for the generation by neural stem cells of various populations of progenitor cell populations in the PNS and CNS, including progenitors in the ventral telencephalon and olfactory epithelium for Mash1, and in the cranial and dorsal root ganglia, the dorsal telencephalon and the ventral spinal cord for Ngn1 and Ngn2 (Cau et al. 1997; Fode et al. 1998, 2000; Ma et al. 1998; Casarosa et al. 1999; Horton et al. 1999; Scardigli et al. 2001). In contrast, several other bHLH genes that are also expressed by neural progenitor cells, including the ato orthologs Math1 and Math5 and NeuroD, do not appear to be involved in this initial step of neurogenesis (Bermingham et al. 1999; Wang et al. 2001).
Although functional studies in Drosophila and vertebrates initially focused on demonstrating a proneural function for bHLH proteins, the restricted expression of proneural genes in distinct populations of neural progenitors that give rise to different subtypes of neurons raised the possibility that they may also influence neuronal fate decisions. Consistent with this idea, gain-of-function (GOF) experiments in Drosophila have shown that the ectopic expression of atonal and achaete-scute results in the generation of different types of sense organs (Jarman et al. 1993; Chien et al. 1996; Jarman and Ahmed 1998). In addition, rescue experiments in the embryonic CNS have demonstrated that the achaete-scute genes differ in their ability to specify neural precursor identities (Parras et al. 1996; Skeath and Doe 1996), and similarly, atonal and a closely related gene, amos promote the development of different types of olfactory sense organs (Goulding et al. 2000; M.L. Huang et al. 2000).
In vertebrates, there is evidence that some bHLH genes have conserved a neuronal subtype specification activity similar to that of their Drosophila counterparts (for review, see Brunet and Ghysen 1999; Cepko 1999; Guillemot 1999; Hassan and Bellen 2000). In the best studied case, Mash1 has been shown by both GOF and LOF analyses to regulate the expression of the homeodomain gene Phox2a, which together with its relative Phox2b is an essential determinant of the noradrenergic neurotransmitter phenotype (Hirsch et al. 1998; Lo et al. 1998). These results have led to the proposition that Mash1 coordinates generic and neuronal subtype specific programs of neurogenesis (Hirsch et al. 1998; Lo et al. 1998). However, both Mash1 and Phox2b are required for Phox2a expression in neural crest-derived autonomic precursors (Hirsch et al. 1998; Lo et al. 1998; Pattyn et al. 1999), and their respective contribution to the activation of an autonomic program of differentiation during normal development has not been addressed directly. In particular, the role of Mash1 in noradrenergic differentiation remains unclear as the loss of Phox2a expression in Mash1 LOF mutants may reflect a requirement for the proneural function of Mash1 to progress past the stage of Phox2a expression, as well as a more direct role in regulating Phox2a expression. Additional evidence for a role of Mash1 in the specification of neuronal subtype identity is its capacity to induce the expression of GABAergic differentiation markers in dorsal telencephalic neurons (Fode et al. 2000). However, as this result is based on GOF analysis, it remains unknown whether it reflects a genuine function of Mash1 during telencephalic development.
A role in the specification of neuronal phenotypes has also been attributed to several of the vertebrate ato-related genes. Ectopic expression of the Ngn genes in migratory neural crest and mesodermal cells has been shown to induce the expression of several sensory neuron markers as well as generic markers of neuronal differentiation, thus implicating Ngn genes in the specification of sensory phenotypes in the PNS (Perez et al. 1999). A role for Ngns in the specification of neuronal identity in the CNS has not been addressed directly, however, particularly in a situation where Ngn activity could be compared directly with that of Mash1, in a manner similar to the parallel GOF analysis of ac-sc and ato genes performed in Drosophila (Chien et al. 1996; Parras et al. 1996; Bray 2000). In contrast, ato-related genes other than Ngns have been shown to have important functions in the specification of particular neuronal subtypes, including Math1 for sensory inner ear cells and Math5 for retinal ganglion cells (Kanekar et al. 1997; Bermingham et al. 1999; Hassan and Bellen 2000; Matter-Sadzinski et al. 2001).
Here, we have directly addressed the roles of Mash1 and Ngn2, two bHLH genes with well characterized proneural functions, in the specification of neuronal subtype identity. To avoid some of the limitations inherent to traditional LOF and GOF approaches, we have used a knock-in (KI) strategy, whereby the coding sequence of one of the two genes was introduced into the locus of the second gene that was simultanously deleted. In the resulting two strains of mice, Mash1 precisely replaces Ngn2 (Ngn2KIMash1 mice) and Ngn2 replaces Mash1 (Mash1KINgn2 mice) in the expression domain specific to the replaced gene. Given that these KI mutations result in the ectopic expression of a proneural gene in a distinct lineage but in a normal context, the resulting phenotypes should be relevant to the normal functions of these two genes. Moreover, the KI mutations allow for a direct comparative analysis of the activities of Mash1 and Ngn2 proneural proteins in the same cells. Finally, in this system we retain a proneural activity in neural progenitors, allowing neuronal subtype specification defects to be distinguished from proneural defects. Using these KI mutant mice, we have asked the following questions: Do both Mash1 and Ngn2 have an intrinsic capacity to specify neuronal subtype identity, and to what extent is their specification function influenced by the cellular context? In other words, can these proneural genes impose a new subtype specification program on progenitors, or are their activities constrained by other determinants with which they cooperate? Finally, how essential is the contribution of proneural genes to the specification of neuronal subtype identity in the different lineages where such a function has been demonstrated?
From this comparative analysis of Mash1 and Ngn2 functions, we conclude first that Mash1 can respecify a variety of distinct neuronal lineages when ectopically expressed in Ngn2+ precursors, whereas Ngn2 cannot respecify Mash1+ progenitors, indicating that Mash1 has an instructive role in specifying the identity of various neuronal subtypes whereas the role of Ngn2 in this process is permissive. Second, the ectopic expression of Ngn2 can only rescue some of the neuronal populations that normally depend on Mash1 activity, likely reflecting the existence of parallel specification pathways in a subset of Mash1-expressing lineages.
Results
Replacement of Mash1 by Ngn2 in mice
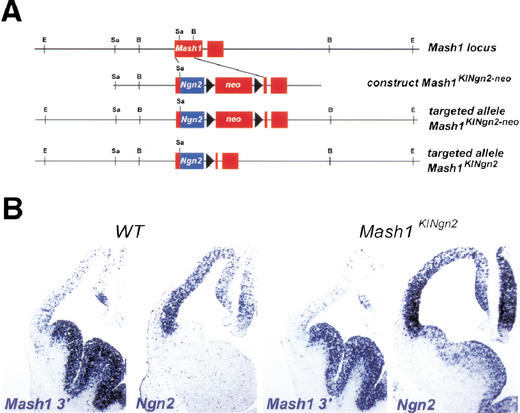
To systematically compare the activities of Ngn2 and Mash1 in contexts where proneural genes are normally active, we generated two KI mutations. An initial characterization of chimeric mice carrying a mutation in which the Mash1 coding sequence replaces the Ngn2 sequence (Ngn2KIMash1) has been reported previously (Fode et al. 2000). The reciprocal mutation, whereby the coding sequence of Mash1 was replaced by the sequence of Ngn2 (Mash1KINgn2; Fig. 1A), was generated by homologous recombination in embryonic stem cells. The mutation was transmitted to the germ line and animals heterozygous for the Mash1KINgn2allele were viable and fertile, whereas homozygous Mash1KINgn2animals died at birth in a manner similar to Mash1 null mutant (Mash1Δ) animals, indicating that Ngn2 does not have the capacity to completely rescue Mash1 function.
Figure 1.
Targeting strategy to introduce the Ngn2 sequence into the Mash1 locus. (A) Targeting vector designed to substitute the coding sequence of Mash1 with the coding sequence of Ngn2, leaving the 5′ and 3′ untranslated regions (UTR) of Mash1 intact. Following transmission of the mutation to the germline, the neomycin resistance gene (Neo) was excised, giving rise to the Mash1KINgn2 allele. (B) Hybridization of RNA probes for the Mash1 3′UTR sequence and the Ngn2 coding sequence to frontal sections of the telencephalic region of E12.5 wild-type and Mash1KINgn2homozygous mutant embryos, showing that Mash1KINgn2transcripts are detected in the ventral telencephalon.
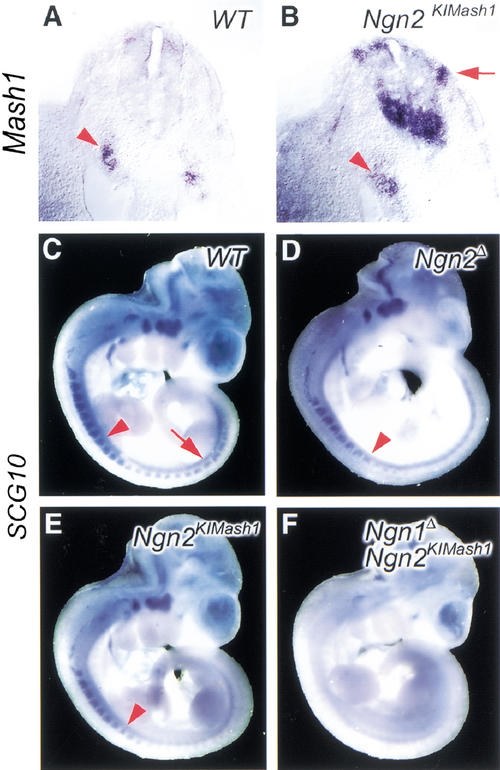
We confirmed that Ngn2 is correctly expressed in the Mash1 expression domain of Mash1KINgn2mice using two RNA probes recognizing respectively the coding sequence of Ngn2 and the 3′ untranslated region (UTR) of Mash1, both of which hybridize to the transcript encoded by the Mash1KINgn2allele (Fig. 1B). In the telencephalon of wild-type mice at E12.5, Ngn2 is expressed exclusively in the dorsal telencephalic ventricular zone (VZ) whereas Mash1 is expressed at high levels in the ventral VZ and at low levels dorsally (Fig. 1B). In embryos homozygous for the Mash1KINgn2allele (referred to below as Mash1KINgn2embryos), chimeric transcripts coding for the Ngn2 protein were found in the VZ and subventricular zone (SVZ) of the lateral and medial ganglionic eminences at levels comparable to those of wild-type Ngn2 transcripts in the mediodorsal telencephalon and of wild-type Mash1 transcripts in the ventral telencephalon (Fig. 1B). Ectopic expression of Ngn2 was also observed in other parts of the Mash1 expression domain, including the olfactory epithelium and the autonomic nervous system (data not shown).
Ectopic Ngn2 compensates for the loss of Mash1 proneural function in ventral telencephalon
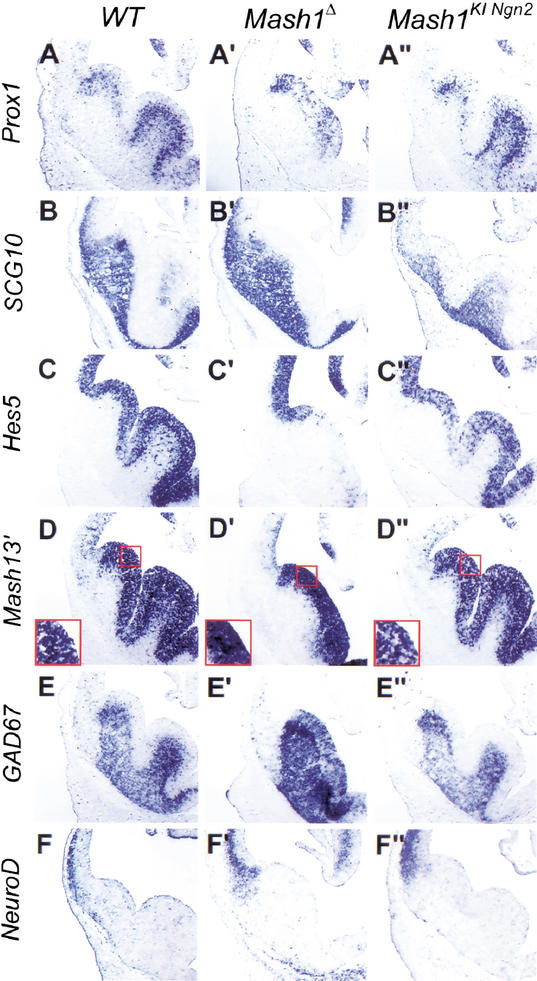
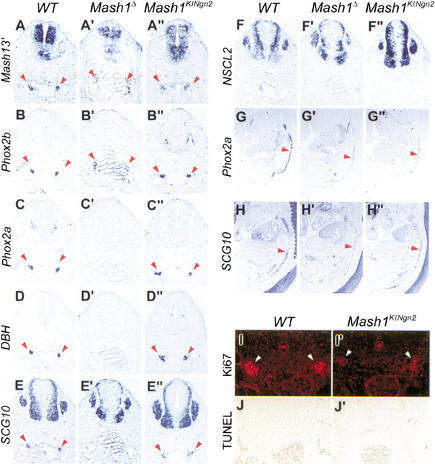
Mash1 and Ngn2 have been shown to act as proneural genes in different regions of the nervous system (Fode et al. 1998, 2000; Ma et al. 1998, 1999; Casarosa et al. 1999). The proneural function of Mash1, i.e., its role in the selection of neural progenitors and the activation of Notch signaling, has been best characterized in the olfactory epithelium (Guillemot et al. 1993; Cau et al. 1997) and ventral telencephalon (Casarosa et al. 1999; Horton et al. 1999). We first asked whether Ngn2 has the capacity to substitute for Mash1 proneural function and to initiate the neurogenesis program normally activated by Mash1 by comparing the development of the ventral telencephalon and olfactory epithelium in Mash1KINgn2and Mash1Δ mutant embryos. Strikingly, Mash1KINgn2embryos presented a complete rescue of the defect in ventral telencephalic progenitors observed in Mash1Δ embryos at E12.5, as shown by the presence of a medial ganglionic eminence (MGE) of normal size (Fig. 2A"–F"), in comparison with the atrophic MGE in Mash1Δ mutants (Fig. 2A‘–F‘). Moreover, MGE progenitors differentiated normally, as shown by normal expression of the homeodomain-containing gene Prox1 in SVZ progenitors and of the panneuronal marker SCG10 in postmitotic neurons in the mantle zone, whereas both markers are reduced in the ventral telencephalon of Mash1Δ embryos, particularly in the pallidal region (Fig. 2A‘, A",B‘,B").
Figure 2.
Ngn2 rescues ventral telencephalic development and does not promote dorsal telencephalic fates when replacing Mash1 in ventral telencephalic progenitors. Frontal sections at telencephalic level of E12.5 wild-type (A–F), homozygous Mash1Δ (A‘–F‘), and homozygous Mash1KINgn2(A"–F") embryos were hybridized with RNA probes for the genes shown on the left. Progenitors in the medial ganglionic eminence (MGE) are missing in Mash1 Δ embryos (A‘–F‘), and MGE formation is rescued by Ngn2 expression from the Mash1 locus (A"–F"). Differentiation of MGE progenitors is also restored, as shown by expression of Prox1 in subventricular zone (SVZ) progenitors (A") and of SCG10 in mantle zone neurons (B"). Ngn2 expression in ventral telencephalic progenitors also restores Notch signaling, as shown by expression of the Notch effector Hes5 in ventricular zone (VZ) progenitors (C") and by the “salt and pepper” pattern of expression of Mash1, as detected by a Mash1 3′UTR probe (D"), whereas Mash1 is transcribed more uniformly by VZ cells in Mash1 Δ embryos (D‘). Oulined areas in D–D" are magnified in the insets. Neurons differentiating from Ngn2-expressing progenitors in the ventral telencephalon acquire a normal phenotype, as shown by their expression of ventral markers such as GAD67 (E") and by the lack of misexpression of dorsal-specific genes such as NeuroD (F").
We then examined whether ectopic Ngn2 in ventral telencephalic progenitors activates Notch signaling, a pathway that is defective in Mash1Δ embryos (Casarosa et al. 1999; Fig. 2). Expression of the Notch ligand, Dll1 and of the transcriptional effector of Notch signaling, Hes5 (Ohtsuka et al. 1999) was rescued in Mash1KINgn2embryos (Fig. 2C"; data not shown). Moreover, transcription at the Mash1 locus, examined with a Mash1 3′UTR probe recognizing transcripts from the wild-type, Mash1Δ and Mash1KINgn2alleles of Mash1, was restricted to a subset of progenitor cells in the VZ of Mash1KINgn2embryos, as observed in wild-type embryos (Fig. 2D,D"), whereas it was more uniform in Mash1Δ embryos (Fig. 2D‘). This supports the conclusion that a Notch-mediated process of lateral inhibition involved in down-regulating proneural gene expression (de la Pompa et al. 1997) is restored in Mash1KINgn2mutants. Finally, the premature expression of the SVZ markers Prox1, GAD67, and Dlx5 in the VZ in Mash1Δ embryos was not observed in Mash1KINgn2embryos (Fig. 2A‘,A",E‘,E"; data not shown).
Together, these data demonstrate that Ngn2 misexpression in ventral telencephalic progenitors can fully compensate for the loss of Mash1 in all aspects of Mash1 proneural function in the ventral telencephalon, that is, the activation of Notch signaling in VZ progenitors, the selection of SVZ progenitors, and the differentiation of postmitotic neurons. In a similar manner, the ectopic expression of Ngn2 resulted in normal development of the olfactory epithelium in Mash1KINgn2embryos (data not shown).
Replacement of Mash1 by Ngn2 does not change the identity of ventral telencephalic neurons
We next addressed the possibility that Mash1 and Ngn2 activate distinct differentiation programs in telencephalic progenitors, as suggested by the observation that the two genes are expressed in complementary ventral and dorsal telencephalic domains that give rise to neuronal populations with different phenotypic characteristics, including different neurotransmitter phenotypes, i.e., GABAergic and glutamatergic, respectively (Wilson and Rubenstein 2000). We have shown previously that when Mash1 is expressed from the Ngn2KIMash1 allele, it induces in a subset of dorsal telencephalic neurons the expression of the markers of GABAergic neurons, GAD67 and Dlx1, suggesting that Mash1 specifies aspects of the neurotransmitter phenotype of telencephalic neurons (Fode et al. 2000). We asked whether reciprocally, the replacement of Mash1 by Ngn2, which rescues the differentiation of ventral telencephalic neurons in the pallidum (Fig. 2B"), results in the misspecification of these neurons.
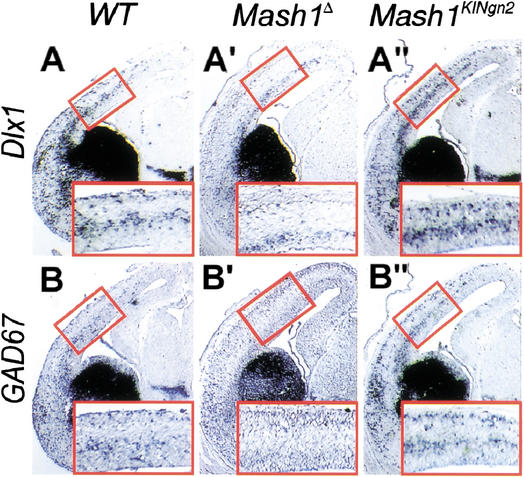
We examined the phenotype of neurons differentiating from Ngn2-expressing MGE progenitors in Mash1KINgn2using ventral-specific markers. Surprisingly, these neurons appeared correctly specified, as shown by the normal expression of GAD67 and Dlx1 at E12.5 (Fig. 2E"; data not shown). Moreover, examination of the cortex of Mash1KINgn2embryos at E15.5 revealed normal expression patterns of GAD67, Dlx1, and Lhx6 (Fig. 3A",B"; data not shown), indicating that interneurons migrating tangentially from the ventral telencephalon into the cortex, a neuronal population that is dramatically reduced in Mash1Δ mutant embryos (Casarosa et al. 1999; Anderson et al. 2001; Fig. 3A‘,B‘), are produced by Ngn2-expressing ventral telencephalic progenitors.
Figure 3.
Ngn2 rescues the formation of tangentially migrating interneurons in the cerebral cortex. Frontal sections through the telencephalon of E15.5 embryos hybridized with RNA probes for markers of tangentially migrating interneurons. Interneurons expressing Dlx1 (A–A") and GAD67 (B–B") are born in the ventral telencephalon and migrate tangentially into the cortex. Many of these neurons are missing in absence of Mash1 (A‘, B‘), and are rescued in Mash1KINgn2 embryos (A", B"). Outlined areas are magnified in the insets.
Finally, to ask whether Ngn2 can impart dorsal telencephalic characteristics when misexpressed in ventral progenitors, we examined the expression of the dorsal telencephalic markers NeuroD, Tbr1, and Math2 in Mash1KINgn2 embryos. Expression of Tbr1 and Math2 is dependent on Ngn2 function in the dorsal telencephalon (Fode et al. 2000), and NeuroD is a direct target of Ngn genes in all Ngn-dependent lineages (Ma et al. 1996, 1998; Fode et al. 1998; H.P. Huang et al. 2000). Surprisingly, none of these genes was ectopically expressed in the ventral telencephalon of Mash1KINgn2embryos at E10.5 and E12.5 (Fig. 2F"; data not shown). Thus, in striking contrast to the previous demonstration that Mash1 can confer ventral characteristics to dorsal telencephalic neurons (Fode et al. 2000), Ngn2 does not have the capacity to respecify ventral telencephalic neurons, and cannot even activate in the ventral telencephalon its direct target NeuroD, which is normally induced in all Ngn-dependent lineages.
Replacement of Ngn2 by Mash1 respecifies a subset of spinal motor neurons
The differences between the KI phenotypes of Mash1 and Ngn2 in the telencephalon may be caused by intrinsic differences in the activities of these two genes, or could reflect different strategies employed to specify ventral and dorsal identities in this region, with proneural genes contributing dorsally but not ventrally. To further address the possibility that Mash1 and Ngn2 have divergent properties in neuronal subtype specification, we examined the phenotypes of Mash1KINgn2and Ngn2KIMash1 embryos in the ventral spinal cord, another region of the CNS where Mash1 and Ngn2 are expressed in distinct progenitor domains.
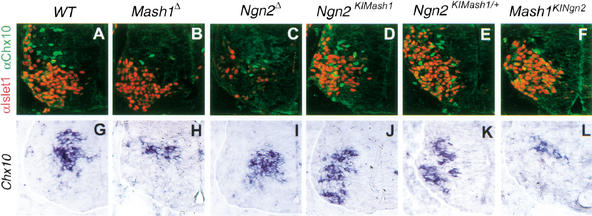
Ngn2 is broadly expressed in the VZ of the ventral spinal cord (Sommer et al. 1996; Scardigli et al. 2001). Loss of Ngn2 function leads to reduced expression of a number of homeodomain proteins throughout the ventral spinal cord, including in motor neurons and V1, V2, and V3 interneurons (Scardigli et al. 2001; Fig. 4C). In contrast, Mash1 expression in the ventral spinal cord is restricted at E10.5 to a narrow region of the VZ, coinciding with the domain that will produce V2 interneurons, characterized by expression of the homeodomain protein Chx10 (Mizuguchi et al. 2001). In Mash1Δ embryos, expression of Chx10 was severely reduced, indicating that Mash1 is required for the specification and/or differentiation of V2 interneurons (Fig. 4B). In contrast, markers for motor neurons and V1 interneurons showed no overt defects (data not shown).
Figure 4.
Mash1 and Ngn2 LOF and GOF phenotypes in the ventral spinal cord. (A–F) Double immunolabeling for the motor neuron marker Isl1 (red) and the V2 interneuron marker Chx10 (green) in transverse sections at brachial level of the ventral spinal cord of E10.5 embryos. There is a drastic reduction in number of V1 interneurons produced in the Mash1 mutant spinal cord (B), and expression of subtype-specific homeodomain proteins is affected in many neuronal populations of Ngn2 mutant embryos (Scardigli et al. 2001), including Isl1 in motor neurons and Chx10 in V1 interneurons (C). Expression of Isl1 is rescued by the ectopic expression of Mash1 in most motor neurons of homozygous Ngn2KIMash1 embryos (D), whereas a few cells in the motor neuron population fail to express Isl1 and instead express Chx10 (D). Ectopic expression of Chx10 in cells ventral to the V2 interneuron population is also observed in embryos heterozygous for the Ngn2KIMash1 allele (E). Expression of Ngn2 from the Mash1 locus in Mash1KINgn2homozygous mutant embryos does not rescue the defect in V2 interneurons resulting from the loss of Mash1 function (F). (G–L) Sections of the brachial spinal cord of E10.5 embryos hybridized with an RNA probe for Chx10. Loss of Mash1 results in a severe reduction in Chx10 expression (H) which is not rescued by ectopic Ngn2 expression in Mash1-expressing progenitors (L). In contrast, ectopic expression of Mash1 in Ngn2-expressing progenitors leads to a ventral expansion of the Chx10 expression domain (J,K).
To determine whether the defects found in the spinal cord of Ngn2Δ and Mash1Δ mutants reflect a generic proneural or differentiation function of Ngn2 and Mash1 in motor neurons and V2 interneurons, or conversely, specific functions of the two genes, we examined whether these defects were rescued in Mash1KINgn2 and Ngn2KIMash1 mice. Labeling of the ventral spinal cord of E10.5 Ngn2KIMash1 embryos with Chx10 and the motor neuron marker Isl1 showed that expression of these proteins was largely normal (Fig. 4D), indicating that Mash1 can replace Ngn2 for the control of the expression of neuron-specific homeodomain proteins. However, a small number of cells expressing Chx10 was intermingled with Isl1+ motor neurons (Fig. 4D), suggestive of a defect in motor neuron specification in these embryos. Ectopic Chx10+ cells, which were also observed in mice heterozygous for the Ngn2KIMash1 allele (Fig. 4E) did not express Isl1, indicating that these neurons have not acquired a mixed phenotype. Irx3 expression, which marks the V2 interneuron progenitor domain and not the motor neuron progenitor domain (Briscoe et al. 2000) was in contrast unaffected in Ngn2KIMash1 embryos (data not shown), thus excluding the possibility that the presence of Chx10+ cells ventral to their normal location is caused by abnormal mixing of progenitor populations for motor neurons and V2 interneurons. These results thus indicate that a subset of neurons in the spinal cord of Ngn2KIMash1 embryos have been converted from a Isl1+ motor neuron phenotype to a Chx10+ V2 interneuron phenotype, demonstrating that in the ventral spinal cord as in the dorsal telencephalon, ectopic expression of Mash1 is capable of respecifying a subset of Ngn2-dependent neurons.
We asked whether, reciprocally, replacement of Mash1 by Ngn2 could rescue or respecify V2 interneurons. Examination of the spinal cord of E10.5 Mash1KINgn2 embryos revealed that the number of Chx10+ cells was reduced to a similar extent in these embryos and in Mash1Δ embryos (Fig. 4B). Moreover, there was no evidence for the ectopic differentiation of Isl1+ motor neurons or En1+ V1 interneurons in the V2 domain (Fig. 4F; data not shown). Therefore, ectopic expression of Ngn2 is not able to rescue the Mash1Δ V2 interneuron defect or to respecify these cells into motor neurons or V1 interneurons. This data thus points to a significant difference in the properties of Mash1 and Ngn2, with Mash1 and not Ngn2 being capable of altering neuronal fates when ectopically expressed.
Ngn2 can replace Mash1 to support sympathetic neuron development
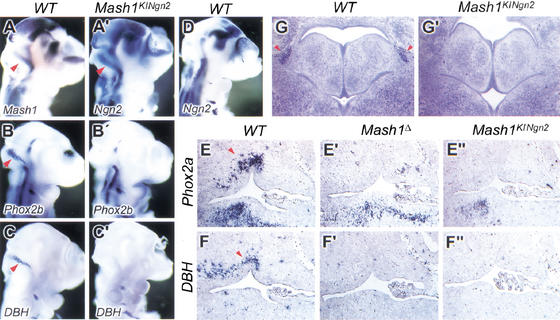
To further compare the role of Mash1 and Ngn2 in the specification of neuronal fates, we analyzed the development of noradrenergic neurons in Mash1KINgn2 mice. Mash1 has an essential role in the specification of the noradrenergic neurotransmitter phenotype through regulation of the homeodomain genes Phox2a and Phox2b, in both peripheral neurons of sympathetic ganglia and central neurons of the locus coeruleus (Hirsch et al. 1998; Lo et al. 1998). The panneuronal marker SCG10 and the noradrenergic markers Phox2a, DBH, and TH were expressed at normal levels in sympathetic precursors of Mash1KINgn2embryos at E10.5 (Fig. 5B"–E"; data not shown), whereas their expression was undetectable in Mash1Δ embryos (Fig. 6B‘–E‘). Furthermore, and in contrast with previous experiments showing that ectopic expression of an Ngn gene can induce the expression of sensory-specific markers in nonsensory neural crest derivatives (Perez et al. 1999), no ectopic expression of the sensory markers NSCL2 and Brn3 was observed in sympathetic ganglia in Mash1KINgn2embryos (Fig. 5F" data not shown). Thus, ectopic expression of Ngn2 in Mash1 mutant precursors supports sympathetic neuron differentiation, including the acquisition of a noradrenergic phenotype, and does not ectopically activate sensory neuronal traits.
Figure 6.
Ngn2 does not rescue the development of noradrenergic neurons of the locus coeruleus. (A–D, A‘–C‘) Hybridization of E10.5 wild-type (A–D) and Mash1KINgn2embryos (A‘–C‘) embryos with probes for Mash1 (A), Phox2b (B) and DBH (C), showing expression of these genes in precursors of the locus coeruleus, located in the rostral hindbrain near the rhombic lip (arrowheads). Ngn2 is expressed in these precursors in Mash1KINgn2embryos (A‘) and not in wild-type embryos (D). Nevertheless, noradrenergic traits, including expression of Phox2b (B‘) and DBH (C‘) are not induced by Ngn2 in Mash1KINgn2embryos. (E–E", F–F") Sagittal sections at the level of the fourth ventricle in wild-type and mutant embryos at E13.5. Phox2a and DBH are normally expressed by the neurons of the locus coeruleus located anterior and dorsal to the fourth ventricle (arrowheads in E and F, respectively), whereas expression of the two genes is missing at the same location in Mash1Δ (E‘, F‘) and Mash1KINgn2embryos (E", F"). (G, G‘) Nissl stained coronal sections at the level of the pons at birth. The locus coeruleus is recognizable as a compact group of darkly stained neurons lateral to the fourth ventricle (arrowhead in G), which is missing in Mash1KINgn2newborns (G‘).
Strikingly, despite the initial rescue of sympathetic neuron development, expression of Phox2a, DBH, and SCG10 was severely reduced in the sympathetic chain of Mash1KINgn2embryos at E13.5, to an extent similar to that seen in Mash1Δ embryos (Fig. 5G‘,H‘,G",H"). Thus, Ngn2 expression is not sufficient to maintain a normal differentiation program in Mash1 mutant sympathetic neurons. The appearance of a sympathetic mutant phenotype in Mash1KINgn2 embryos between E10.5 and E13.5 could be attributable to a defect in proliferation or to a degeneration of Ngn2-expressing sympathetic precursors. TUNEL analysis revealed no increase in cell death in sympathetic ganglia of E11.5 and E12.5 Mash1KINgn2mutants (Fig. 5J,J‘; data not shown). In contrast, a marked reduction in the number of sympathetic precursors staining with the mitotic cell-specific antibody Ki67 was observed in Mash1KINgn2embryos at E11.5 (Fig. 5I,I‘), and 46% fewer Phox2b+ precursors incorporated BrdU in Mash1KINgn2mutant embryos in comparison to wild-type controls (n>3000; data not shown). These results implicate a cell proliferation defect as a major cause for the incomplete generation of the sympathetic chain in Mash1KINgn2 embryos, and suggest that Ngn2 has a greater capacity than Mash1 to arrest proliferation of sympathetic precursors.
Figure 5.
Ngn2 rescues the differentiation of noradrenergic neuronal precursors in sympathetic ganglia but does not support their proliferation. Transverse sections at forelimb level of wild-type and mutant embryos at E10.5 (A–F), E13.5 (G–H) and E12.5 (I–J). In Mash1Δ embryos, Phox2b is expressed independently of Mash1 in sympathetic ganglia (B‘), whereas other genes associated with the noradrenergic phenotype, including Phox2a (C‘) and DBH (D‘), are not expressed, and postmitotic SCG10+ neurons (E‘) are not generated. In Mash1KINgn2embryos, Ngn2 supports the noradrenergic differentiation of sympathetic precursors, as shown by expression of Phox2a (C") and DBH (D"), and the generation of postmitotic SCG10+ neurons (E"). Hybridization on sagittal sections of E13.5 embryos with probes for Phox2a (G–G") and SCG10 (H–H") shows that the sympathetic ganglia of Mash1KINgn2embryos (G", H") are reduced compared with wild-type ganglia (G, H), to a level similar to that seen in Mash1Δ embryos (G‘, H‘). Antibody staining for the mitotic marker Ki67 reveals a reduced number of dividing cells in sympathetic ganglia of Mash1KINgn2embryos at E12.5 (I‘) as compared with wild-type embryos (I). TUNEL analysis shows that there is no increase in cell death in sympathetic ganglia of Mash1KINgn2embryos at E11.5 (J‘). Arrowheads indicate sympathetic ganglia
Ngn2 does not rescue locus coeruleus development in Mash1KINgn2 mutant embryos
The initial rescue of noradrenergic differentiation in Mash1KINgn2embryos could reflect the capacity of Ngn2 to directly activate the noradrenergic regulatory cascade when expressed in sympathetic precursors. Alternatively, noradrenergic differentiation in Mash1KINgn2embryos may be driven by the independent Phox2b pathway (Pattyn et al. 1999), with Ngn2 providing only a generic proneural/neuronal differentiation activity to sympathetic precursors. To further examine the potential of Ngn2 to promote the noradrenergic phenotype, we studied locus coeruleus development in Mash1KINgn2embryos. In E10.5 wild-type embryos, locus coeruleus precursors form a characteristically shaped group of cells in the rostral hindbrain that express Mash1, Phox2a, Phox2b, and DBH (Fig. 6A–C; data not shown). Ngn2 is expressed with a similar pattern in Mash1KINgn2embryos (Fig. 6A‘). In E10.5 Mash1KINgn2mutant embryos, expression of Phox2a, Phox2b, and DBH was undetectable in this region (Fig. 6B‘,C‘; data not shown), as reported previously for Mash1Δ embryos (Hirsch et al. 1998; data not shown). The locus coeruleus was also absent in Mash1KINgn2mice at E13.5 (Fig. 6E",F") and at P0 (Fig. 6G‘). Thus, Ngn2 does not rescue noradrenergic neuron development in the locus coeruleus, suggesting that Ngn2 does not have the potential to activate the noradrenergic differentiation pathway. Therefore, the rescue of sympathetic neuron differentiation in Mash1KINgn2embryos is likely caused by the combined activities of Ngn2, compensating for the loss of the generic neuronal determination/differentiation function of Mash1, and of an independent noradrenergic specification pathway driven by Phox2b, which is active in symathetic precursors and not in the locus coeruleus.
Mash1 does not rescue dorsal root ganglia development in Ngn1Δ, Ngn2KIMash1 mutant embryos
Ngn1 and Ngn2 have been shown to control the generation of different populations of sensory neurons in dorsal root ganglia (DRGs), resulting in a transient defect in neurogenesis in Ngn2 mutant mice, whereas the loss of both Ngn1 and Ngn2 completely blocks DRG development (Ma et al. 1999). A similar delay in onset of neuronal differentiation in DRGs was observed in Ngn2KIMash1 mice, using a probe for the panneuronal marker SCG10 (Fig. 7D,E). Moreover, no differentiation of DRG neurons was observed in Ngn1Δ; Ngn2KIMash1 double mutant embryos (Fig. 7F), a phenotype similar to that of Ngn1Δ; Ngn2Δ double mutants. Thus ectopic expression of Mash1 from the Ngn2 locus does not rescue DRG defects, indicating that Ngn2 has a unique activity in the development of peripheral sensory ganglia that Mash1 cannot carry out.
Figure 7.
Mash1 does not rescue the development of Ngn2-dependent sensory neurons in dorsal root ganglia. (A, B). Transverse section of E9.5 embryos sectioned following whole mount in situ hybridization with a Mash1 probe. Mash1 is expressed in sympathetic ganglia (arrowheads) of both wild-type and Ngn1Δ; Ngn2KIMash1 double mutant embryos. Mash1 expression is also observed in migrating neural crest cells entering DRGs in a Ngn2KIMash1 embryo. (C–F) Mouse embryos at E10.5 are probed with the pan-neuronal marker SCG10. A wild-type embryo shows DRG staining well beyond the 20th somite (C, arrow). SCG10 expression in Ngn2KIMash1 (E) is delayed in comparison to the wild-type embryo, as also observed in Ngn2Δ mutants (D). Arrowhead points to the eighth somite (C–E). Ngn1Δ, Ngn2KIMash1 embryo (F) shows no SCG10 staining in DRGs, indicating that no sensory neurons are generated by Mash1 expression from the Ngn2 locus.
Discussion
Mash1 and Ngn2, two proneural genes belonging respectively to the achaete-scute and atonal families of bHLH factors are expressed in distinct and complementary progenitor populations throughout the nervous system (Fig. 8), suggesting that these genes contribute to the specification of neuronal phenotypes in the CNS and PNS. We have used a KI approach in mouse to systematically compare the activities of Ngn2 and Mash1 when expressed in the same cellular contexts and at stages when proneural function is normally provided. We discuss here the divergent roles of Mash1 and Ngn2 in neuronal subtype specification, and the variable contribution of these genes to the specification of neuronal identity in different lineages (Fig. 8).
Figure 8.
. Summary of the phenotypic analysis of Ngn2KIMash1 and Mash1KINgn2mice. The different regions of the CNS and PNS analyzed in the two KI mouse strains are schematized. Neurogenesis defects observed in null mutant mice are rescued in KI mice (indicated by RN +), except in tissues that are missing due to specification defects (indicated by RN nd). The rescue of specification defects (indicated by RSS +) varies from lineage to lineage in both KI strains, reflecting the variable contribution of proneural genes to the specification of neuronal subtype identity in different lineages. Respecification of neuronal subtypes caused by ectopic proneural gene expression (indicated by RNS +) is observed only in the CNS of Ngn2KIMash1 mice, indicating that Mash1 but not Ngn2 has an instructive role in the specification of neuronal identity in the CNS.
Mash1 is a determinant of neuronal identity
Evidence that Mash1 has a role in the specification of neuronal identity has been obtained in different regions of the nervous system. The strongest evidence comes from GOF experiments showing that Mash1 can induce expression of (1) markers of GABAergic neurons, Dlx1 and GAD67, in the cerebral cortex (Fode et al. 2000), (2) the noradrenergic determinant Phox2a and an autonomic phenotype in neural crest stem cell and neural tube cultures (Lo et al. 1998, 2002), and (3) the marker of V2 interneuron identity Chx10, when expressed in motor neuron progenitors (this paper). Induction of ectopic markers in several regions of Ngn2KIMash1 embryos correlates with a down-regulation of markers that define neuronal phenotype in these regions (e.g., Islet1 in motor neurons, Math2 in cortical neurons), suggesting that ectopic expression of Mash1 in Ngn2-expressing progenitors results in substitution of neuronal subtype-specific differentiation programs. Respecification of cortical neurons and spinal motor neurons is also observed to some extent in heterozygous Ngn2KIMash1 embryos (Fode et al. 2000; Fig. 4E), indicating that this phenotype is indeed caused by the ectopic expression of Mash1 and that the expression of Ngn2 in the same cells does not significantly interfere. Moreover, it is important to notice that only a subset of neurons are respecified in both the dorsal telencephalon and motor neuron domains of homozygous Ngn2KIMash1 embryos whereas the majority of neurons differentiate normally (Fode et al. 2000; Fig. 4D), clearly indicating that instructive cues for the specification of cortical neurons and motor neurons persist in these mutant embryos in the absence of Ngn2 function.
From the above data, we conclude that Mash1 is an instructive determinant of neuronal subtype identity, in the sense that it can override endogenous differentiation programs when ectopically expressed, thus demonstrating that it conveys neuronal subtype information and acts to some extent independently from the cellular context. A striking feature of the Ngn2KIMash1 mutant phenotype is that ectopic expression of Mash1 results in the induction of different neuronal phenotypes in different regions of the nervous system, i.e., GABAergic neurons in the telencephalon and V2 interneurons in the spinal cord. If one includes the induction of Phox2a in neural crest cultures, then Mash1 is able to induce at least three different phenotypes in different progenitor populations. Within each of these regions, however, Mash1 expression and activity are associated with only one neuronal phenotype. This suggests that the specificity of Mash1 is modulated by regionally restricted cofactors, a hypothesis already proposed to account for the context-dependent specificity of Drosophila proneural proteins in sensory organ induction (Chien et al. 1996; Jarman and Ahmed 1998; Goulding et al. 2000). The identification of modulators of proneural gene activity will be critical to understand how neuronal identities are specified.
A permissive role for Ngn2 in specification of neuronal fates
In contrast with Mash1, Ngn2 does not have an instructive role in the specification of neuronal identity, based on both GOF (this study; Mizugushi et al. 2001) and LOF studies (Scardigli et al. 2001). First, none of the tissues examined in Mash1KINgn2mice showed evidence of respecification of neuronal identity (Fig. 8), indicating that the replacement of Mash1 by Ngn2 is not sufficient to override the subtype-specific differentiation programs activated in Mash1-expressing progenitors. This is in clear contrast with the ectopic expression of subtype-specific markers in the telencephalon and spinal cord in Ngn2KIMash1 embryos (Fode et al. 2000; Fig. 8). Second, electroporation of Ngn2 in the neural tube of chick embryos results in premature cell cycle exit and premature neuronal differentiation of neuroepithelial cells, as expected for a gene with proneural activity, but Ngn2-induced ectopic neurons do not express markers of motor neurons or ventral interneurons, consistent with the idea that Ngn2 does not have the capacity to endow progenitor cells with neuronal subtype information in an ectopic context. In contrast, the bHLH gene Olig2 has the capacity in the same assay to induce the expression of a number of motor neuron markers (Mizugushi et al. 2001; Novitch et al. 2001).
One interpretation of the above data could be that the function of Ngn2 in neurogenesis is strictly that of a proneural gene being involved in the selection of progenitor cells (Fode et al. 1998) and their commitment to a “generic” neuronal fate (Nieto et al. 2001), but having no role in the specification of neuronal identity. We do not think that this interpretation is likely for several reasons. First, LOF analysis of Ngns has revealed that although Ngn1 and Ngn2 have redundant proneural functions in the ventral spinal cord (as only Ngn1Δ;Ngn2Δ double mutants present a marked reduction in neurogenesis; Scardigli et al. 2001), Ngn2Δ single mutants present a severe reduction in expression of neuronal subtype-specific homeodomain proteins throughout the ventral spinal cord. Thus, Ngn2 function is required in neuronal fate specification programs independantly of its proneural activity (Scardigli et al. 2001). In the dorsal spinal cord, loss of Ngn1 and Ngn2 functions results in the substitution of a specific population of interneurons (D3A neurons) by another (D1 neurons), but this could be attributable to repression by Ngns of the subtype determinant Math1 rather than to a direct role of Ngns in specification of dorsal interneuron identity (Gowan et al. 2001).
GOF experiments also support the idea that Ngns contribute to the specification of neuronal identity, in particular for peripheral sensory neurons. Forced expression of Ngn1 in vivo by retroviral infection in the chick results in ectopic expression of several sensory neuron markers in tissues of neural and mesodermal origin (Perez et al. 1999). Expression of Ngn1 or Ngn2 in dissociated neural tube cell cultures induces sensory neurons more efficiently than it promotes neuronal differentiation, indicating that Ngns promote the sensory identity in peripheral neurons (Lo et al. 2002). Importantly, this activity requires specific culture conditions (i.e., low BMP concentrations). In other conditions (i.e., high BMP concentrations), Ngns promote instead an autonomic fate, revealing that the role of Ngns in neuronal subtype specification is strongly influenced by the cellular context, which dictates the quality of the subtype that Ngns promote (Lo et al. 2002). Another striking illustration of the dependence of Ngn function on the cellular context is the finding that ectopic expression of Ngn2 in the ventral telencephalon of Mash1KINgn2 mice rescues the generation of Mash1-dependent progenitors but does not induce NeuroD expression, although NeuroD is a direct target of Ngn genes throughout the embryo (H.P. Huang et al. 2000). This result highlights the critical role played by regionally expressed cofactors in determining the specificity of Ngn activity and the identity of the target genes that Ngns activate.
The inability of Ngn2 to override endogenous differentiation programs when ectopically expressed may be due in some situations to its late expression with respect to the period of competence of neural progenitors for subtype specification. In the sympathetic lineage in particular, Mash1 is first expressed in progenitor cells that may already be restricted to an autonomic fate (Lo et al. 2002), and this alone could explain that in Mash1KINgn2 embryos, Ngn2 expression from the Mash1 locus does not lead to a respecification of sympathetic neurons to a sensory fate. However, no systematic difference in temporal pattern of expression has been reported between Mash1 and Ngn2 in CNS progenitors, and it is therefore likely that differences in intrinsic properties rather than in timing of expression account for the different KI phenotypes of Mash1 and Ngn2.
Together, results from LOF and GOF analysis support the idea that Ngns are important components of the transcription machinery underlying the specification of neuronal identity. However, because Ngns do not interfere with neuronal subtype-specific differentiation programs when ectopically expressed (this study), and the outcome of their activity is largely controlled by other factors (this study; Lo et al. 2002), they do not have the characteristics of instructive determinants of neuronal identity. Instead, they appear to function as permissive factors that are required for the normal progression of subtype specification programs, as indicated by the role of Ngn2 in the expression of homeodomain neuronal determinant in the spinal cord (Scardigli et al. 2001), but that must act in combination with instructive factors to specify neuronal fates. In the spinal cord, the bHLH protein Olig2, which is expressed in the motor neuron progenitor domain, is a likely candidate as it can induce motor neuron differentiation when ectopically expressed in the chick neural tube (Novitch et al. 2001; Mizugushi et al. 2001). Similarly, Mash1 is expressed in the V2 interneuron progenitor domain (Mizugushi et al. 2001), and we show here that it is involved in the specification of V2 interneuron identity. Ngn2 is coexpressed with Olig2 and Mash1 in progenitors for motor neurons and V2 neurons, respectively (Mizugushi et al. 2001), suggesting that it may function in cooperation with these two factors, and possibly other bHLH proteins in different progenitor populations, to promote the differentiation of the different types of spinal cord neurons.
It has been proposed that the functions of progenitor selection and neuronal subtype specification, which are carried in parallel by the ac-sc and ato genes in Drosophila, have been uncoupled and are controlled by different bHLH genes in vertebrates (Hassan and Bellen 2000). As discussed above, the results of our study do not support this model and suggest on the contrary that vertebrate bHLH genes with proneural activity (i.e., Mash1 and Ngns) also have important and diverse functions in neuronal subtype specification, which likely involve interactions with many other determinants of neuronal identity.
Variable contribution of Mash1 to the specification of neuronal subtype identities
The rescue of the Mash1 null mutant phenotype by ectopic expression of Ngn2 varies in different regions of the nervous system (Fig. 8). Although development of the ventral telencephalon, olfactory epithelium and, transiently, sympathetic ganglia is normal in Mash1KINgn2mice, there is no rescue of V2 interneurons and locus coeruleus development. The correct differentiation of some Mash1-dependent noradrenergic neurons (e.g., sympathetic neurons) but not others (locus coeruleus) is striking, and can be explained by the difference in the genetic pathways specifying the noradrenergic phenotype in these two tissues (Goridis and Brunet 1999; Pattyn et al. 2000). Mash1 and the Phox2 genes are required for the production of noradrenergic neurons in both sympathetic ganglia and the locus coeruleus, but their interactions differ drastically in the two tissues. In the locus coeruleus, Mash1, Phox2a, and Phox2b act sequentially in a linear pathway, so that in absence of Mash1, neither Phox2a nor Phox2b is expressed (Hirsch et al. 1998; Pattyn et al. 2000). The replacement of Mash1 by Ngn2 in Mash1KINgn2 mice does not rescue locus coeruleus development, most likely because Ngn2 does not share with Mash1 the property of sufficiency to activate Phox2a expression and the noradrenergic differentiation pathway. This result therefore supports the conclusion that in the absence of instructive cofactors, Ngn2 does not have the capacity to induce the ectopic expression of inappropriate neuronal-subtype specific genes or the appropriate expression of subtype-specific genes normally dependent on Mash1.
In contrast with the complete dependence of Phox2a and Phox2b expression on Mash1 activity in the locus coeruleus, Phox2b expression is activated independently of Mash1 in sympathetic neuron precursors, and noradrenergic differentiation in these cells requires both and independently Mash1 and Phox2b activity (Hirsch et al. 1998; Pattyn et al. 2000). The rescue of Phox2a expression and noradrenergic differentiation that we observe in sympathetic precursors of Mash1KINgn2 mice is not caused by Ngn2 activity alone, based on the conclusion drawn from the locus coeruleus phenotype that Ngn2 does has the capacity to induce this phenotype (see above). More likely, Ngn2 provides a proneural/differentiation activity that is missing in Mash1Δ mutants, as well as a context-dependent subtype-determining function. Expression of the instructive cofactor Phox2b is then presumably sufficient, in conjunction with Ngn2, to induce noradrenergic differentiation. Thus, the rescue of the Mash1-null phenotype by ectopic expression of Ngn2 in sympathetic ganglia and not in the locus coeruleus probably reflects the different strategies underlying noradrenergic development in these two tissues (Goridis and Brunet 1999).
Similar to the rescue of sympathetic neuron development, the normal development of the ventral telencephalon in Mash1KINgn2mice presents a paradox. As already discussed, Mash1 has been implicated in the specification of the GABAergic phenotype of ventral telencephalic neurons, based on its capacity to induce in dorsal neurons the ectopic expression of Dlx genes, which are essential regulators of ventral telencephalic neuron differentiation (Anderson et al. 1997) and of the GABAergic neuron marker GAD67 (Fode et al. 1998). It was therefore unexpected that replacement of Mash1 by Ngn2 rescues the expression of Dlx1 and GAD67 and the normal differentiation of GABAergic neurons in the cortex and ventral telencephalon. As discussed above in the context of sympathetic development, this result clearly points to the existence of a Mash1-independent pathway of induction of Dlx gene expression and GABAergic neuron specification. Whereas this putative pathway cannot alone compensate for the loss of Mash1 function in Mash1Δ mice, it is sufficient to rescue telencephalic development in Mash1KINgn2 mice in combination with the proneural activity and cofactor-dependent subtype specification function of Ngn2. The respective contributions of Mash1 and other putative determinants to the specification of GABAergic neurons during normal telencephalic development remain to be established.
The role of Mash1 in the development of V2 spinal cord interneurons appears to be more similar to that discussed above for the locus coeruleus. The ectopic expression of Chx10 observed in Ngn2KIMash1 mice indicates that Mash1 has an instructive role in the specification of V2 interneurons, and the reduction in number of Chx10+ cells in Mash1Δ mice likely reflects a requirement for this specification function, although an additional role for Mash1 in the selection of V2 interneuron progenitors cannot be excluded. In any case, the lack of rescue of this defect in Mash1KINgn2mice indicates that Ngn2 alone cannot compensate for the loss of a specification function of Mash1, and in addition that no other instructive determinant of V2 interneuron identity can replace Mash1 function, even when Ngn2 activity is provided. Our analysis of Mash1KINgn2mice therefore points to important differences in the role played by Mash1 in subtype specification programs, with Mash1 being the major subtype determinant in some neuronal populations (locus coeruleus, V2 interneurons) and sharing this role with other factors in other populations (ventral telencephalon, sympathetic neurons).
Variable efficiency in the rescue of Ngn-dependent lineages by Mash1
The capacity of Mash1 to rescue the development of Ngn2-dependent lineages also varies in different regions of the nervous system (Fig. 8). The normal level of neurogenesis observed in the dorsal telencephalon of Ngn2KIMash1 demonstrates the capacity of Mash1 to compensate for the loss of Ngn2 proneural function (Fode et al. 2000). Similarly, Mash1 rescues the defects in homeodomain protein expression observed in the ventral spinal cord of Ngn2 null mutant embryos (Scardigli et al. 2001; Fig. 4), and analysis of embryos homozygous for both the Ngn2KIMash1 allele and a Ngn1 null mutant allele (Ngn1Δ; Ngn2KIMash1 embryos) shows that ectopic expression of Mash1 in Ngn2-expressing progenitors is sufficient to rescue the neurogenesis defect observed in the spinal cord of Ngn1; Ngn2 double mutants (Scardigli et al. 2001; data not shown). More surprisingly, ectopic expression of Mash1 does not rescue the development of dorsal root ganglia in Ngn1Δ; Ngn2KIMash1 embryos (Fig. 7). One possibility, supported by GOF studies in dissociated neural tube cell cultures (Lo et al. 2002), is that Ngns have a function in the specification of the sensory identity of DRG neurons that Mash1 cannot provide, and that in the low BMP environment of DRGs, Mash1 cannot promote an autonomic differentiation of DRG neurons.
An alternative interpretation of the lack of DRG development in Ngn1Δ, Ngn2KIMash1 mice is suggested by the finding that Mash1 has a greater capacity than Ngn2 to sustain the proliferation of sympathetic neuron precursors (Fig. 5I,I‘; Lo et al. 2002), and conversely that atonal-related genes such as Ngns and NeuroD are more efficient than Mash1 at promoting neuronal differentiation in ectodermal cells due to a lower sensitivity to Notch-mediated lateral inhibition (Chitnis and Kintner 1996; Ma et al. 1996; Lo et al. 2002). Because of the different capacities of the two genes to promote the transition from proliferation to differentiation, the substitution of Ngn2 by Mash1 in DRG cells may lead to an excess of cell proliferation and a delay of differentiation that may interfere with cell survival. The idea that Mash1 is more compatible with cell proliferation than are the Ngns is supported by the observation that expression of these genes generally correlates with the proliferative status of progenitor populations. Mash1 tends to be expressed in PNS and CNS progenitors undergoing extensive proliferation (e.g., sympathetic precursors, ganglionic eminence in the ventral telencephalon, SVZ progenitors in the dorsal telencephalon; C. Schuurmans and F. Guillemot, unpubl.) whereas Ngns tend to be expressed in progenitors that differentiate rapidely (e.g., sensory precursors in dorsal root and cranial ganglia, VZ progenitors in the dorsal telencephalon). Further investigation will be necessary to elucidate the mechanisms underlying the divergent properties of Mash1 and Ngn2 in regulating the transition from proliferation to differentiation and in specifying neuronal subtype identity.
Materials and methods
Construction of Mash1KINgn2 targeting vector
The Mash1 genomic clone (gb1.4) used to generate the Mash1KINgn2targeting vector was described previously (Guillemot et al. 1993). A 5.5-kb SacI fragment ending with the SacI site located three nucleotides downstream of the start ATG was used as the 5′ arm of the targeting construct. Ngn2 coding sequences were cloned directly downstream and in-frame with the endogenous start site of Mash1 by introducing an EcoRI cloning site immediately downstream of the SacI site by PCR. The 3′ arm was a 1.4-kb BamH1 fragment corresponding to the 3′-most sequence of the gb1.4 clone. These fragments were cloned into pKSloxPNT, which contains a loxP-flanked PGK-neomycin cassette (Hanks et al. 1995). The targeting vector was linearized by XhoI and electroporated into R1 ES cells as described previously (Guillemot et al. 1993). Homologous recombination events were identified by Southern blotting using EcoRI to digest the genomic DNA and a 1 kb HpaI fragment as a 5′- external probe, as previously described (Guillemot et al. 1993x). Positive clones were then verified with a 3′ 300-bp SacI/NdeI external probe from pλM1B7 (kind gift of Jane Johnson, University of Texas). One recombinant ES cell clone was injected into C57BL/6J females and gave germ-line transmission of the mutation. The mutant strain was maintained by backcrossing into a CD1 outbred background.
Genotyping of Mash1KINgn2, Ngn2KIMash1, Ngn2Δ, and Mash1Δ mutant mice
Ngn2KIMash1 and Ngn2Δ embryos were generated by crossing heterozygous animals and genotyping was performed by PCR as described previously (Fode et al. 1998, 2000). Wild-type, heterozygous and homozygous Mash1 mutant embryos were obtained from intercrosses of Mash1Δ/+ mice (Guillemot et al. 1993) or Mash1KINgn2/+ mice. For staging of embryos, midday of the vaginal plug was considered as E0.5. Genotyping of the different Mash1 alleles was preformed by PCR using the following primers and conditions. For the Mash1Δ allele, upper primer CCAG GACTCAATACGCAGGG and lower primer GCAGCGCATC GCCTTCTATC, with 30 cycles of 94°C/1 min, 60°C/1 min, and 72°C/1 min. For the Mash1KINgn2allele, upper primer ACAGTTTGGCCCGGCATGGA and lower primer AGATG TAATTGTGGGCGAAG with 30 cycles of 94°C/1 min, 60°C/1 min, and 72°C/1 min. For the Mash1 wild-type allele, upper primer CTCCGGGAGCATGTCCCCAA and lower primer CCAGGACTCAATACGCAGGG with 30 cycles of 94°C/1 min, 64°C/1 min, and 72°C/1 min.
RNA in situ hybridization and immunohistochemistry
Embryos were fixed at 4°C in 4% paraformaldehyde for 2 h (up to E11.5) or overnight (E12.5 and older), rinsed in phosphate-buffered saline (PBS), cryoprotected overnight in 20% sucrose in PBS and embedded in OCT (Tissue-Tek, Miles). Embedded embryos were sectioned on a cryostat at 10 μm. Section RNA in situ hybridization was performed as described in Cau et al. (1997). The cRNA probes used in this study were the following: Dll1, Hes5, GAD67, Dlx1, Dlx5, Lhx2, and SCG10, as described in Casarosa et al. (1999); Mash1 and NeuroD as described in Cau et al. (1997); Phox2a, Phox2b, and DBH as described in Pattyn et al. (1999); Ngn2 (Gradwohl et al. 1996); Prox1 (Torii et al. 1999); Lhx6 (Grigoriou et al. 1998); and Nscl2 (Perez et al. 1999). Double-label immunofluorescence was performed by simultaneous incubation with the antibodies of interest, which included monoclonal antibodies against Pax6 (Pax6), Islet-1 (40.2D6), Engrailed-1 (4G11) MNR2/HB9 (81.5C10) and Lim 1/2, all of which were obtained from the Developmental Studies Hybridoma Bank, and a Chx10 rabbit antiserum that was a gift from Johan Ericson (Karolinska Institute, Stockholm). Goat anti-mouse and goat anti-rabbit secondary antibodies conjugated to AlexA and Cy3 (Jackson Immunoresearch) were used for double labeling experiments. Confocal analysis was carried out on a Leica TCS 4D microscope.
Histology, BrdU incorporation, and TUNEL experiments
For histological analysis, embryos were fixed in Bouin’s fixative for 3–4 days, processed for wax embedding, cut at 7 μm, and stained with cresyl violet. For BrdU incorporation experiments, pregnant females were injected intraperitoneally with 100 μg/g of body weight of BrdU (Sigma) and sacrificed after 30 min. Embryos were processed as described above and BrdU labeling was exposed by 40 min treatment in HCl 2N at 37°C. The anti-BrdU monoclonal antibody (1:100 dilution) was from Boehringer Mannheim. The TUNEL procedure was performed as described in Cau et al. (1997).
Acknowledgments
We thank members of the lab for their advice during the progress of the work and Jean-François Brunet for his critical comments on the manuscript. We also thank Jean-Luc Vonesch and Didier Hentsch for help with confocal microscopy, and Marianne LeMeur and the transgenic facility staff for the generation of the knock-in mouse strains. We thank J. Ericson, S. Hodge, C.C. Hui, R.R. McInnes, S. Pfaff, and J. Rubenstein for the gifts of cDNAs and antibodies. The monoclonal antibodies Pax6, 74.5A5, 40.2D6, 4G11, 81.5C10, and 67.4E12, developed by A. Kawakami, T.M. Jessell, and S. Brenner-Morton were obtained from the Developmental Studies Hybridoma Bank maintained by the Iowa University, Department of Biological Sciences. C.P was supported by long term postdoctoral fellowships from the Spanish Ramón Areces Foundation and from the EMBO, C.S. by long term postdoctoral fellowships from the Human Frontiers Science Program and the Medical Research Council of Canada, and R. S. by the Italian Telethon Foundation and the European Community TRM Program. Note the change of name from Carol Fode to Carol Schuurmans. This work was supported by grants from the European Community “Quality of Life and Management of Living Resources” Research and Technological Development Program, the Human Frontiers Science Program, the Association pour la Recherche sur le Cancer and the Ministère de l'Enseignement et de la Recherche to F.G. and by institutional funds from INSERM, CNRS, and Hôpital Universitaire de Strasbourg.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL francois@igbmc.u-strasbg.fr; FAX 33-388-65-32-01.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.940902.
References
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bray S. Specificity and promiscuity among proneural proteins. Neuron. 2000;25:1–2. doi: 10.1016/s0896-6273(00)80862-4. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Ghysen A. Deconstructing cell determination: proneural genes and neuronal identity. Bioessays. 1999;21:313–318. doi: 10.1002/(SICI)1521-1878(199904)21:4<313::AID-BIES7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. The genetics of the Drosophila achaete-scute gene complex: A historical appraisal. Int J Dev Biol. 1998;42:291–297. [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Chien CT, Hsiao CD, Jan LY, Jan YN. Neuronal type information encoded in the basic-helix-loop-helix domain of proneural genes. Proc Natl Acad Sci. 1996;93:13239–13244. doi: 10.1073/pnas.93.23.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis A, Kintner C. Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development. 1996;122:2295–2301. doi: 10.1242/dev.122.7.2295. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes & Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- Goridis C, Brunet JF. Transcriptional control of neurotransmitter phenotype. Curr Opin Neurobiol. 1999;9:47–53. doi: 10.1016/s0959-4388(99)80006-3. [DOI] [PubMed] [Google Scholar]
- Goulding SE, zur Lage P, Jarman AP. amos, a proneural gene for Drosophila olfactory sense organs that is regulated by lozenge. Neuron. 2000;25:69–78. doi: 10.1016/s0896-6273(00)80872-7. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Fode C, Guillemot F. Restricted expression of a novel murine atonal-related bHLH protein in undifferentiated neural precursors. Dev Biol. 1996;180:227–241. doi: 10.1006/dbio.1996.0297. [DOI] [PubMed] [Google Scholar]
- Grigoriou M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–2074. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Vertebrate bHLH genes and the determination of neuronal fates. Exp Cell Res. 1999;253:357–364. doi: 10.1006/excr.1999.4717. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Hanks M, Wurst W, Anson-Cartwright L, Auerbach AB, Joyner AL. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2. Science. 1995;269:679–682. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- Hassan BA, Bellen HJ. Doing the MATH: Is the mouse a good model for fly development? Genes & Dev. 2000;14:1852–1865. [PubMed] [Google Scholar]
- Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- Horton S, Meredith A, Richardson JA, Johnson JE. Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol Cell Neurosci. 1999;14:355–369. doi: 10.1006/mcne.1999.0791. [DOI] [PubMed] [Google Scholar]
- Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by Neurogenin3. Mol Cell Biol. 2000;20:3292–307. doi: 10.1128/mcb.20.9.3292-3307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ML, Hsu CH, Chien CT. The proneural gene amos promotes multiple dendritic neuron formation in the Drosophila peripheral nervous system. Neuron. 2000;25:57–67. doi: 10.1016/s0896-6273(00)80871-5. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Ahmed I. The specificity of proneural genes in determining Drosophila sense organ identity. Mech Dev. 1998;76:117–125. doi: 10.1016/s0925-4773(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Brand M, Jan LY, Jan YN. The regulation and function of the helix-loop-helix gene, asense, in Drosophila neural precursors. Development. 1993;119:19–29. doi: 10.1242/dev.119.Supplement.19. [DOI] [PubMed] [Google Scholar]
- Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125:609–620. doi: 10.1242/dev.125.4.609. [DOI] [PubMed] [Google Scholar]
- Lo, L, Dormand, E., Greenwood, A., and Anderson, D.J. 2002. Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development. In press. [DOI] [PubMed]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes & Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter-Sadzinski L, Matter JM, Ong MT, Hernandez J, Ballivet M. Specification of neurotransmitter receptor identity in developing retina: The chick ATH5 promoter integrates the positive and negative effects of several bHLH proteins. Development. 2001;128:217–231. doi: 10.1242/dev.128.2.217. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K, Nakafuku M. Combinatorial roles of Olig2 and Neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Modolell J. Patterning of the adult peripheral nervous system of Drosophila. Perspect Dev Neurobiol. 1997;4:285–296. [PubMed] [Google Scholar]
- Morrison SJ. Neuronal differentiation: Proneural genes inhibit gliogenesis. Curr Biol. 2001;11:349–351. doi: 10.1016/s0960-9822(01)00191-9. [DOI] [PubMed] [Google Scholar]
- Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras C, Garcia-Alonso LA, Rodriguez I, Jimenez F. Control of neural precursor specification by proneural proteins in the CNS of Drosophila. EMBO J. 1996;15:6394–6399. [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci. 2000;15:235–243. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- Perez SE, Rebelo S, Anderson DJ. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development. 1999;126:1715–1728. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Schuurmans C, Gradwohl G, Guillemot F. Cross regulation between Neurogenin2 and pathways specifying progenitor identity in the spinal cord. Neuron. 2001;31:203–217. doi: 10.1016/s0896-6273(01)00358-0. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Doe CQ. The achaete-scute complex proneural genes contribute to neural precursor specification in the Drosophila CNS. Curr Biol. 1996;6:1146–1152. doi: 10.1016/s0960-9822(02)70681-7. [DOI] [PubMed] [Google Scholar]
- Sommer L, Ma Q, Anderson DJ. neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes & Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]