Abstract
Antinuclear antibody (ANA) is found in connective tissue disorders and in autoimmune liver disease. While ANA-positive connective tissue disorders are subdivided according to possession of specific antibodies to extractable nuclear antigens (ENA) (anti-ribonucleoprotein (anti-RNP), anti-Smith (anti-Sm), anti-Ro, anti-La), little is known about the presence and significance of ENA in autoimmune liver disease. To investigate this, we have tested 35 children with autoimmune hepatitis (AIH) (19 ANA and/or smooth muscle antibody-positive (ANA/SMA+ve); 16 liver kidney microsomal 1-positive (LKM-1 + ve)) and 14 with ANA/SMA+ve autoimmune sclerosing cholangitis (ASC), using both double dimension immunodiffusion and ELISAs. Eighty children with non-autoimmune liver disease (20 alpha 1-antitrypsin deficiency, 20 Wilson's disease, 20 Alagille's syndrome and 20 chronic hepatitis B virus infection) and 20 healthy controls were also tested. ENA were detected in seven (20%) patients with AIH: two ANA-positive, one SMA-positive and four LKM-1-positive. Three were positive for anti-Sm, two for anti-La, one for anti-Sm/anti-La and one for anti-Sm/anti-La/anti-Ro. ENA-positive had more severe liver disease than ENA-negative patients (P < 0.03). ENA were not detected in ASC, non-autoimmune liver diseases and controls. Our results indicate that ENA reactivity, including anti-Sm and anti-La, characteristic of systemic lupus erythematosus and Sjögren's syndrome, respectively, are present in some patients with AIH even in the absence of ANA, and may characterize a particularly severe form of the disease.
Full text
PDF

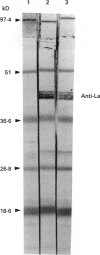
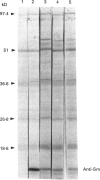
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cervera R., Khamashta M. A., Font J., Sebastiani G. D., Gil A., Lavilla P., Doménech I., Aydintug A. O., Jedryka-Góral A., de Ramón E. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1993 Mar;72(2):113–124. [PubMed] [Google Scholar]
- Clotet B., Guardia J., Pigrau C., Lience E., Murcia C., Pujol R., Bacardí R. Incidence and clinical significance of anti-ENA antibodies in systemic lupus erythematosus. Estimation by counterimmunoelectrophoresis. Scand J Rheumatol. 1984;13(1):15–20. doi: 10.3109/03009748409102662. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Saito I. Criteria for diagnosis of Sjögren's syndrome. Rheum Dis Clin North Am. 1994 May;20(2):391–407. [PubMed] [Google Scholar]
- Garry R. F. New evidence for involvement of retroviruses in Sjögren's syndrome and other autoimmune diseases. Arthritis Rheum. 1994 Apr;37(4):465–469. doi: 10.1002/art.1780370405. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Scofield R. H., Reichlin M. Anti-Ro in Sjögren's syndrome and systemic lupus erythematosus. Rheum Dis Clin North Am. 1992 May;18(2):337–358. [PubMed] [Google Scholar]
- Hochberg M. C. Systemic lupus erythematosus. Rheum Dis Clin North Am. 1990 Aug;16(3):617–639. [PubMed] [Google Scholar]
- James K., Meek G. Evaluation of commercial enzyme immunoassays compared to immunofluorescence and double diffusion for autoantibodies associated with autoimmune diseases. Am J Clin Pathol. 1992 Apr;97(4):559–565. doi: 10.1093/ajcp/97.4.559. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., McFarlane I. G. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993 Oct;18(4):998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- Konikoff F., Shoenfeld Y., Isenberg D. A., Barrison I., Sobe T., Theodor E., Slor H. Anti-Rnp antibodies in chronic liver diseases. Clin Exp Rheumatol. 1987 Oct-Dec;5(4):359–361. [PubMed] [Google Scholar]
- Kurki P., Gripenberg M., Teppo A. M., Salaspuro M. Profiles of antinuclear antibodies in chronic active hepatitis, primary biliary cirrhosis and alcoholic liver disease. Liver. 1984 Apr;4(2):134–138. doi: 10.1111/j.1600-0676.1984.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Mackay I. R. The hepatitis-lupus connection. Semin Liver Dis. 1991 Aug;11(3):234–240. doi: 10.1055/s-2008-1040441. [DOI] [PubMed] [Google Scholar]
- Maddison P. J. ANA-negative SLE. Clin Rheum Dis. 1982 Apr;8(1):105–119. [PubMed] [Google Scholar]
- Maddison P. J., Provost T. T., Reichlin M. Serological findings in patients with "ANA-negative" systemic lupus erythematosus. Medicine (Baltimore) 1981 Mar;60(2):87–94. doi: 10.1097/00005792-198103000-00002. [DOI] [PubMed] [Google Scholar]
- Mieli-Vergani G., Lobo-Yeo A., McFarlane B. M., McFarlane I. G., Mowat A. P., Vergani D. Different immune mechanisms leading to autoimmunity in primary sclerosing cholangitis and autoimmune chronic active hepatitis of childhood. Hepatology. 1989 Feb;9(2):198–203. doi: 10.1002/hep.1840090206. [DOI] [PubMed] [Google Scholar]
- Mieli-Vergani G., Vergani D. Progress in pediatric autoimmune hepatitis. Semin Liver Dis. 1994 Aug;14(3):282–288. doi: 10.1055/s-2007-1007318. [DOI] [PubMed] [Google Scholar]
- Mills J. A. Systemic lupus erythematosus. N Engl J Med. 1994 Jun 30;330(26):1871–1879. doi: 10.1056/NEJM199406303302608. [DOI] [PubMed] [Google Scholar]
- Olsen M. L., Arnett F. C., Reveille J. D. Contrasting molecular patterns of MHC class II alleles associated with the anti-Sm and anti-RNP precipitin autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 1993 Jan;36(1):94–104. doi: 10.1002/art.1780360117. [DOI] [PubMed] [Google Scholar]
- Penner E., Kindas-Mügge I., Hitchman E., Sauermann G. Nuclear antigens recognized by antibodies present in liver disease sera. Clin Exp Immunol. 1986 Feb;63(2):428–433. [PMC free article] [PubMed] [Google Scholar]
- Struckmann J., Worning P., Manthorpe R., Bendixen G. Detection of antibody against extractable nuclear antigen by an enzyme-linked immuno-sorbent assay. Results from patients with rheumatic and internal medical diseases. Allergy. 1985 Aug;40(6):442–446. doi: 10.1111/j.1398-9995.1985.tb02683.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- el-Shabrawi M., Wilkinson M. L., Portmann B., Mieli-Vergani G., Chong S. K., Williams R., Mowat A. P. Primary sclerosing cholangitis in childhood. Gastroenterology. 1987 May;92(5 Pt 1):1226–1235. doi: 10.1016/s0016-5085(87)91082-1. [DOI] [PubMed] [Google Scholar]