Abstract
Human mastocytosis is characterized by increased mast cells. It usually occurs as a sporadic disease that is often transient and limited in children and persistent or progressive in adults. The c-KIT protooncogene encodes KIT, a tyrosine kinase that is the receptor for mast cell growth factor. Because mutated KIT can transform cells, we examined c-KIT in skin lesions of 22 patients with sporadic mastocytosis and 3 patients with familial mastocytosis. All patients with adult sporadic mastocytosis had somatic c-KIT mutations in codon 816 causing substitution of valine for aspartate and spontaneous activation of mast cell growth factor receptor (P = 0.0001). A subset of four pediatric onset cases with clinically unusual disease also had codon 816 activating mutations substituting valine, tyrosine, or phenylalanine for aspartate. Typical pediatric patients lacked 816 mutations, but limited sequencing showed three of six had a novel dominant inactivating mutation substituting lysine for glutamic acid in position 839, the site of a potential salt bridge that is highly conserved in receptor tyrosine kinases. No c-KIT mutations were found in the entire coding region of three patients with familial mastocytosis. We conclude that c-KIT somatic mutations substituting valine in position 816 of KIT are characteristic of sporadic adult mastocytosis and may cause this disease. Similar mutations causing activation of the mast cell growth factor receptor are found in children apparently at risk for extensive or persistent disease. In contrast, typical pediatric mastocytosis patients lack these mutations and may express inactivating c-KIT mutations. Familial mastocytosis, however, may occur in the absence of c-KIT coding mutations.
Mastocytosis and mast cell disease are synonymous terms applied to a heterogeneous group of conditions that have in common increased numbers of mast cells in various organs and characteristic clinical findings. Mastocytosis usually occurs as a sporadic disease and is rarely familial (1–3). The skin is the organ most commonly involved, and cutaneous lesions usually consist of discrete macular and papular collections of dermal mast cells with increased melanin pigment in the overlying epidermis. Cutaneous disease presenting with lesions of this type has been called urticaria pigmentosa because the pigment is visible clinically and the lesions tend to urticate when the mast cells degranulate and release their vasoactive products. Although individual cutaneous lesions may show some similarities in all patients, there may be marked differences in lesional morphology and in the clinical course of individual patients.
Mastocytosis traditionally has been divided into two groups based on the age of onset of the disease. A majority of cases occur in infants and children as transient disease, with papular lesions that are confined to the skin and that usually disappear by adolescence. A second group of patients has adult onset cutaneous disease, which persists or progresses and which may be associated with small papular and macular lesions or infiltrative plaques, involvement of other organs (systemic mastocytosis), and/or hematopoietic abnormalities. However, some cases of pediatric disease do not fit into this dichotomous classification scheme and may persist or progress; some pediatric cases may be of later onset or have larger lesions, diffuse cutaneous involvement, or systemic involvement.
The cause and nature of mastocytosis have not been determined experimentally, although it is clear that some cases of mastocytosis are true neoplasms in which the neoplastic mast cells contain heterozygous somatic mutations of the c-KIT protooncogene (4). The mutations described in patients thus far cause constitutive activation of the c-KIT protein product (KIT), which is the receptor for mast cell growth factor (4–6).
KIT is a type III transmembrane tyrosine kinase with an extracellular domain that binds mast cell growth factor, also known as steel factor or stem cell factor (SCF) (7, 8). KIT is expressed by several cell types, including mast cells and melanocytes (7–9). Activation of KIT by ligand binding results in dimerization and phosphorylation of specific tyrosine residues in the intracellular portion of KIT and initiates a sequence of events that may effect cell proliferation and differentiated functions (10, 11). Activated KIT stimulates proliferation of mast cells and prevents their apoptosis in vitro and in vivo (12, 13). However, although KIT activation is widely thought of as stimulating growth, signaling through KIT may have different effects on cells in different physiologic states. For instance, KIT stimulates proliferation of cultured melanocytes and is critical for melanocyte development (9, 14–17). However, early ectopic KIT expression by melanocytes in murine dermatomes may actually interfere with melanocyte development (18), and KIT activation may stimulate apoptosis in melanoma cells (9, 19, 20).
Most known c-KIT mutations cause loss of function of KIT, resulting in piebaldism in humans (21, 22) and the W spotted phenotype in the mouse (16, 17). However, c-KIT mutations in codon 816 causing substitution of aspartate or tyrosine for valine, and a codon 559 mutation causing substitution of valine for glycine, have been found in neoplastic mast cell lines and have been reported to cause constitutive phosphorylation and activation of KIT (23–28). A number of additional c-KIT somatic activating mutations have been found clustered in the juxtamembrane region in human gastrointestinal stromal tumors (29). These mutations can transform cell lines from factor-dependent growth to factor-independent in vitro growth (25, 28, 29, 30). Furthermore, animal studies have shown that these activating mutations can confer tumorigenicity in vivo (24, 30), suggesting that these or similar mutations might cause or contribute to the development of mastocytosis. We have reported isolated cases in which an activating mutation, causing substitution of valine (V) for aspartate (D) in codon 816 (V816D), was found in the peripheral blood of adult patients with mastocytosis and hematopoietic disorders (5) as well as in the neoplastic mast cells of a patient with systemic mastocytosis (4) and in a skin lesion of a child with extensive cutaneous involvement and systemic symptoms (6). However, no systematic survey of mastocytosis patients for c-KIT mutations has been reported to date, and current classification schemes of mastocytosis do not take these important molecular genetic data into account.
To determine whether c-KIT mutations are associated with various clinical forms of mastocytosis, we screened the coding sequences of c-KIT mRNA from cutaneous lesions of patients with sporadic adult, sporadic childhood, and familial mastocytosis. We found that mutations of c-KIT codon 816 resulting in constitutive activation of the mast cell growth factor receptor were characteristic of all cases of sporadic adult cutaneous mastocytosis and a small group of sporadic pediatric patients with distinctive clinical features. Seemingly paradoxically, limited sequencing showed that almost half of the patients with classic pediatric mastocytosis had a novel mutation causing loss of KIT function, suggesting c-KIT may be capable of functioning as a tumor suppressor as well as an oncogene.
METHODS
Patients.
The 25 patients with cutaneous mastocytosis (Table 1) included 3 adult patients with sporadic systemic mastocytosis with skin and visceral involvement (patients 1–3), 8 adults with sporadic disease confined to the skin (patients 4–11), 4 pediatric cases with extensive or progressive disease (patients 12–15) including one who was studied as an adult, 6 children with classical pediatric mastocytosis (patients 16–22), and 3 extremely rare patients, members of a kindred with familial cutaneous mastocytosis (patients 23–25). Of the familial patients, two were adults and the third was a child with diffuse cutaneous involvement.
Table 1.
Patient characteristics
| Patient | Type | Age of onset | Cutaneous involvement | Systemic involvement |
|---|---|---|---|---|
| 1* | Sporadic | 40 years | Progressive | Marrow, spleen, liver, lymph nodes |
| 2 | Sporadic | 56 years | Progressive | Marrow with fibrosis, spleen, liver, gastrointestinal |
| 3 | Sporadic | 20 years | Progressive | Marrow with fibrosis |
| 4 | Sporadic | 27 years | Slowly progressive (10 years)† | None |
| 5 | Sporadic | 25 years | Persistent (11 years) | None |
| 6 | Sporadic | 49 years | Persistent (10 years) | None |
| 7 | Sporadic | 41 years | Persistent (11 years) | None |
| 8 | Sporadic | 33 years | Persistent (3 years) | None |
| 9 | Sporadic | 35 years | Persistent (10 years) | None |
| 10 | Sporadic | 12 years | Slowly progressive (15 years)†‡ | None |
| 11 | Sporadic | 34 years | Persistent | None |
| 12 | Sporadic | 6 months | Progressive§ | Marrow, spleen |
| 13 | Sporadic | Birth | Massive diffuse cutaneous | Liver, spleen, (clinically) |
| 14 | Sporadic | 1 week | Cutaneous papules, nodules, and plaques | None |
| 15 | Sporadic | 2 years | Progressive¶ | None |
| 16 | Sporadic | 6 months | Transient‖ | None |
| 17 | Sporadic | 11 months | Transient‖ | None |
| 18 | Sporadic | 5 months | Transient‖ | None |
| 19 | Sporadic | 1 year | Transient‖ | None |
| 20 | Sporadic | 3 months | Transient‖ | None |
| 21 | Sporadic | 18 months | Transient‖ | None |
| 22 | Sporadic | 6 months | Transient‖ | None |
| 23 | Familial | 2 week | Persistent** | None |
| 24 | Familial | <6 months | Persistent** | None |
| 25 | Familial | 6 months | Persistent | None |
Previously reported limited sequencing (4).
Slowly progressive cases have shown urticaria pigmentosa type lesions that significantly increased over 10 or more years, without systemic involvement. Persistent cases have similar lesions but have remained stable.
This patient also could be classified as a late case of pediatric onset (age 12) (Table 3, Group 1c).
This patient had a history of infantile onset and was referred for study as an adult.
Slightly later onset than usual, this child has continued to develop lesions over 4 years and shows an adult type mutation.
Patients presenting to primary care physician/dermatologist with typical childhood urticaria pigmentosa, assumed to be transient (as are most cases). Average follow-up 2 years, range 6 months to 4 years.
Patients 23, 24, and 25 are now 4, 21, and 41 years old, respectively, and still have disease.
Tissue Samples.
Lesional skin biopsies were available from all patients. (Institutional Review Board: CU-M1356, YU-M1452, M1000). Most patients with sporadic disease (patients 1–11 and 13–22) had formalin-fixed archival tissue available. In addition, fresh biopsies were taken and frozen from patients 1–9, 12–16, and 23–25. Buccal epithelial cells were also available from patients 1–6, 12–15, and 23–25.
Mutation Screening.
RNA was extracted from microtome sections cut from either paraffin-embedded or frozen biopsies, and genomic DNA was extracted from buccal epithelial cells, as described (4, 21). Copy DNA was synthesized with reverse transcriptase by using random hexamers as primers, and c-KIT coding sequences were amplified from copy DNA with the PCR by using primer pairs indicated in Table 2 (31). The entire KIT coding region was sequenced in cases in which frozen biopsy material was available, including patients in each of the different clinical groups. Because only a small amount of copy DNA could be generated from the paraffin-embedded material, and because the resulting individual copy DNAs were short, sequencing of archival material was limited to targeted areas within the region encoding the KIT phosphotransferase and juxtamembrane domains, in which activating mutations have been identified (4–7). Sequences were determined by direct amplimer sequencing and by subcloning followed by plasmid sequencing. It has been shown that mast cells are the main source of c-KIT mRNA in lesions of mastocytosis and that a dual signal in direct amplimer sequencing and a mixed population of mutant and wild-type sequences in corresponding subclones indicates heterozygosity in the mast cells (4).
Table 2.
Oligonucleotide primers used for DNA amplification and sequencing
| Primer name | Oligonucleotide | Position | Bases sequenced* |
|---|---|---|---|
| Kit A | 5′-CAAGATCTATCGCAGCTACCGCGATGAGAGGC | 7–30 | 394 |
| Kit B | 3′-TTCCCATACAAGGAGCGGTCAAC | 401–379 | |
| Kit C | 5′-TGAATGGATCACGGAAAAGGCAG | 261–383 | 353 |
| Kit D | 3′-TCCGACAGCACTGACTTGCC | 614–595 | |
| Kit E | 5′-TGCATTGTTCTGTGGACCAGG | 572–592 | 485 |
| Kit F | 3′-GGTGTTCAGGTTTGGGGAATG | 1057–1037 | |
| Kit G | 5′-GACTTCAATTATGAACGTCA | 817–836 | 504 |
| Kit H | 3′-GGAATCCTGCTGCCACACAT | 1321–1302 | |
| Kit I | 5′-TCCTGACTTACGACAGGCTC | 1265–1248 | 585 |
| Kit J | 3′-CCATAAGCAGTTGCCTCAAC | 1850–1832 | |
| Kit K | 5′–CCTTATGATCACAAATGGGA | 1750–1769 | 466 |
| Kit L | 3′-ACAACATAAGAAACTCCAGG | 2216–2197 | |
| Kit M | 5′-GATCATGCAGAAGCTGCACT | 2107–2126 | 486 |
| Kit N | 3′-AAAATCCCATAGGACCAGAC | 2594–2575 | |
| Kit O | 5′-CATCATGGAGGATGACGAGTTGG | 2286–2308 | 342 |
| Kit P | 5′-TGTATTCACAGAGACTTGGC | 2383–2402 | 214 |
| Kit Q | 3′-GGGGCTGCTTCCTAAAGAGAACAG | 2628–2605 | |
| Kit R | 5′-CTGTGAAGTGGATGGCACCT | 2516–2535 | 479 |
| Kit S | 3′-GAAGAGATCATTCCTGGAGG | 2995–2976 |
Bases sequenced = amplimer size minus primers.
Statistical Analysis.
Associations were tested in two-way tables by using two-sided Fisher exact tests in sas 6.11 (SAS Institute, Cary, NC).
In Vitro Expression of KIT.
A normal human c-KIT copy DNA was subcloned into the PCDNAIII mammalian cell expression vector (Invitrogen), and mutants were generated by site-directed mutagenesis: base 2467 G → T, tyrosine (Y) for aspartate at position 816 (D816Y); base 2468 A → T, valine for aspartate at position 816 (D816V); bases 2467 G → T and 2468 A → T, phenylalanine (F) for aspartate at position 816 (D816F); and base 2536 G → A, lysine (K) for glutamic (E) acid at position 839 (E839K). A double mutant, D816V/E839K, also was made to determine whether E839K is intramolecularly dominant. Normal and mutated copy DNAs were transfected and expressed in COS-7 cells. KIT was immunoprecipitated and blotted with antiphosphotyrosine or anti-KIT antibodies as described (4). Treatment with SCF was 200 mg/ml, 37°C, for 10 minutes after serum starvation for 18 h.
RESULTS
Prevalence of Mutations.
Sequencing of copy DNAs from skin lesions showed that 18 of 22 sporadic patients had c-KIT coding mutations resulting in amino acid substitutions. Direct amplimer sequencing showed dual signals at the indicated bases, and subcloning showed a mixture of wild-type and mutated clones, indicative of heterozygosity of the lesional mast cells (4). Eleven adult cases (patients 1–11) and four pediatric cases with extensive or progressive disease (patients 12–15) were heterozygous for mutations in codon 816, which result in constitutively activated mast cell growth factor receptor (Table 3, Group 1). The presence of codon 816 mutations did not discriminate between cutaneous involvement only or cutaneous and extracutaneous involvement. This was true in both the adult patients (Table 3, Groups 1a and 1b) and the pediatric patients (Groups 1c and 1d). Adult patients with activating 816 mutations included three with cutaneous and systemic disease (Table 3, patients 1, 2, and 3, Group 1a) and eight with mastocytosis confined to the skin (patients 4–11, Group 1b). These 11 adult onset cases all had a transversion in base 2468 (A → T) that causes substitution of valine for aspartate (KITV816D) that had been reported in isolated cases (4–6). Thus, activating mutations in codon 816 of c-KIT were found in all adults with sporadic cutaneous mastocytosis, whether or not they had systemic involvement, and therefore appear characteristic of this disease.
Table 3.
Patients grouped by mutational status
| Group | Mutation/substitution | Spontaneous phosphorylation | Percent of KIT sequenced | Primer pair(s) | Patients | Common features |
|---|---|---|---|---|---|---|
| 1a | 2468/V816D | Yes | 100% | All* | 1–3 | Sporadic progressive adult urticaria pigmentosa with systemic involvement |
| 1b | 2468/V816D | Yes | 100% 7% | All PQ | 4–9 10, 11 | Sporadic slowly progressive or persistent adult urticaria pigmentosa without systemic involvement |
| 1c | 2467/V816Y | Yes | 100% | All | 12, 13 | Extensive pediatric cutaneous disease with systemic involvement |
| 1d | 2467, 2468/V816F 2468/V816D | Yes Yes | 100% 7% | All PQ | 14 15 | Extensive or progressive pediatric cutaneous disease without systemic involvement |
| 2a | 2536/E839K | No, dominant loss of function | 100% 11% | All OP | 16 17, 18 | Sporadic pediatric urticaria pigmentosa (presumed transient) |
| 2b | None found | Unknown (partial sequence revealed only wild-type sequence) | 11% | OP | 19–22 | Sporadic pediatric urticaria pigmentosa (presumed transient) |
| 3 | None present | No (wild-type) | 100% | All | 23–25 | Familial persistent urticaria pigmentosa |
Complete sequencing of c-KIT coding regions used a combination of primers O and Q and/or P and Q in phosphotransferase region.
Four cases with childhood onset and unusual clinical presentations also had codon 816 mutations (Table 3, Groups 1c and 1d). One pediatric case (patient 12) had an unusual history of infantile onset cutaneous mastocytosis that persisted and progressed to adult systemic mastocytosis. Sequences of c-KIT from skin lesions and spleen of this patient, sampled as an adult, were heterozygous for a transversion in base 2467 (G → T), resulting in substitution of tyrosine for aspartate in codon 816 (KITV816Y). KITV816Y has been detected and studied in vitro and was reported to be highly constitutively activated (23, 28, 32) but has not been demonstrated in vivo. A second pediatric case (patient 13), who had diffuse cutaneous mastocytosis with clinical involvement of liver and spleen, had the same tyrosine substitution at codon 816 found in patient 12. The third pediatric patient in this group (patient 14) had several large plaques in additional to smaller discrete lesions and showed novel codon 816 mutations (GA → TT in bases 2467 and 2468) resulting in substitution of phenylalanine for aspartate (KITV816F) and constitutive activation of KIT (32). The same KITV816D mutation found in sporadic adult cases was found in one pediatric patient who had relatively late onset of adult type macular lesions and progressive disease after 4 years (patient 15). Thus, the pediatric patients with codon 816-activating mutations had more extensive and/or persistent disease than typical pediatric patients.
Codon 816 mutations were not present in any of the seven children with classic pediatric sporadic cutaneous mastocytosis (Table 3, Group 2) or in the three patients (two adults and one child) with familial mastocytosis (Group 3). However, lesions of three children with sporadic cutaneous mastocytosis contained a novel transition in base 2536 (G → A), resulting in substitution of lysine for glutamic acid in at position 839 (KITE839K) (Table 3, Group 2a). Targeted sequencing of the limited material available from the other four sporadic pediatric patients (Table 3, Group 2b) and complete sequencing of c-KIT from lesions of the three patients with familial mastocytosis (Group 3) showed no detectable mutations in this region or in the juxtamembrane domain.
Statistical analysis showed that codon 816 mutations are significantly associated with persistent and extensive disease, with P = 0.0001 (Table 3, Group 1 vs. Group 2). Codon 839 mutations are associated less strongly with typical pediatric mastocytosis (Table 3, Group 2), with P = 0.023. Overall, sporadic mastocytosis is associated with c-KIT mutations, with P = 0.0008 (Table 3, Groups 1 and 2 vs. Group 3).
Fresh frozen biopsy material, sufficient for sequencing of the entire KIT coding region, was available from lesions of 11 of the patients, including all 3 patients with sporadic adult cutaneous and systemic mastocytosis (patients 1–3), 6 patients with sporadic adult cutaneous mastocytosis (patients 4–9), 4 pediatric cases with sporadic cutaneous mastocytosis (patients 12, 13, 14, 16), and the 3 patients with familial mastocytosis (patients 23–25). It is especially noteworthy that all of the sporadic patients in whom the entire KIT coding region could be sequenced were heterozygous for a single c-KIT mutation. No c-KIT mutations were identified in three patients with familial mastocytosis, demonstrating that familial cutaneous mastocytosis can occur in children and in adults in the absence of c-KIT coding mutations. When available, sequencing of genomic DNA in cases with identified mutation (cases 1–6, 10, 12–14) showed that neither the 816 nor 839 mutations were present in the germ line, indicating that these are somatic mutations (4).
The absence of any of c-KIT mutations in lesions of the kindred with familial mastocytosis also emphasizes the specific association of individual mutations with specific forms of mastocytosis and underscores the differences between classic sporadic mastocytosis and familial mastocytosis. In this context, the absence of c-KIT coding mutations in these patients demonstrates that other factors must be responsible for some cases of mastocytosis and confirms the clinical impression that mastocytosis is actually a heterogeneous group of diseases.
In Vitro Function of Mutant KITs.
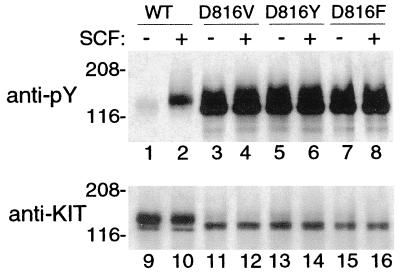
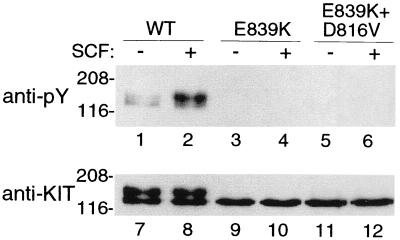
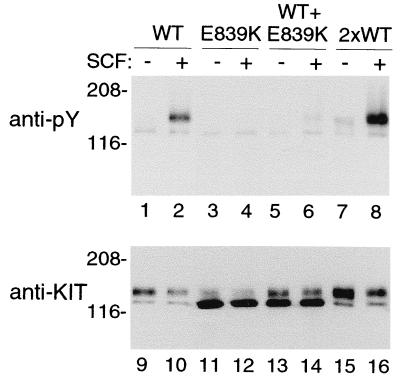
We confirmed that all of the KIT mutations associated with persistent or extensive disease, (KITV816D, KITV816Y, and KITV816F) caused spontaneous phosphorylation of KIT and were present predominantly as a 145-kDa form (Fig. 1). In contrast, KITE839K, the novel mutation identified in lesions of three sporadic pediatric patients, had unique properties. KITE839K was present predominately as a 125-kDa protein that did not mature into the 145-kDa protein characteristic of both wild-type and mutated KITs (Fig. 2). Furthermore, KITE839K was not phosphorylated spontaneously or in response to exogenous SCF. Adding a codon 816 activating mutation to KITE839K (KITV816D/E839K) failed to restore phosphorylation, indicating that the E839K mutation causes a loss of function and is intramolecularly dominant over an activating mutation. Coexpression of KITE839K with KITWT showed that the 839 mutation was dominant–negative in an intermolecular sense (dominant–interfering, codominant–negative), suppressing phosphorylation of coexpressed KITWT exposed to exogenous SCF (Fig. 3).
Figure 1.
KITWT and KIT mutant phosphorylation in COS-7 cells. Blotting of immunoprecipitated KIT with an antiphosphotyrosine antibody (anti-PY, lanes 1–8) confirms in our system the previously reported results of other researchers (23, 24, 32) showing minimal spontaneous tyrosine phosphorylation of KIT in COS-7 cells expressing KITWT (lane 1) and high levels of spontaneous tyrosine phosphorylation of mutant KITs with substitution in position 816 (lanes 3, 5, and 7). These results serve as controls for Fig. 2. Increased tyrosine phosphorylation of KITWT is seen after exposure to exogenous SCF (+, lane 2), but tyrosine phosphorylation of codon 816 mutants is already maximal, so there is no increase in response to SCF (compare lanes 4, 6, and 8 with lanes 3, 5, and 7). After stripping, reprobing of the blot with anti-KIT antibody demonstrates the relative amounts of protein in each lane (lanes 9–16). Note that much greater amounts of KITWT are needed to demonstrate spontaneous tyrosine phosphorylation than are needed with codon 816 mutants (compare lane 9 with lanes 11, 13, and 15) and that maximal KIT tyrosine phosphorylation is higher in the mutant KITs than in KITWT (compare lane pairs 2 and 10 with 4 and 12, 6 and 14, and 8 and 16).
Figure 2.
Codon 839 mutation inactivates KIT. Blotting of immunoprecipitated KIT with an antiphosphotyrosine antibody (anti-Py, lanes 1–6) shows a low level of spontaneous KIT tyrosine phosphorylation in COS-7 cells expressing high levels of KITWT (lane 1) but no spontaneous phosphorylation of KITE839K (lane 3). KITWT is phosphorylated in response to exogenous SCF, but KITE839K is not (lanes 2 and 4, respectively). A double mutant, KITD816V/E839K, which incorporates both the activating D816V mutation and E839K loss-of-function mutation, is not phosphorylated spontaneously or in response to exogenous SCF, indicating that the E839K has an intramolecular dominant–inactivating effect (lanes 5 and 6). Blotting with anti-KIT antibody after stripping the antiphosphotyrosine antibody (anti-KIT, lanes 7–12) shows KIT protein in all transfectants. Note also that KITWT is present as both a 125-kDa form and as a fully glycosylated, mature, 145-kDa form (lanes 7 and 8) but that KITE839K is present predominately as a lower molecular weight form in the steady state (lanes 9, 10, 11, and 12).
Figure 3.
Dominant–negative effect of Glu839Lys mutant on wild-type KIT phosphorylation. Anti-py blot of immunoprecipitated wild-type and mutant KITs expressed in COS-7 cells. Again, KITWT shows minimal spontaneous phosphorylation but is phosphorylated in response to exogenous SCF (lanes 1 and 2). The E839K mutant is not phosphorylated spontaneously or in response to SCF (lanes 3 and 4). SCF-induced KITWT phosphorylation (lane 2) is decreased dramatically when the wild-type receptor plasmid is transfected with an equal amount of the E839K mutant plasmid (lanes 5 and 6). As a control, a double amount of KITWT plasmid (2×) was transfected (lanes 7 and 8). Spontaneous and SCF-induced phosphorylation were both increased compared with the lower amounts of KITWT plasmid (compare lanes 1 and 2 with 7 and 8). Reprobing of the anti-Py blot (after stripping) with anti-KIT Ab shows the amount of protein in each lane. Long exposure documents a minor component of 145-kDa KITE839K (lanes 11 and 12). The amounts of 125- and 145-kDa KIT in lanes 13 and 14 equals approximately the sum of these two forms expressed separately (lanes 9 and 10, KITWT; lanes 11 and 12, KITE839K). The increase in protein in the control 2× transfected KITWT (lanes 15 and 16) over the 1× KITWT transfectant is comparable to the increase in phosphorylation (lanes 15 and 16 vs. lanes 9 and 10). Molecular markers are indicated in kilodaltons on the left.
DISCUSSION
We have identified somatic mutations of c-KIT, the gene encoding the mast cell growth factor receptor, in lesions of the majority of our patients with sporadic cutaneous mastocytosis. These mutations are not polymorphisms because they are somatic mutations and are not present in the germ line DNA of the patients. Mutations of codon 816, which cause ligand independent constitutive activation of KIT (23–26, 28, 30), were present in all adults with sporadic cutaneous mastocytosis, whether or not the patients had systemic involvement.
We have suggested that these mutations are causal events in the development of some forms of mastocytosis (4). Because of their known ability to provide a growth advantage by stimulating autonomous cell proliferation (28) and the ability of activated KIT to transform the cells in vitro and in vivo (23–26, 28, 30), we hypothesize that cells containing activating c-KIT mutations are positively selected, perhaps via both enhanced proliferation and inhibition of apoptosis (12, 13), thus explaining their presence and persistence in the skin and other organs. Because codon 816-activating mutations were found in all patients with sporadic adult mastocytosis, they appear to be a feature of this form of the disease, and we therefore hypothesize that codon 816 mutations are necessary for persistent or progressive adult sporadic disease. The presence of these mutations, however, does not appear to differentiate between patients with and without extracutaneous disease. Other as yet undetermined factors, or perhaps the stage of mast cell development at which mutation occurs, may determine whether there is systemic involvement.
The result of analysis of pediatric sporadic mastocytosis is more complex. These patients fall into three groups: those with codon 816 activating mutations (combined Groups 1c and 1d in Table 3), those with codon 839 inactivating mutations, and those with no identified mutations. The Group 1 pediatric patients, with activating codon 816 mutations, are clinically distinct in that these patients tend to have disease that is more extensive and/or more persistent than typical pediatric patients. It should be stressed that, although this group represents a significant percentage of our pediatric patients, they are probably quite rare because all but one were referred because of an unusual clinical presentation. As is the case with the adult Group 1 patients, the presence of 816-activating mutations does not distinguish between those with or without systemic involvement.
The second group of pediatric patients (Table 3, Group 2) is distinct because of the presence of a dominant inactivating mutation in codon 839. This mutation is dominant in an intramolecular sense because KIT with both E839K and D816V is not spontaneously activated and is nonfunctional. E839K is also dominant–negative in a intermolecular (dominant–interfering) sense because it suppresses SCF-induced phosphorylation of coexpressed KITWT. In studies reported separately, we have shown that KITE839K is not glycosylated fully and is retained predominately in the Golgi apparatus (Y.M., X.W., and B.J.L., unpublished work). It is notable that the glutamic acid at position 839 is conserved at homologous positions in all known receptor tyrosine kinases (33). Based on crystallization studies of the insulin receptor (34), this amino acid appears to form a salt bridge with an arginine at position 914, which also is conserved completely in receptor tyrosine kinases. Our in vitro results show that the glutamic acid at 839 is necessary for KIT autophosphorylation, suggesting that this putative salt bridge helps maintain critical tertiary structure of the enzymatic portion of KIT and presumably other tyrosine kinases.
Although the role the novel mutation in codon 839 might play in the pathogenesis of the three sporadic pediatric patients is not intuitively obvious, it is clear that it does not have the same role that the 816 mutations have in persistent disease. There are several possible explanations for its presence. First, the 839 mutation may be an epiphenomenon not causally related to mastocytosis. This seems unlikely because this mutation is not found in adult sporadic mastocytosis or in familial cases and therefore does not seem to occur simply in proliferating mast cells. Second, it is possible that activation of mast cell growth factor receptor may be required to suppress mast cell growth at some stage in development. This possibility is suggested by the known ability of activated KIT to suppress proliferation of melanoma cells despite its ability to stimulate proliferation of normal melanocytes and by the ability of ectopic KIT expression to interfere with normal melanoblast development (9, 18, 19, 20). Furthermore, it has been observed that melanocytes explanted from skin of mice homozygous for the mild loss-of-function c-KIT mutant Wf exhibit malignant transformation at a much higher rate than melanocytes explanted from skin of normal mice (35). Thus, there is precedent for an association of loss of KIT function, tumorigenesis, and tumor progression both in vivo and in vitro. These considerations are all important because the onset of pediatric mastocytosis is usually congenital or in early infancy, when many cells are undergoing unique developmental programs that could be affected differentially by KIT activation. Understanding the role of this mutation may, therefore, require in vivo analysis in a developmentally appropriate animal model.
Because we could only examine a limited region of c-KIT in most of our sporadic pediatric cases, it is possible that other activating or inactivating mutations occur in these patients. However, our data indicate that E839K is dominant, and we can say definitively that one pediatric case (patient 16) contains only the E839K mutation because we sequenced the entire c-KIT coding region and found no other mutation. As more sporadic pediatric cases become available for complete analysis, the association of specific c-KIT mutations with these cases may become stronger and may be correlated with prognosis. Our current data, however, are sufficient to indicate the need to assign pediatric cases with 816 mutations into a special group because of the possibility of disseminated, extensive, or persistent disease and to propose reevaluating the classification of mastocytosis incorporating molecular genetic data. Regardless of its role in the pathogenesis of pediatric mastocytosis, the segregation of the codon 839 mutation into a single clinical group of patients strengthens the association of specific mutations with specific clinical forms of mastocytosis and emphasizes the differences between persistent adult type mastocytosis and transient pediatric type disease.
A recent report of the analysis of a kindred with multiple gastrointestinal stromal tumors is of interest (36). Sporadic gastrointestinal stromal tumors are associated with somatic c-KIT mutations causing amino acid changes or deletions in the juxtamembrane region and constitutive activation of KIT (29). Affected members of this kindred carry similar germline mutations and have gastrointestinal stromal tumors but not mastocytosis (36). This is in contrast to our patients with familial mastocytosis, in whom no c-KIT mutations were identified. The lack of somatic c-KIT mutations in this family suggests that additional pathologic mechanisms exist in some patients with mastocytosis, and in this kindred are inherited.
Acknowledgments
We acknowledge Drs. David N. Silvers, Nancy Esterly, Maria Garzon, and Alan Halperin for contributing cases, Dr. Ruth Halaban for critical discussions, and Dr. Gerald Lazarus for insisting that typical pediatric mastocytosis is a different disease from adult mastocytosis. This work was funded in part by National Institutes of Health Grant RO1 AR 43356-01A2 and the Yale Skin Disease Research Center Grant.
ABBREVIATION
- SCF
stem cell factor
References
- 1.Longley B J, Duffy T P, Kohn S. J Am Acad Dermatol. 1995;32:545–561. doi: 10.1016/0190-9622(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe D D. J Invest Dermatol. 1991;96:2S–4S. [PubMed] [Google Scholar]
- 3.Soter N A. J Invest Dermatol. 1991;96:32S–39S. [PubMed] [Google Scholar]
- 4.Longley B J, Tyrrell L, Lu S-Z, Ma Y-S, Langley K, Ding T G, Duffy T, Jacobs P, Tang L H, Modlin I. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 5.Nagata H, Worobec A S, Oh C K, Chowdhury B A, Tannenbaum S, Suzuki Y, Metcalfe D D. Proc Natl Acad Sci USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata H, Okada T, Worobec A S, Semere T, Metcalfe D D. Int Arch Allergy Immunol. 1997;113:184–186. doi: 10.1159/000237541. [DOI] [PubMed] [Google Scholar]
- 7.Yarden Y, Kuang W J, Yang-Feng T, Coussens L, Munemitsu S, Dull T J, Chen E, Schlessinger J, Francke U, Ullrich A. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qui F, Ray P, Brown K, Barker P E, Jhanwar S, Ruddle F H, Besmer P. EMBO J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakut R, Perlis R, Eliyahu S, Yarden Y, Givol D, Lyman S D, Halaban R. Oncogene. 1993;8:2221–2229. [PubMed] [Google Scholar]
- 10.Koch C A, Anderson D, Morgan M F, Ellis C, Pawson T. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 11.Pawson T. Science. 1995;252:573–580. [Google Scholar]
- 12.Iemura A, Tsai M, Ando A, Wershil B K, Galli S J. Am J Pathol. 1994;144:321–328. [PMC free article] [PubMed] [Google Scholar]
- 13.Mekori Y A, Oh C K, Metcalfe D D. J Immunol. 1993;151:3775–3784. [PubMed] [Google Scholar]
- 14.Funasaka Y, Boulton T, Cobb M, Yarden Y, Fan B, Lyman S D, Williams D E, Anderson D M, Zakut R, Mishima Y, et al. Mol Biol Cell. 1992;3:197–209. doi: 10.1091/mbc.3.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galadari I, el Komy M, Mousa A, Hashimoto K, Mehregan A H. Int J Dermatol. 1992;31:253–256. doi: 10.1111/j.1365-4362.1992.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 16.Geissler E N, Ryan M A, Housman D E. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 17.Chabot B, Stephenson D A, Chapman V M, Besmer P, Bernstein A. Nature (London) 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 18.Kluppel M, Nagel D L, Bucan M, Bernstein A. Development (Cambridge, UK) 1997;124:65–77. doi: 10.1242/dev.124.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Lassam N, Bickford S. Oncogene. 1992;7:51–56. [PubMed] [Google Scholar]
- 20.Huang S Y, Luca M, Gutman M, McConkey D J, Langley K E, Lyman S D, Bar-Eli M. Oncogene. 1996;13:2339–2347. [PubMed] [Google Scholar]
- 21.Giebel L B, Spritz R A. Proc Natl Acad Sci USA. 1991;88:8696–8699. doi: 10.1073/pnas.88.19.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spritz R A, Giebel L B, Holmes S A. Am J Hum Genet. 1992;50:261–269. [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujimura T, Furitsu T, Morimoto M, Isozaki K, Nomura S, Matsuzawa Y, Kitamura Y, Kanakura Y. Blood. 1994;83:2619–2626. [PubMed] [Google Scholar]
- 24.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield J H, Ashman L K, Kanayama Y, et al. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitayama H, Kanakura Y, Furitsu T, Tsujimura T, Oritani K, Ikeda H, Sugahara H, Mitsui H, Kanayama Y, Kitamura Y, et al. Blood. 1995;85:790–798. [PubMed] [Google Scholar]
- 26.Tsujimura T, Furitsu T, Morimoto M, Kanayama Y, Nomura S, Matsuzawa Y, Kitamura Y, Kanakura Y. Int Arch Allergy Immunol. 1995;106:377–385. doi: 10.1159/000236870. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimura T, Morimoto M, Hashimoto K, Moriyama Y, Kitayama H, Matsuzawa Y, Kitamura Y, Kanakura Y. Blood. 1996;87:273–283. [PubMed] [Google Scholar]
- 28.Hashimoto K, Tsujimura T, Kimura M, Tohya K, Morimoto M, Kitayama H, Kanakura Y, Kitamura Y. Am J Pathol. 1996;148:189–200. [PMC free article] [PubMed] [Google Scholar]
- 29.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Kurata A, Takeda M, Tunio G M, Matsuzawa Y, Kanakura Y, et al. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 30.Piao X, Bernstein A. Blood. 1996;87:3117–3123. [PubMed] [Google Scholar]
- 31.Longley J, Ding T G, Cuono C, Durden F, Crooks C, Hufeisen S, Eckert R, Wood G S. J Invest Dermatol. 1991;97:974–979. doi: 10.1111/1523-1747.ep12491890. [DOI] [PubMed] [Google Scholar]
- 32.Moriyama Y, Tsujimura T, Hashimoto K, Morimoto M, Kitayama H, Matasuzawa Y, Kanakura Y. J Biol Chem. 1996;271:3347–3350. doi: 10.1074/jbc.271.7.3347. [DOI] [PubMed] [Google Scholar]
- 33.Hanks S, Quinn A M, Hunter T. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 34.Hubbard S, Wei L, Ellis L, Hendrickson W. Nature (London) 1994;372:746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- 35.Larue L, Dougherty N, Porter S, Mintz B. Proc Natl Acad Sci USA. 1992;89:7816–7820. doi: 10.1073/pnas.89.16.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida T, Hirata S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A, Matsuda H, Kitamura Y. Nat Genet. 1998;19:323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]