Abstract
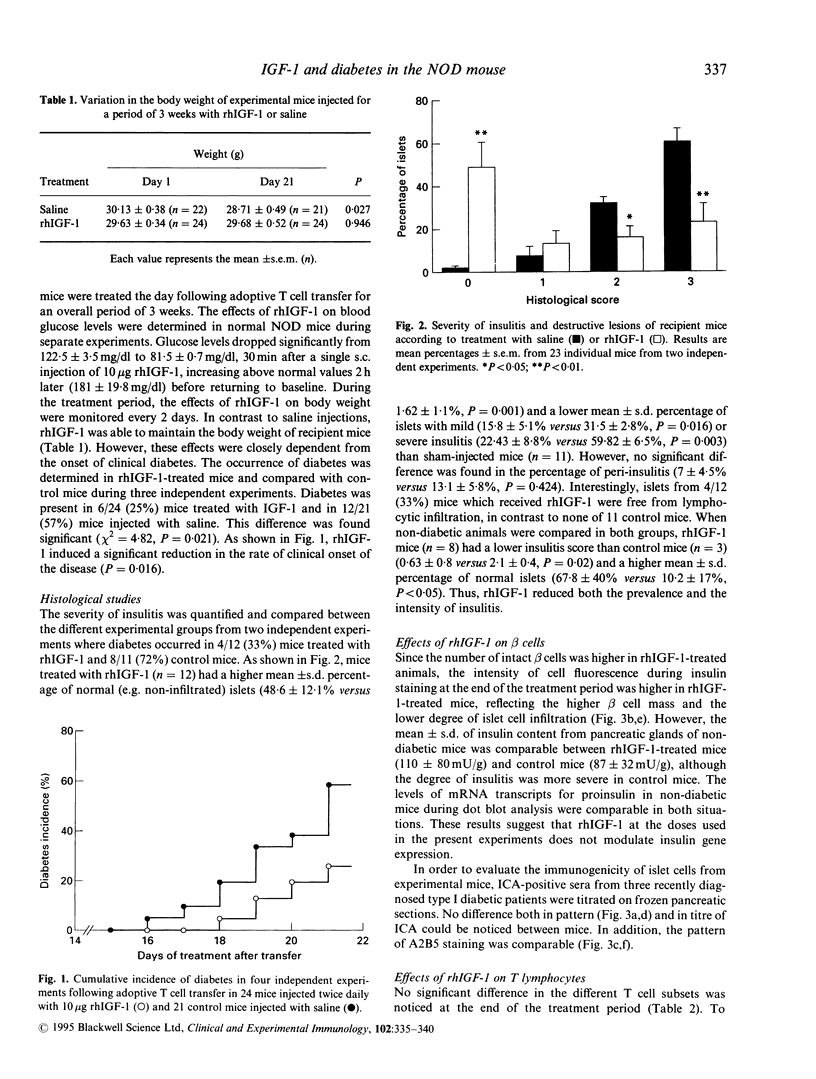
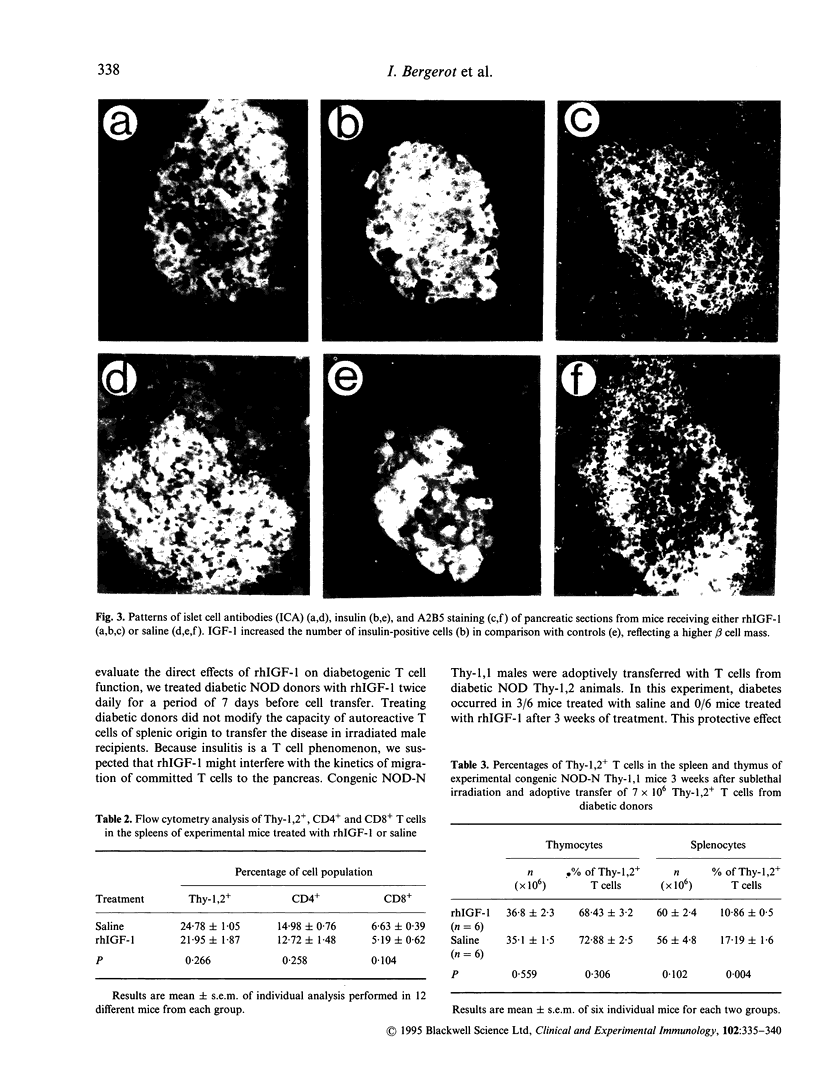
To evaluate the effect of IGF-1 on the autoimmune process of beta cell destruction, permissive non-obese diabetic (NOD) recipients were adoptively transferred with 7 x 10(6) autoreactive T cells from diabetic NOD mice and were administered subcutaneously 10 micrograms rhIGF-1, twice daily for 3 weeks. Administration of rhIGF-1 reduced the final incidence of successful transfers of diabetes observed in only 6/24 mice (25%) versus 12/21 (57%) in control mice. A marked reduction of insulitis during histological analysis of pancreatic glands was also observed. Mice treated with rhIGF-1 had a higher percentage of intact islets (48.6 +/- 12% versus 1.6 +/- 1.1%, P = 0.001) and a lower percentage of infiltrated islets. Islets from rhIGF-1-treated mice had a more intense insulin staining reflecting a higher beta cell mass, but no difference was observed in the amount of insulin content of pancreatic extracts and in the amounts of mRNA transcripts for proinsulin. No difference was also observed in the titres of three islet cell antibody (ICA)-positive sera and in the pattern of A2B5 staining. Some mice developed diabetes and severe islet cell infiltration despite rhIGF-1, thus indicating that some committed T cells were still able to invade the islets and cause beta cell destruction. The percentages of CD4+ and CD8+ T cells in the spleen of experimental mice were similar. To evaluate the effects of rhIGF-1 on cell trafficking in recipient mice, T cells from diabetic NOD Thy-1,2 mice injected into congenic NOD-N Thy-1,1 mice were monitored 3 weeks after adoptive cell transfer. The percentage of Thy-1,2+ T cells was significantly reduced in the spleen (10.8 +/- 1.3% versus 17.2 +/- 3.9%, P = 0.004) of rhIGF-1 treated mice in contrast to the thymus (68.4 +/- 7.9% versus 72.87 +/- 6.2%, P = 0.306), suggesting that rhIGF-1 could influence T cell trafficking to the lymphoid organs. The findings that rhIGF-1 has protective effects in autoimmune diabetes opens new perspectives for future experiments as well as for preventive strategies in human type I diabetes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Maclaren N. K., Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990 Aug;39(8):933–937. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- Baxter J. B., Blalock J. E., Weigent D. A. Characterization of immunoreactive insulin-like growth factor-I from leukocytes and its regulation by growth hormone. Endocrinology. 1991 Oct;129(4):1727–1734. doi: 10.1210/endo-129-4-1727. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Bendelac A., Bach J. F., Carnaud C. Syngeneic T cell transfer of diabetes into NOD newborn mice: in situ studies of the autoimmune steps leading to insulin-producing cell destruction. Eur J Immunol. 1989 Oct;19(10):1947–1951. doi: 10.1002/eji.1830191028. [DOI] [PubMed] [Google Scholar]
- Bertrand S., De Paepe M., Vigeant C., Yale J. F. Prevention of adoptive transfer in BB rats by prophylactic insulin treatment. Diabetes. 1992 Oct;41(10):1273–1277. doi: 10.2337/diab.41.10.1273. [DOI] [PubMed] [Google Scholar]
- Binz K., Joller P., Froesch P., Binz H., Zapf J., Froesch E. R. Repopulation of the atrophied thymus in diabetic rats by insulin-like growth factor I. Proc Natl Acad Sci U S A. 1990 May;87(10):3690–3694. doi: 10.1073/pnas.87.10.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño L., Eisenbarth G. S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Clark R., Strasser J., McCabe S., Robbins K., Jardieu P. Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest. 1993 Aug;92(2):540–548. doi: 10.1172/JCI116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Shimizu K., Bowring M. A., Wells S. Expression of receptors for tetanus toxin and monoclonal antibody A2B5 by pancreatic islet cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5066–5070. doi: 10.1073/pnas.79.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Schmid C., Zapf J., Froesch E. R. Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2868–2872. doi: 10.1073/pnas.86.8.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P., Eardley D. D. Suppressive effects of insulin and insulin-like growth factor-1 (IGF1) on immune responses. J Immunol. 1986 Jun 1;136(11):3994–3999. [PubMed] [Google Scholar]
- Jacob R. J., Sherwin R. S., Bowen L., Fryburg D., Fagin K. D., Tamborlane W. V., Shulman G. I. Metabolic effects of IGF-I and insulin in spontaneously diabetic BB/w rats. Am J Physiol. 1991 Feb;260(2 Pt 1):E262–E268. doi: 10.1152/ajpendo.1991.260.2.E262. [DOI] [PubMed] [Google Scholar]
- Kawada J., Toide K., Nishida M., Yoshimura Y., Tsujihara K. New diabetogenic streptozocin analogue, 3-O-methyl-2-([(methylnitrosoamino) carbonyl]amino)-D-glucopyranose. Evidence for a glucose recognition site on pancreatic B-cells. Diabetes. 1986 Jan;35(1):74–77. doi: 10.2337/diab.35.1.74. [DOI] [PubMed] [Google Scholar]
- Kooijman R., Willems M., De Haas C. J., Rijkers G. T., Schuurmans A. L., Van Buul-Offers S. C., Heijnen C. J., Zegers B. J. Expression of type I insulin-like growth factor receptors on human peripheral blood mononuclear cells. Endocrinology. 1992 Nov;131(5):2244–2250. doi: 10.1210/endo.131.5.1425423. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Worthen S. M., Leiter E. H. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes. 1989 Nov;38(11):1446–1455. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- Smith F. E., Rosen K. M., Villa-Komaroff L., Weir G. C., Bonner-Weir S. Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6152–6156. doi: 10.1073/pnas.88.14.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart C. A., Meehan R. T., Neale L. S., Cintron N. M., Furlanetto R. W. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab. 1991 May;72(5):1117–1122. doi: 10.1210/jcem-72-5-1117. [DOI] [PubMed] [Google Scholar]
- Tapson V. F., Boni-Schnetzler M., Pilch P. F., Center D. M., Berman J. S. Structural and functional characterization of the human T lymphocyte receptor for insulin-like growth factor I in vitro. J Clin Invest. 1988 Sep;82(3):950–957. doi: 10.1172/JCI113703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivolet C., Bendelac A., Bedossa P., Bach J. F., Carnaud C. CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J Immunol. 1991 Jan 1;146(1):85–88. [PubMed] [Google Scholar]
- Timsit J., Savino W., Safieh B., Chanson P., Gagnerault M. C., Bach J. F., Dardenne M. Growth hormone and insulin-like growth factor-I stimulate hormonal function and proliferation of thymic epithelial cells. J Clin Endocrinol Metab. 1992 Jul;75(1):183–188. doi: 10.1210/jcem.75.1.1619008. [DOI] [PubMed] [Google Scholar]
- Van Schravendijk C. F., Foriers A., Van den Brande J. L., Pipeleers D. G. Evidence for the presence of type I insulin-like growth factor receptors on rat pancreatic A and B cells. Endocrinology. 1987 Nov;121(5):1784–1788. doi: 10.1210/endo-121-5-1784. [DOI] [PubMed] [Google Scholar]
- Welsh M., Welsh N., Bendtzen K., Mares J., Strandell E., Oberg C., Sandler S. Comparison of mRNA contents of interleukin-1 beta and nitric oxide synthase in pancreatic islets isolated from female and male nonobese diabetic mice. Diabetologia. 1995 Feb;38(2):153–160. doi: 10.1007/BF00400089. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986 Aug;35(8):855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]