Abstract
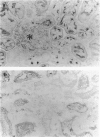
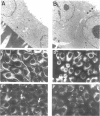
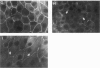
The subepithelial immune deposits of Dorus Zadel Black (DZB) rats with mercury-induced membranous nephropathy consist of autoantibodies directed to laminin P1 and of complement. The animals develop massive proteinuria within 10-14 days which is associated with obliteration of foot processes of glomerular visceral epithelial cells (GVEC), or podocytes. Previous studies indicate that these autoantibodies are probably not the sole mediator of proteinuria and GVEC damage. In this study we investigated whether circulating or macrophage-derived cytokines can contribute to the GVEC changes as detected in vivo. In vivo at the height of the proteinuria, increased intraglomerular IFN-gamma immunoreactivity was found. In diseased rats a five-fold increase in intraglomerular macrophages was found, but we could not detect intraglomerular IFN-alpha, IFN-beta, IL-1 beta or tumour necrosis factor-alpha (TNF-alpha) by using immunohistology. Subsequently, we exposed cultured GVEC to these cytokines to investigate their cytotoxic effects on several physiological and structural parameters. IFN-gamma and IL-4 were the only cytokines that exerted toxic effects, resulting in a rapidly decreased transepithelial resistance of confluent monolayers, which was closely associated with altered immunoreactivity of the tight junction protein ZO-1. IL-4 also affected vimentin and laminin immunoreactivity. IFN-gamma and IL-4 only interfered with monolayer integrity when added to the basolateral side of the GVEC, indicating specific (receptor-mediated) effects. Only IL-4 decreased the viability of the cells, and treated monolayers demonstrated an increased passage of the 44-kD protein horseradish peroxidase. From our experiments we concluded that IFN-gamma subtly affected monolayer integrity at the level of the tight junctions, and that IL-4 additionally induced cell death. We hypothesize that the toxic effects of the cytokines IFN-gamma and IL-4 as seen with cultured podocytes are necessary together with the autoantibodies, for the ultimate induction of proteinuria in mercury nephropathy in the DZB rat.
Full text
PDF



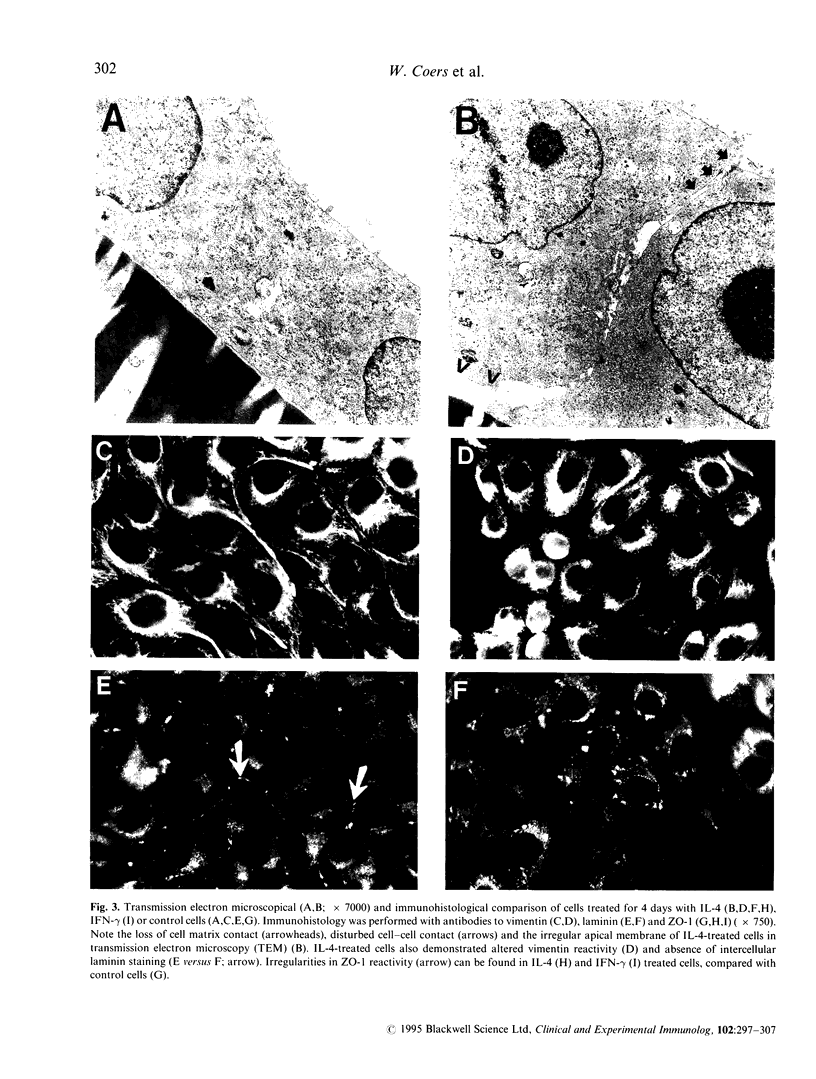
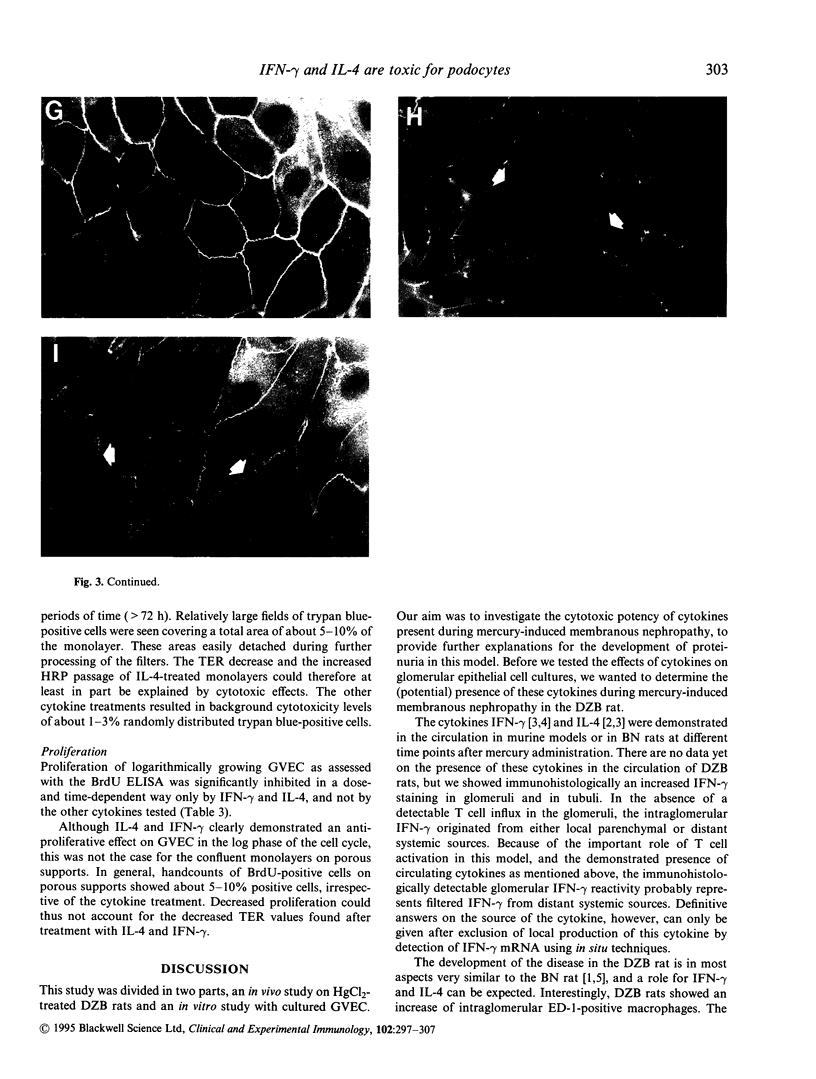
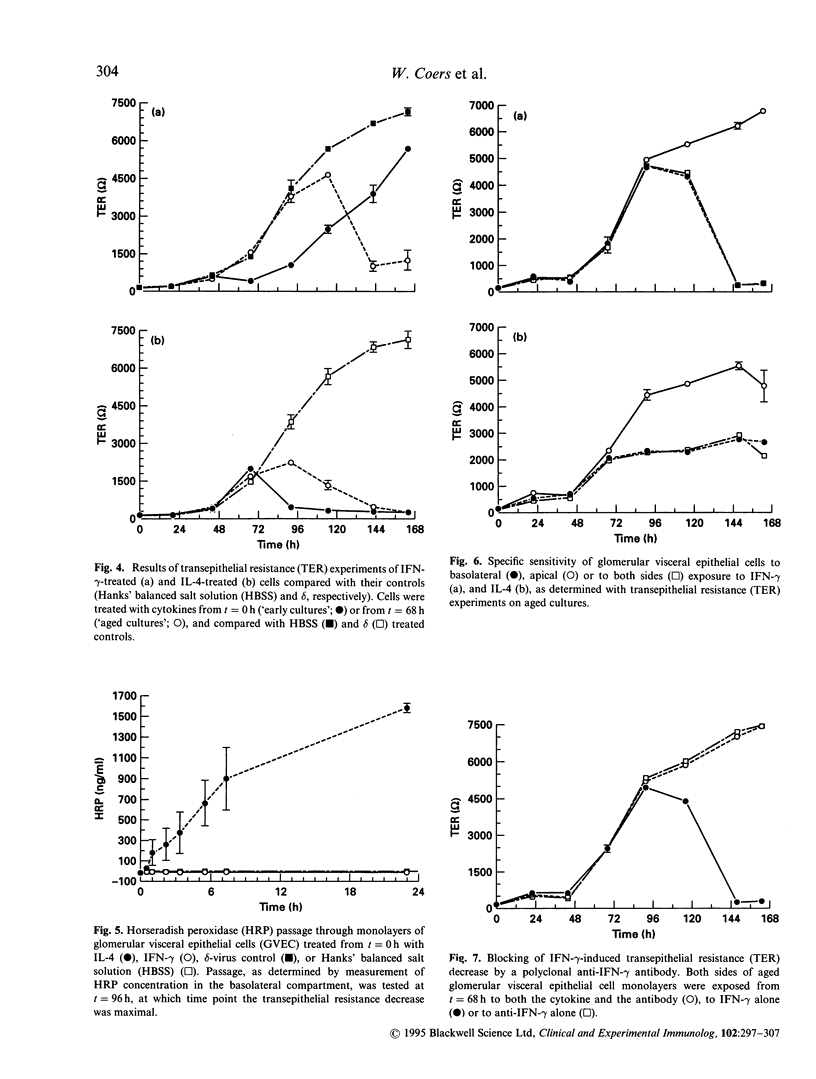
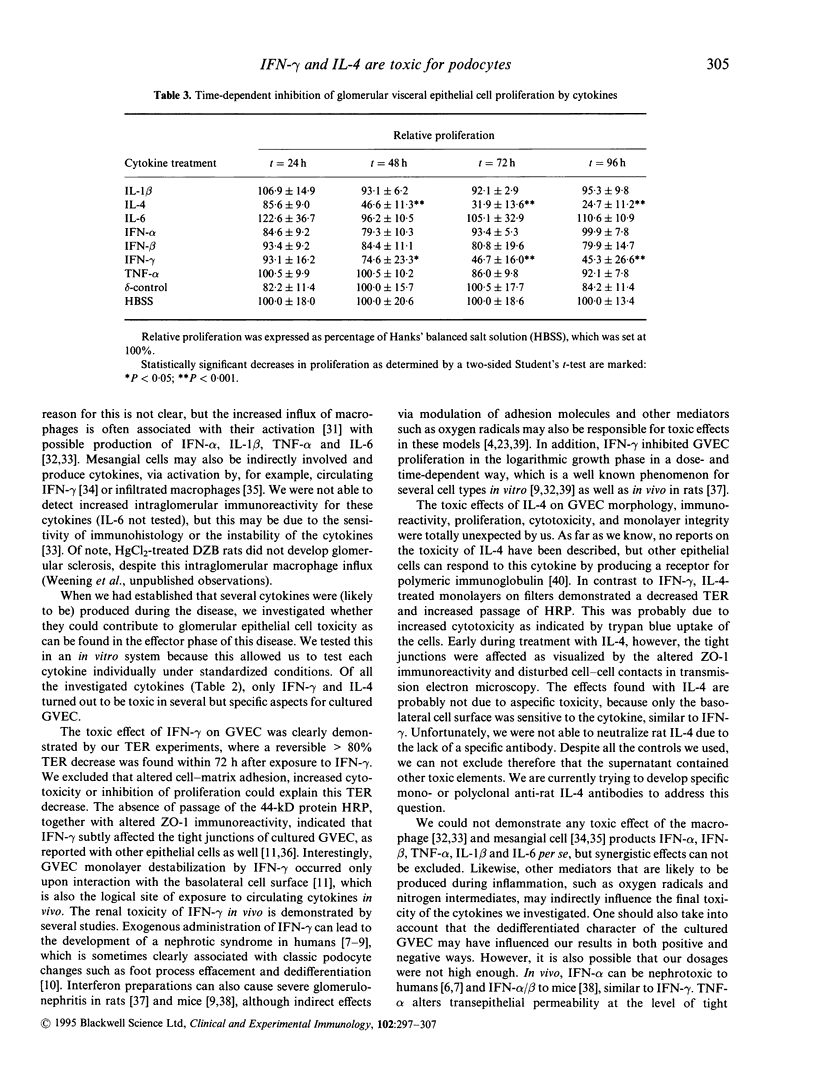


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. B., Planchon S. M., Roche J. K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993 Mar 15;150(6):2356–2363. [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Aten J., Bosman C. B., Rozing J., Stijnen T., Hoedemaeker P. J., Weening J. J. Mercuric chloride-induced autoimmunity in the brown Norway rat. Cellular kinetics and major histocompatibility complex antigen expression. Am J Pathol. 1988 Oct;133(1):127–138. [PMC free article] [PubMed] [Google Scholar]
- Aten J., Veninga A., Bruijn J. A., Prins F. A., de Heer E., Weening J. J. Antigenic specificities of glomerular-bound autoantibodies in membranous glomerulopathy induced by mercuric chloride. Clin Immunol Immunopathol. 1992 Apr;63(1):89–102. doi: 10.1016/0090-1229(92)90098-9. [DOI] [PubMed] [Google Scholar]
- Ault B. H., Stapleton F. B., Gaber L., Martin A., Roy S., 3rd, Murphy S. B. Acute renal failure during therapy with recombinant human gamma interferon. N Engl J Med. 1988 Nov 24;319(21):1397–1400. doi: 10.1056/NEJM198811243192107. [DOI] [PubMed] [Google Scholar]
- Coers W., Brouwer E., Vos J. T., Chand A., Huitema S., Heeringa P., Kallenberg C. G., Weening J. J. Podocyte expression of MHC class I and II and intercellular adhesion molecule-1 (ICAM-1) in experimental pauci-immune crescentic glomerulonephritis. Clin Exp Immunol. 1994 Nov;98(2):279–286. doi: 10.1111/j.1365-2249.1994.tb06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers W., Huitema S., Smeenk R. J., Salant D. J., Grond J., Weening J. J. Quantification of glomerular epithelial cell adhesion by using anti-DNA antibodies in ELISA. Hybridoma. 1992 Aug;11(4):529–537. doi: 10.1089/hyb.1992.11.529. [DOI] [PubMed] [Google Scholar]
- Cook J. R., Crute B. E., Patrone L. M., Gabriels J., Lane M. E., Van Buskirk R. G. Microporosity of the substratum regulates differentiation of MDCK cells in vitro. In Vitro Cell Dev Biol. 1989 Oct;25(10):914–922. doi: 10.1007/BF02624004. [DOI] [PubMed] [Google Scholar]
- Cybulsky A. V., Carbonetto S., Huang Q., McTavish A. J., Cyr M. D. Adhesion of rat glomerular epithelial cells to extracellular matrices: role of beta 1 integrins. Kidney Int. 1992 Nov;42(5):1099–1106. doi: 10.1038/ki.1992.393. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Egido J., Gómez-Chiarri M., Ortíz A., Bustos C., Alonso J., Gómez-Guerrero C., Gómez-Garre D., López-Armada M. J., Plaza J., Gonzalez E. Role of tumor necrosis factor-alpha in the pathogenesis of glomerular diseases. Kidney Int Suppl. 1993 Jan;39:S59–S64. [PubMed] [Google Scholar]
- Goldman M., Druet P., Gleichmann E. TH2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991 Jul;12(7):223–227. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- Gresser I. Biologic effects of interferons. J Invest Dermatol. 1990 Dec;95(6 Suppl):66S–71S. doi: 10.1111/1523-1747.ep12874776. [DOI] [PubMed] [Google Scholar]
- Halloran P. F., Urmson J., Van der Meide P. H., Autenried P. Regulation of MHC expression in vivo. II. IFN-alpha/beta inducers and recombinant IFN-alpha modulate MHC antigen expression in mouse tissues. J Immunol. 1989 Jun 15;142(12):4241–4247. [PubMed] [Google Scholar]
- Harms G., van Goor H., Koudstaal J., de Ley L., Hardonk M. J. Immunohistochemical demonstration of DNA-incorporated 5-bromodeoxyuridine in frozen and plastic embedded sections. Histochemistry. 1986;85(2):139–143. doi: 10.1007/BF00491761. [DOI] [PubMed] [Google Scholar]
- Ijzermans J. N., Marquet R. L. Interferon-gamma: a review. Immunobiology. 1989 Oct;179(4-5):456–473. doi: 10.1016/S0171-2985(89)80049-X. [DOI] [PubMed] [Google Scholar]
- Kakizaki Y., Kraft N., Atkins R. C. Interferon-gamma stimulates the secretion of IL-1, but not of IL-6, by glomerular mesangial cells. Clin Exp Immunol. 1993 Mar;91(3):521–525. doi: 10.1111/j.1365-2249.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop J. Immunologic effects of interferon. J Invest Dermatol. 1990 Dec;95(6 Suppl):72S–74S. doi: 10.1111/1523-1747.ep12874780. [DOI] [PubMed] [Google Scholar]
- Langeler E. G., van Hinsbergh V. W. Characterization of an in vitro model to study the permeability of human arterial endothelial cell monolayers. Thromb Haemost. 1988 Oct 31;60(2):240–246. [PubMed] [Google Scholar]
- Lederer E., Truong L. Unusual glomerular lesion in a patient receiving long-term interferon alpha. Am J Kidney Dis. 1992 Nov;20(5):516–518. doi: 10.1016/s0272-6386(12)70268-8. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrenas J., Parfrey N. A., Halloran P. F. Interferon gamma-mediated renal MHC expression in mercuric chloride-induced glomerulonephritis. Kidney Int. 1991 Feb;39(2):273–281. doi: 10.1038/ki.1991.33. [DOI] [PubMed] [Google Scholar]
- Mattila P. M., Nietosvaara Y. A., Ustinov J. K., Renkonen R. L., Häyry P. J. Antigen expression in different parenchymal cell types of rat kidney and heart. Kidney Int. 1989 Aug;36(2):228–233. doi: 10.1038/ki.1989.184. [DOI] [PubMed] [Google Scholar]
- Mendrick D. L., Kelly D. M., Rennke H. G. Antigen processing and presentation by glomerular visceral epithelium in vitro. Kidney Int. 1991 Jan;39(1):71–78. doi: 10.1038/ki.1991.9. [DOI] [PubMed] [Google Scholar]
- Moss J., Shore I., Woodrow D., Gresser I. Interferon-induced glomerular basement membrane and endothelial cell lesions in mice. An immunogold ultrastructural study of basement membrane components. Am J Pathol. 1988 Dec;133(3):557–563. [PMC free article] [PubMed] [Google Scholar]
- Mullin J. M., Snock K. V. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990 Apr 1;50(7):2172–2176. [PubMed] [Google Scholar]
- Nair S., Ernstoff M. S., Bahnson R. R., Arthur S., Johnston J., Downs M. A., Neuhart J., Kirkwood J. M. Interferon-induced reversible acute renal failure with nephrotic syndrome. Urology. 1992 Feb;39(2):169–172. doi: 10.1016/0090-4295(92)90277-4. [DOI] [PubMed] [Google Scholar]
- O'Meara Y. M., Natori Y., Minto A. W., Goldstein D. J., Manning E. C., Salant D. J. Nephrotoxic antiserum identifies a beta 1-integrin on rat glomerular epithelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):F1083–F1091. doi: 10.1152/ajprenal.1992.262.6.F1083. [DOI] [PubMed] [Google Scholar]
- Ochel M., Vohr H. W., Pfeiffer C., Gleichmann E. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991 May 1;146(9):3006–3011. [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Quigg R. J., Cybulsky A. V., Jacobs J. B., Salant D. J. Anti-Fx1A produces complement-dependent cytotoxicity of glomerular epithelial cells. Kidney Int. 1988 Jul;34(1):43–52. doi: 10.1038/ki.1988.143. [DOI] [PubMed] [Google Scholar]
- Reivinen J., Holthöfer H., Miettinen A. A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int. 1992 Sep;42(3):624–631. doi: 10.1038/ki.1992.327. [DOI] [PubMed] [Google Scholar]
- Richter G., Blankenstein T., Diamantstein T. Evolutionary aspects, structure, and expression of the rat interleukin 4 gene. Cytokine. 1990 May;2(3):221–228. doi: 10.1016/1043-4666(90)90020-t. [DOI] [PubMed] [Google Scholar]
- Sacks S. H., Zhou W., Pani A., Campbell R. D., Martin J. Complement C3 gene expression and regulation in human glomerular epithelial cells. Immunology. 1993 Jul;79(3):348–354. [PMC free article] [PubMed] [Google Scholar]
- Uitto J. Cell-cell and cell-matrix interactions in the skin. Lab Invest. 1993 Aug;69(2):133–135. [PubMed] [Google Scholar]
- Vanderlaan M., Thomas C. B. Characterization of monoclonal antibodies to bromodeoxyuridine. Cytometry. 1985 Nov;6(6):501–505. doi: 10.1002/cyto.990060603. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kamei S., Ohkubo A., Yamanaka M., Ohsawa S., Makino K., Tokuda K. Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clin Chem. 1986 Aug;32(8):1551–1554. [PubMed] [Google Scholar]
- Weiss K. S. Nephrotic range of proteinuria and interferon therapy. Ann Intern Med. 1992 Feb 15;116(4):347–347. doi: 10.7326/0003-4819-116-4-347_1. [DOI] [PubMed] [Google Scholar]
- Wolthuis A., Boes A., Rodemann H. P., Grond J. Vasoactive agents affect growth and protein synthesis of cultured rat mesangial cells. Kidney Int. 1992 Jan;41(1):124–131. doi: 10.1038/ki.1992.16. [DOI] [PubMed] [Google Scholar]
- Wolthuis A., van Goor H., Weening J. J., Grond J. Pathobiology of focal sclerosis. Curr Opin Nephrol Hypertens. 1993 May;2(3):458–464. doi: 10.1097/00041552-199305000-00014. [DOI] [PubMed] [Google Scholar]
- van Goor H., van der Horst M. L., Fidler V., Grond J. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992 May;66(5):564–571. [PubMed] [Google Scholar]
- van den Born J., van den Heuvel L. P., Bakker M. A., Veerkamp J. H., Assmann K. J., Berden J. H. A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int. 1992 Jan;41(1):115–123. doi: 10.1038/ki.1992.15. [DOI] [PubMed] [Google Scholar]
- van der Meide P. H., Borman A. H., Beljaars H. G., Dubbeld M. A., Botman C. A., Schellekens H. Isolation and characterization of monoclonal antibodies directed to rat interferon-gamma. Lymphokine Res. 1989 Winter;8(4):439–449. [PubMed] [Google Scholar]