Abstract
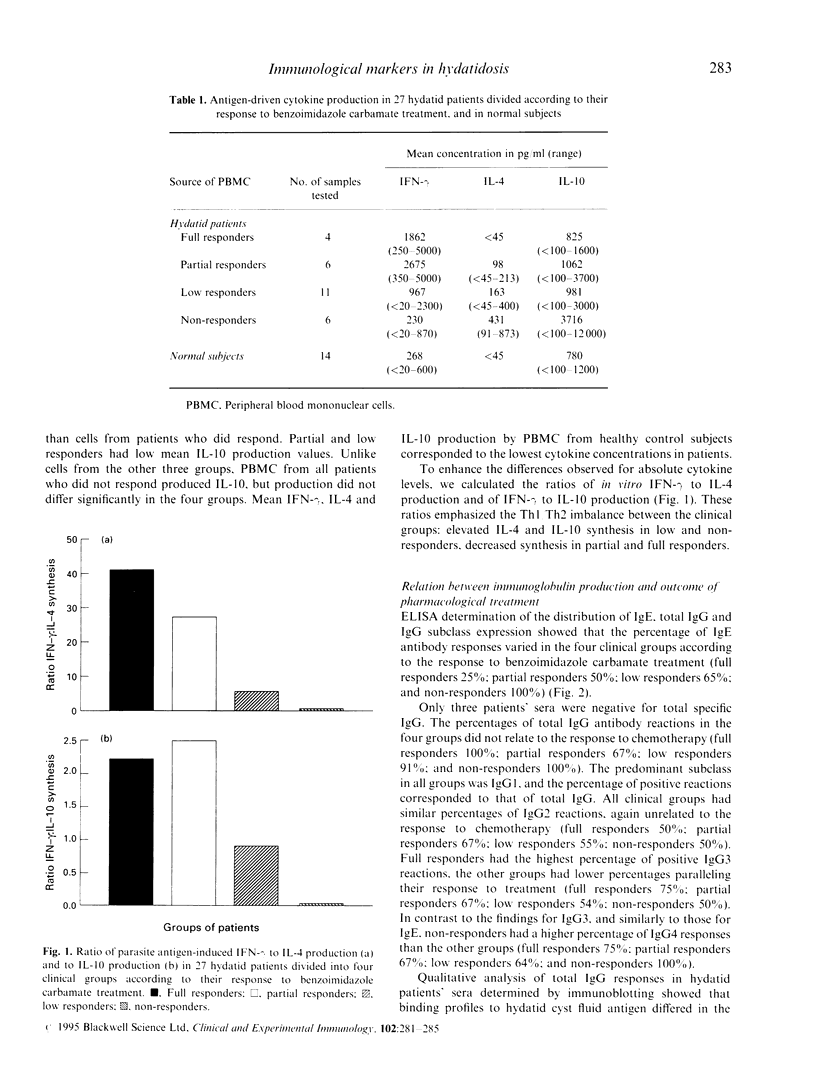
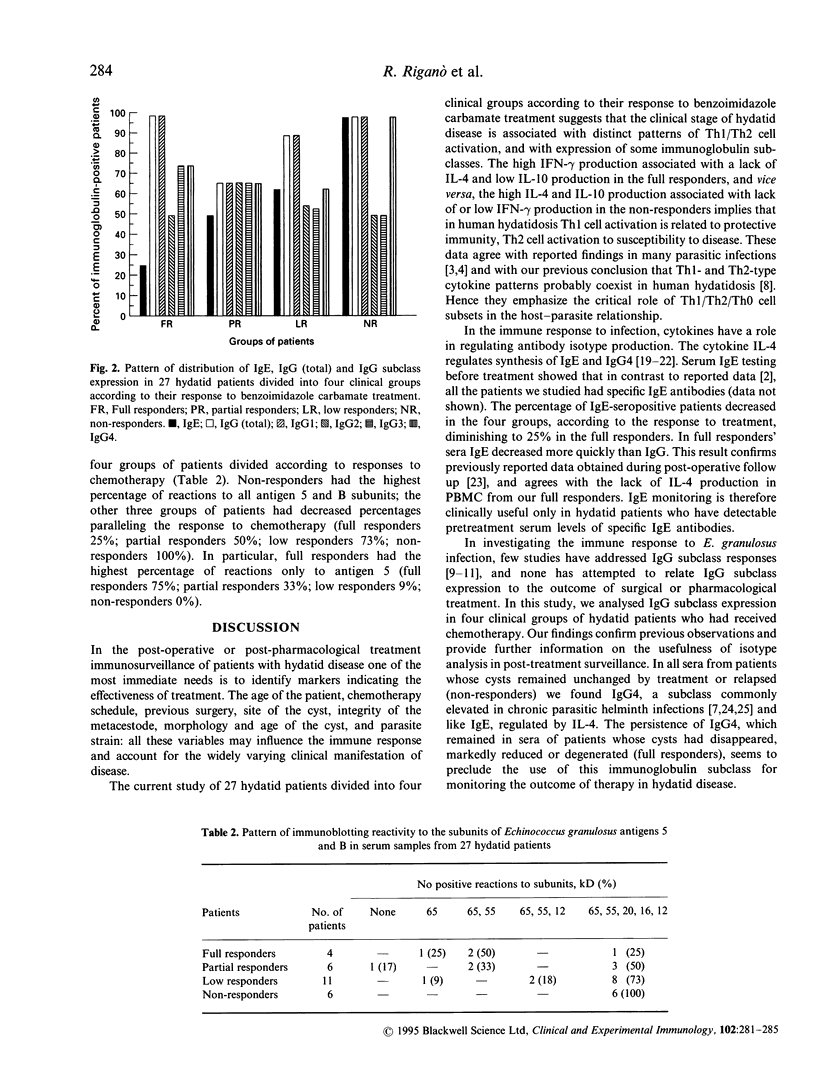
The relation of interferon-gamma (IFN-gamma), IL-4, IL-10 production and specific IgE, total IgG, IgG subclass expression to the effectiveness of pharmacological treatment in human hydatid disease (Echinococcus granulosus infection) was evaluated in 27 hydatid patients divided into four clinical groups according to their response to albendazole/mebendazole therapy (full, partial, low and non-responders). After parasite antigen stimulation, peripheral blood mononuclear cells (PBMC) from full responders produced significantly more IFN-gamma (P = 0.038), significantly less IL-4 (P = 0.001) and less IL-10 than PBMC from non-responders. PBMC from partial and low responders produced intermediate cytokine concentrations. ELISA determining immunoglobulin production showed that sera from all non-responders had IgE and IgG4 antibodies, both regulated by IL-4. In contrast to IgG4, IgE decreased rapidly in full responders. Full responders also showed the highest percentage of IgG3 reactions. Qualitative analysis of total IgG responses in hydatid patients' sera determined by immunoblotting showed that binding profiles to hydatid cyst fluid antigen differed in the four groups of treated patients. Non-responders had the highest percentage of reactions to all subunits of antigens 5 and B, and full responders had the highest percentage of reactions to antigen 5 alone. The high IFN-gamma production associated with a lack of IL-4 and low IL-10 production in the full responders, and vice versa the high IL-4 and IL-10 production associated with lack of or low IFN-gamma production in the non-responders implies Th1 cell activation in protective immunity and Th2 cell activation in susceptibility to hydatid disease. IgE may be a useful marker of therapeutic success in hydatid patients with pretreatment specific IgE antibodies. IgG subclass responses and differential immunoglobulin subclass binding pattern to hydatid antigens may also be useful in the immunosurveillance of hydatid disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceti A., Pennica A., Teggi A., Fondacaro L. M., Caferro M., Leri O., Tacchi G., Celestino D., Quaranta G., De Rosa F. IgG subclasses in human hydatid disease: prominence of the IgG4 response. Int Arch Allergy Immunol. 1993;102(4):347–351. doi: 10.1159/000236582. [DOI] [PubMed] [Google Scholar]
- Baldelli F., Papili R., Francisci D., Tassi C., Stagni G., Pauluzzi S. Post operative surveillance of human hydatidosis: evaluation of immunodiagnostic tests. Pathology. 1992 Apr;24(2):75–79. doi: 10.3109/00313029209063628. [DOI] [PubMed] [Google Scholar]
- Boctor F. N., Peter J. B. IgG subclasses in human chronic schistosomiasis: over-production of schistosome-specific and non-specific IgG4. Clin Exp Immunol. 1990 Dec;82(3):574–578. doi: 10.1111/j.1365-2249.1990.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardieri S., Giordano F., Ingrao F., Ioppolo S., Siracusano A., Vicari G. An evaluation of an agar gel diffusion test with crude and purified antigens in the diagnosis of hydatid disease. Bull World Health Organ. 1974;51(5):525–530. [PMC free article] [PubMed] [Google Scholar]
- Boyer A. E., Tsang V. C., Eberhard M. L., Zea-Flores G., Hightower A., Pilcher J. B., Zea-Flores R., Zhou W., Reimer C. B. Guatemalan human onchocerciasis. II. Evidence for IgG3 involvement in acquired immunity to Onchocerca volvulus and identification of possible immune-associated antigens. J Immunol. 1991 Jun 1;146(11):4001–4010. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Challacombe S. J., Biggerstaff M., Greenall C., Kemeny D. M. ELISA detection of human IgG subclass antibodies to Streptococcus mutans. J Immunol Methods. 1986 Feb 27;87(1):95–102. doi: 10.1016/0022-1759(86)90348-0. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Modlin R. L. Immune mechanisms in bacterial and parasitic diseases: protective immunity versus pathology. Curr Opin Immunol. 1991 Aug;3(4):480–485. doi: 10.1016/0952-7915(91)90007-n. [DOI] [PubMed] [Google Scholar]
- Hussain R., Grögl M., Ottesen E. A. IgG antibody subclasses in human filariasis. Differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. J Immunol. 1987 Oct 15;139(8):2794–2798. [PubMed] [Google Scholar]
- Ishizaka A., Joh K., Shibata R., Wagatsuma Y., Nakanishi M., Tomizawa K., Kojima K., Kandil E., Sakiyama Y., Matsumoto S. Regulation of IgE and IgG4 synthesis in patients with hyper IgE syndrome. Immunology. 1990 Jul;70(3):414–416. [PMC free article] [PubMed] [Google Scholar]
- King C. L., Ottesen E. A., Nutman T. B. Cytokine regulation of antigen-driven immunoglobulin production in filarial parasite infections in humans. J Clin Invest. 1990 Jun;85(6):1810–1815. doi: 10.1172/JCI114639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan A., Yazdanbakhsh M., van Ree R., Aalberse R., Selkirk M. E., Partono F., Maizels R. M. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993 May 1;150(9):3941–3950. [PubMed] [Google Scholar]
- Oriol R., Williams J. F., Pérez Esandi M. V., Oriol C. Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg. 1971 Jul;20(4):569–574. doi: 10.4269/ajtmh.1971.20.569. [DOI] [PubMed] [Google Scholar]
- Riganò R., Profumo E., Di Felice G., Ortona E., Teggi A., Siracusano A. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol. 1995 Mar;99(3):433–439. doi: 10.1111/j.1365-2249.1995.tb05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Regulation and deregulation of human IgE synthesis. Immunol Today. 1990 Sep;11(9):316–321. doi: 10.1016/s0167-5699(10)80004-0. [DOI] [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Short J. A., Heiner D. C., Hsiao R. L., Andersen F. L. Immunoglobulin E and G4 antibodies in cysticercosis. J Clin Microbiol. 1990 Jul;28(7):1635–1639. doi: 10.1128/jcm.28.7.1635-1639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusano A., Ioppolo S., Notargiacomo S., Ortona E., Riganó R., Teggi A., De Rosa F., Vicari G. Detection of antibodies against Echinococcus granulosus major antigens and their subunits by immunoblotting. Trans R Soc Trop Med Hyg. 1991 Mar-Apr;85(2):239–243. doi: 10.1016/0035-9203(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Teggi A., Lastilla M. G., De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993 Aug;37(8):1679–1684. doi: 10.1128/aac.37.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Craig P. S. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg. 1994 Dec;51(6):741–748. doi: 10.4269/ajtmh.1994.51.741. [DOI] [PubMed] [Google Scholar]


