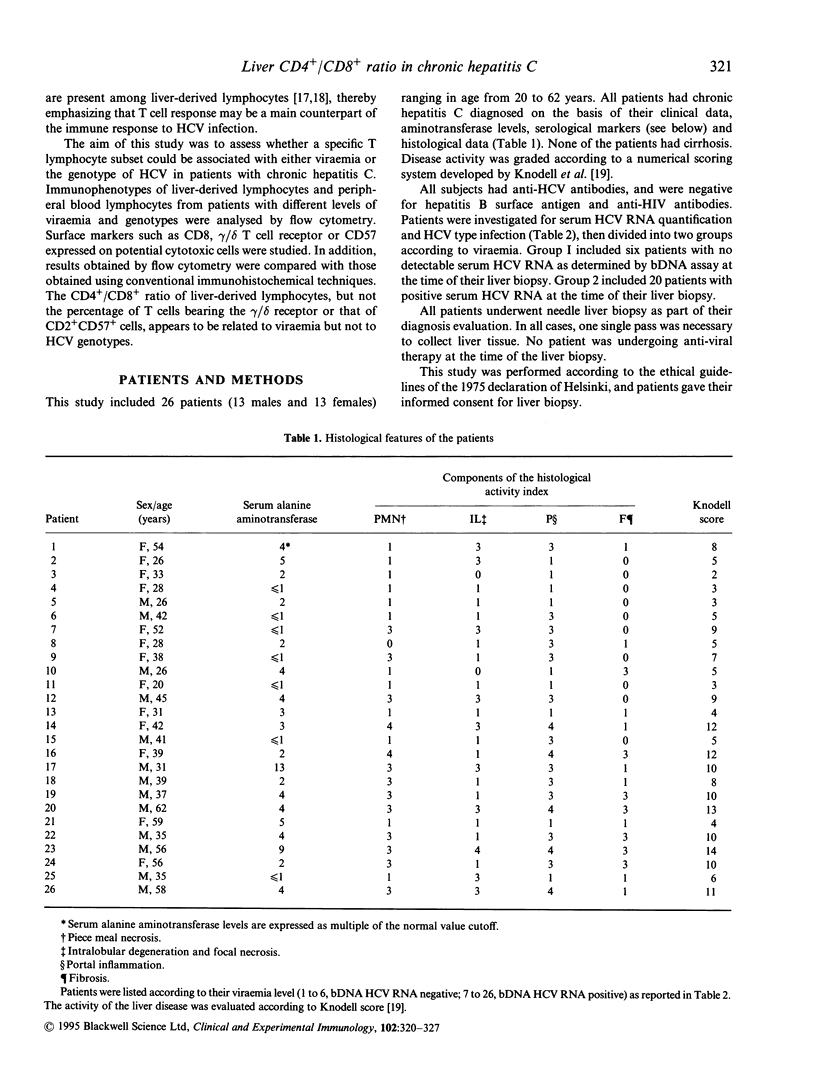
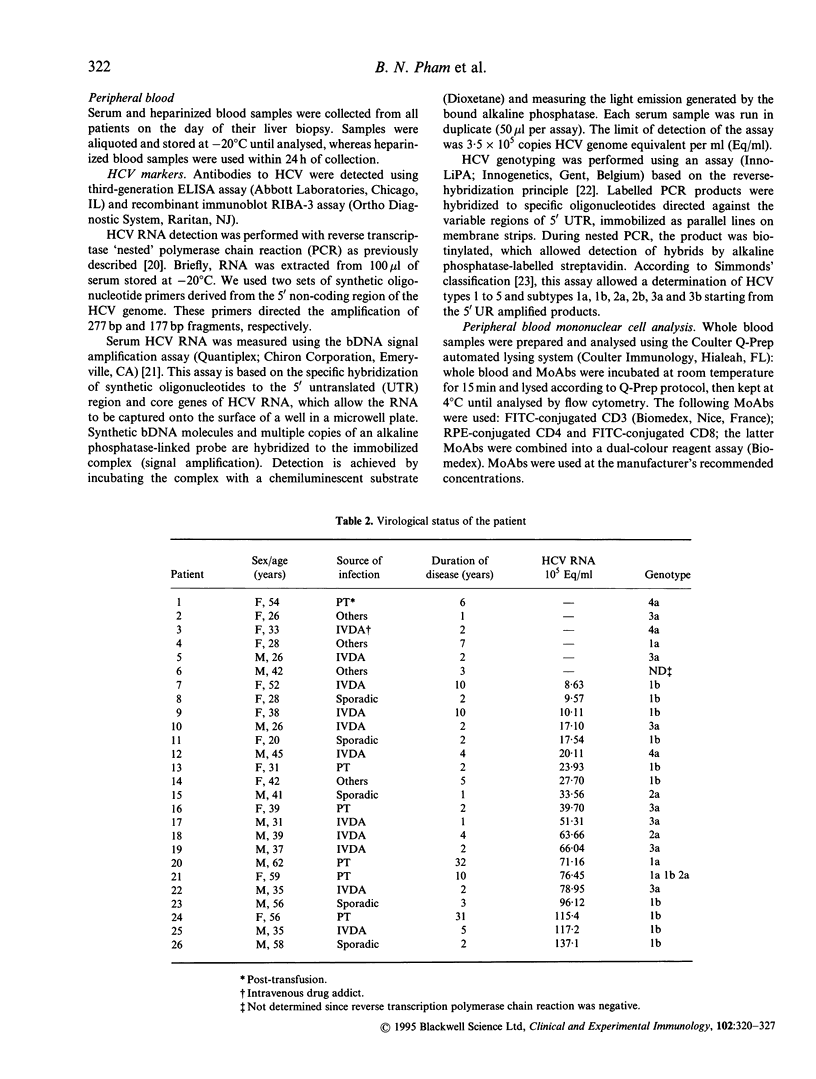
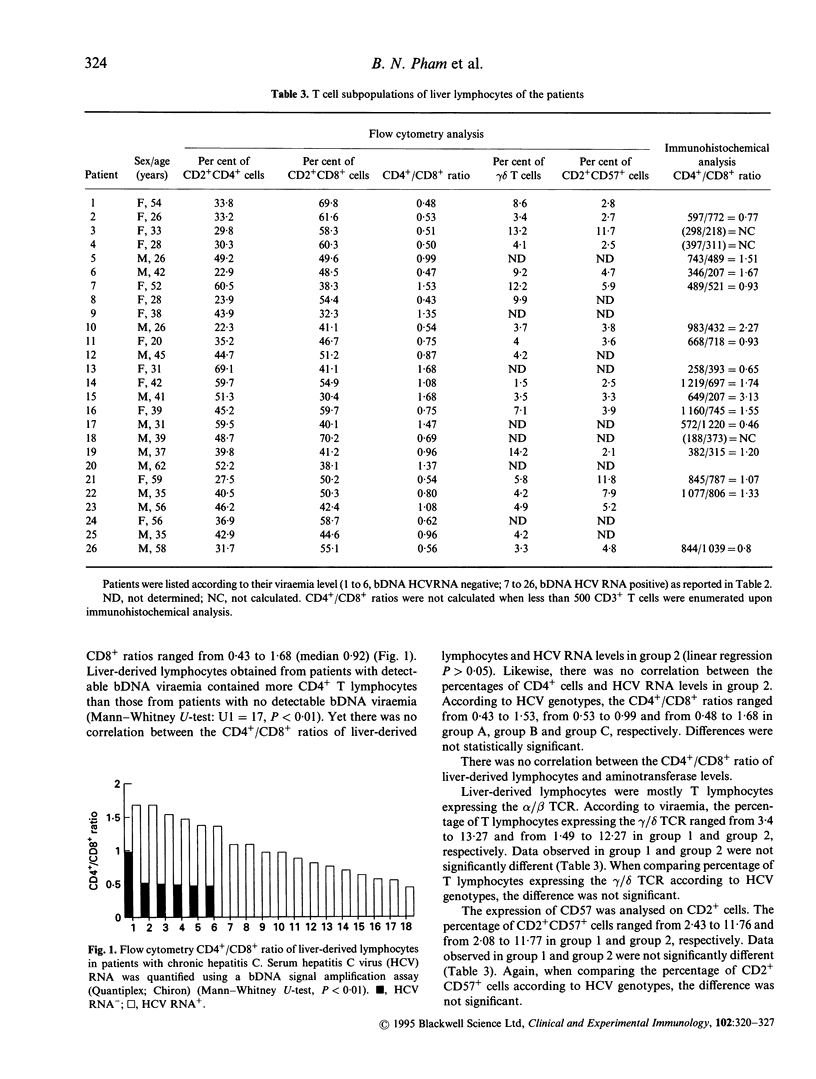
Abstract
The pathogenic mechanisms that lead to chronic hepatitis C are unknown. As hepatitis C virus (HCV) has been shown to induce T cell response, we assessed whether a particular T lymphocyte subset could be preferentially detected in the liver of patients with chronic hepatitis C in relation to viraemia or HCV genotypes. The immunophenotypes of liver-derived lymphocytes were analysed in 26 patients by flow cytometry and immunohistochemistry. Viraemia was quantified by branched DNA assay. Using this assay, HCV RNA was not detectable in six patients. HCV RNA was detected in 20 patients, and titres ranged from 8 to 137 x 10(6) Eq/ml. Genotyping was performed using a line probe assay. Type 1a, 1b, 2a, 3a and 4a were found to infect 2, 10, 2, 7 and 3 patients, respectively. The CD4+/CD8+ ratio of liver-derived lymphocytes was significantly higher (P < 0.01) in patients with detectable viraemia than in patients without detectable viraemia. In contrast, neither the percentage of gamma/delta T lymphocytes nor that of CD2+CD57+ cells was different in the groups. When comparing the CD4+/CD8+ ratio, the percentage of gamma/delta T lymphocytes or CD2+CD57+ cells according to genotype, the differences were not significant. These results suggest that the CD4+/CD8+ ratio of liver-derived lymphocytes is related to viraemia but not to HCV genotypes in patients with chronic hepatitis C, and that T lymphocytes may be involved in the pathogenesis of liver lesions in chronic hepatitis C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukh J., Purcell R. H., Miller R. H. At least 12 genotypes of hepatitis C virus predicted by sequence analysis of the putative E1 gene of isolates collected worldwide. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8234–8238. doi: 10.1073/pnas.90.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Hahn C. S., Galler R., Rice C. M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Chan S. W., McOmish F., Holmes E. C., Dow B., Peutherer J. F., Follett E., Yap P. L., Simmonds P. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J Gen Virol. 1992 May;73(Pt 5):1131–1141. doi: 10.1099/0022-1317-73-5-1131. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag J. L. Non-A, non-B hepatitis. I. Recognition, epidemiology, and clinical features. Gastroenterology. 1983 Aug;85(2):439–462. [PubMed] [Google Scholar]
- Dusheiko G., Schmilovitz-Weiss H., Brown D., McOmish F., Yap P. L., Sherlock S., McIntyre N., Simmonds P. Hepatitis C virus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology. 1994 Jan;19(1):13–18. [PubMed] [Google Scholar]
- Erickson A. L., Houghton M., Choo Q. L., Weiner A. J., Ralston R., Muchmore E., Walker C. M. Hepatitis C virus-specific CTL responses in the liver of chimpanzees with acute and chronic hepatitis C. J Immunol. 1993 Oct 15;151(8):4189–4199. [PubMed] [Google Scholar]
- Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984 Mar 22;308(5957):365–367. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Haas W., Kaufman S., Martinez C. The development and function of gamma delta T cells. Immunol Today. 1990 Oct;11(10):340–343. doi: 10.1016/0167-5699(90)90133-t. [DOI] [PubMed] [Google Scholar]
- Hata K., Van Thiel D. H., Herberman R. B., Whiteside T. L. Natural killer activity of human liver-derived lymphocytes in various liver diseases. Hepatology. 1991 Sep;14(3):495–503. [PubMed] [Google Scholar]
- Hata K., Van Thiel D. H., Herberman R. B., Whiteside T. L. Phenotypic and functional characteristics of lymphocytes isolated from liver biopsy specimens from patients with active liver disease. Hepatology. 1992 May;15(5):816–823. doi: 10.1002/hep.1840150512. [DOI] [PubMed] [Google Scholar]
- Imawari M., Nomura M., Kaieda T., Moriyama T., Oshimi K., Nakamura I., Gunji T., Ohnishi S., Ishikawa T., Nakagama H. Establishment of a human T-cell clone cytotoxic for both autologous and allogeneic hepatocytes from chronic hepatitis patients with type non-A, non-B virus. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2883–2887. doi: 10.1073/pnas.86.8.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Moriyama T., Kaneko T., Harase I., Nomura M., Miura H., Nakamura I., Yazaki Y., Imawari M. HLA B44-restricted cytotoxic T lymphocytes recognizing an epitope on hepatitis C virus nucleocapsid protein. Hepatology. 1993 Nov;18(5):1039–1044. [PubMed] [Google Scholar]
- Knodell R. G., Ishak K. G., Black W. C., Chen T. S., Craig R., Kaplowitz N., Kiernan T. W., Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981 Sep-Oct;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Watanabe S., Konishi M., Yokoi M., Kakehashi R., Kaito M., Kondo M., Hayashi Y., Jomori T., Suzuki S. Quantitation and typing of serum hepatitis C virus RNA in patients with chronic hepatitis C treated with interferon-beta. Hepatology. 1993 Dec;18(6):1319–1325. [PubMed] [Google Scholar]
- Koziel M. J., Dudley D., Wong J. T., Dienstag J., Houghton M., Ralston R., Walker B. D. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992 Nov 15;149(10):3339–3344. [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Spits H., Phillips J. H. The developmental relationship between NK cells and T cells. Immunol Today. 1992 Oct;13(10):392–395. doi: 10.1016/0167-5699(92)90087-N. [DOI] [PubMed] [Google Scholar]
- Lau J. Y., Davis G. L., Kniffen J., Qian K. P., Urdea M. S., Chan C. S., Mizokami M., Neuwald P. D., Wilber J. C. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993 Jun 12;341(8859):1501–1504. doi: 10.1016/0140-6736(93)90635-t. [DOI] [PubMed] [Google Scholar]
- Martinot-Peignoux M., Marcellin P., Gournay J., Gabriel F., Courtois F., Branger M., Wild A. M., Erlinger S., Benhamou J. P. Detection and quantitation of serum HCV-RNA by branched DNA amplification in anti-HCV positive blood donors. J Hepatol. 1994 May;20(5):676–678. doi: 10.1016/s0168-8278(05)80360-5. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Sammons R. E. The labeled antigen method of immunoenzymatic staining. J Histochem Cytochem. 1979 Apr;27(4):832–840. doi: 10.1177/27.4.109496. [DOI] [PubMed] [Google Scholar]
- Minutello M. A., Pileri P., Unutmaz D., Censini S., Kuo G., Houghton M., Brunetto M. R., Bonino F., Abrignani S. Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J Exp Med. 1993 Jul 1;178(1):17–25. doi: 10.1084/jem.178.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C. A., Palmiter R. D., Brinster R. L., Kanagawa O., Chisari F. V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990 Apr 20;248(4953):361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Kurai K., Okada S., Yamamoto K., Lizuka H., Tanaka T., Fukuda S., Tsuda F., Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992 May;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S. Cytotoxic T-lymphocytes. Adv Intern Med. 1988;33:17–44. [PubMed] [Google Scholar]
- Pham B. N., Mosnier J. F., Walker F., Njapoum C., Bougy F., Degott C., Erlinger S., Cohen J. H., Degos F. Flow cytometry CD4+/CD8+ ratio of liver-derived lymphocytes correlates with viral replication in chronic hepatitis B. Clin Exp Immunol. 1994 Sep;97(3):403–410. doi: 10.1111/j.1365-2249.1994.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Alberti A., Alter H. J., Bonino F., Bradley D. W., Brechot C., Brouwer J. T., Chan S. W., Chayama K., Chen D. S. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994 May;19(5):1321–1324. [PubMed] [Google Scholar]
- Simmonds P., Holmes E. C., Cha T. A., Chan S. W., McOmish F., Irvine B., Beall E., Yap P. L., Kolberg J., Urdea M. S. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993 Nov;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- Tsubota A., Chayama K., Ikeda K., Yasuji A., Koida I., Saitoh S., Hashimoto M., Iwasaki S., Kobayashi M., Hiromitsu K. Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology. 1994 May;19(5):1088–1094. [PubMed] [Google Scholar]
- Urdea M. S., Horn T., Fultz T. J., Anderson M., Running J. A., Hamren S., Ahle D., Chang C. A. Branched DNA amplification multimers for the sensitive, direct detection of human hepatitis viruses. Nucleic Acids Symp Ser. 1991;(24):197–200. [PubMed] [Google Scholar]
- Whiteside T. L., Herberman R. B. The role of natural killer cells in human disease. Clin Immunol Immunopathol. 1989 Oct;53(1):1–23. doi: 10.1016/0090-1229(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Yamada M., Kakumu S., Yoshioka K., Higashi Y., Tanaka K., Ishikawa T., Takayanagi M. Hepatitis C virus genotypes are not responsible for development of serious liver disease. Dig Dis Sci. 1994 Feb;39(2):234–239. doi: 10.1007/BF02090191. [DOI] [PubMed] [Google Scholar]



