Abstract
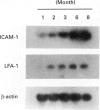
We have analysed the role of ICAM-1 and LFA-1 during development of autoimmune sialadenitis in MRL/lpr mice by direct analysis of RNA obtained from the salivary gland tissues, and the therapeutic effects with antibody administration on adoptive transfer system into SCID mice. The expression of cell adhesion molecules was assessed by using reverse transcriptase polymerase chain reaction (RT-PCR) and Southern blot analysis, and immunohistochemical analysis. Up-regulated expression of ICAM-1 mRNA was observed before the onset of inflammatory lesions in the salivary glands at 1 month and 2 months old, and thereafter LFA-1 mRNA was expressed within the typical inflammatory lesions, resembling human Sjögren's syndrome in MRL/lpr mice. Immunohistochemically, ICAM-1 was localized exclusively in the endothelial cells of varying sized blood vessels before the onset of disease, and LFA-1 expressing inflammatory cells were found within these lesions. When the therapeutic effects in vivo were examined, antibodies to ICAM-1 in combination with anti-LFA-1 prevented adoptive transfer of Sjögren's syndrome in MRL/lpr mice into SCID mice, while no significant effect was found when treated with either antibody. These findings indicate that in Sjögren's syndrome-like autoimmune lesions in MRL/lpr mice the ICAM-1/LFA-1 pathway may play a crucial role in the initiation and subsequent progression of T cell-mediated autoimmunity in the salivary and lacrimal glands of MRL/lpr mice.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Blanchard D., van Els C., Borst J., Carrel S., Boylston A., de Vries J. E., Spits H. The role of the T cell receptor, CD8, and LFA-1 in different stages of the cytolytic reaction mediated by alloreactive T lymphocyte clones. J Immunol. 1987 Apr 15;138(8):2417–2421. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Staunton D. E., Springer T. A. Supergene families meet in the immune system. Immunol Today. 1988 Jul-Aug;9(7-8):213–215. doi: 10.1016/0167-5699(88)91216-9. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Robinson C. A., Curd J. G., Kozin F., Howell F. V. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986 May;29(5):577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- Hamano H., Saito I., Haneji N., Mitsuhashi Y., Miyasaka N., Hayashi Y. Expressions of cytokine genes during development of autoimmune sialadenitis in MRL/lpr mice. Eur J Immunol. 1993 Oct;23(10):2387–2391. doi: 10.1002/eji.1830231002. [DOI] [PubMed] [Google Scholar]
- Haskard D., Cavender D., Beatty P., Springer T., Ziff M. T lymphocyte adhesion to endothelial cells: mechanisms demonstrated by anti-LFA-1 monoclonal antibodies. J Immunol. 1986 Nov 1;137(9):2901–2906. [PubMed] [Google Scholar]
- Hayashi Y., Haneji N., Hamano H., Yanagi K. Transfer of Sjögren's syndrome-like autoimmune lesions into SCID mice and prevention of lesions by anti-CD4 and anti-T cell receptor antibody treatment. Eur J Immunol. 1994 Nov;24(11):2826–2831. doi: 10.1002/eji.1830241137. [DOI] [PubMed] [Google Scholar]
- Hoffman R. W., Alspaugh M. A., Waggie K. S., Durham J. B., Walker S. E. Sjögren's syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984 Feb;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- Ishikura H., Takahashi C., Kanagawa K., Hirata H., Imai K., Yoshiki T. Cytokine regulation of ICAM-1 expression on human renal tubular epithelial cells in vitro. Transplantation. 1991 Jun;51(6):1272–1275. doi: 10.1097/00007890-199106000-00024. [DOI] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Jonsson R., Tarkowski A., Bäckman K., Holmdahl R., Klareskog L. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987 Apr;60(4):611–616. [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K., Yaoita E., Yamamoto T., Tamatani T., Miyasaka M., Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993 Feb 1;150(3):1074–1083. [PubMed] [Google Scholar]
- Koopman G., Parmentier H. K., Schuurman H. J., Newman W., Meijer C. J., Pals S. T. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991 Jun 1;173(6):1297–1304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman P., Moy V. T., Lollo B. A., Brian A. A. The accessory function of murine intercellular adhesion molecule-1 in T lymphocyte activation. Contributions of adhesion and co-activation. J Immunol. 1991 Mar 15;146(6):1773–1782. [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Chused T. M., Mann D. L., Klippel J. H., Fauci A. S., Frank M. M., Lawley T. J., Hamburger M. I. Sjögren's syndrome (Sicca syndrome): current issues. Ann Intern Med. 1980 Feb;92(2 Pt 1):212–226. doi: 10.7326/0003-4819-92-2-212. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Guo Y. J., Miyasaka M., Tamatani T., Collins A. B., Sy M. S., McCluskey R. T., Andres G. Antibodies to intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 prevent crescent formation in rat autoimmune glomerulonephritis. J Exp Med. 1993 Mar 1;177(3):667–677. doi: 10.1084/jem.177.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Itoh T. Higher level expression of lymphocyte function-associated antigen-1 (LFA-1) on in vivo natural killer cells. Eur J Immunol. 1988 Dec;18(12):2077–2080. doi: 10.1002/eji.1830181231. [DOI] [PubMed] [Google Scholar]
- Pardi R., Bender J. R., Dettori C., Giannazza E., Engleman E. G. Heterogeneous distribution and transmembrane signaling properties of lymphocyte function-associated antigen (LFA-1) in human lymphocyte subsets. J Immunol. 1989 Nov 15;143(10):3157–3166. [PubMed] [Google Scholar]
- Piela T. H., Korn J. H. ICAM-1-dependent fibroblast-lymphocyte adhesion: discordance between surface expression and function of ICAM-1. Cell Immunol. 1990 Aug;129(1):125–137. doi: 10.1016/0008-8749(90)90192-t. [DOI] [PubMed] [Google Scholar]
- Piela T. H., Korn J. H. Lymphocyte-fibroblast adhesion induced by interferon-gamma. Cell Immunol. 1988 Jun;114(1):149–160. doi: 10.1016/0008-8749(88)90262-6. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Seko Y., Matsuda H., Kato K., Hashimoto Y., Yagita H., Okumura K., Yazaki Y. Expression of intercellular adhesion molecule-1 in murine hearts with acute myocarditis caused by coxsackievirus B3. J Clin Invest. 1993 Apr;91(4):1327–1336. doi: 10.1172/JCI116333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- St Clair E. W., Angellilo J. C., Singer K. H. Expression of cell-adhesion molecules in the salivary gland microenvironment of Sjögren's syndrome. Arthritis Rheum. 1992 Jan;35(1):62–66. doi: 10.1002/art.1780350110. [DOI] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- White S. C., Casarett G. W. Induction of experimental autoallergic sialadenitis. J Immunol. 1974 Jan;112(1):178–185. [PubMed] [Google Scholar]
- Wuthrich R. P., Jevnikar A. M., Takei F., Glimcher L. H., Kelley V. E. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990 Feb;136(2):441–450. [PMC free article] [PubMed] [Google Scholar]