Abstract
Pre-mRNA splicing is a widely used regulatory mechanism for controlling gene expression, and a family of conserved proteins, SR proteins, participate in both constitutive and alternative splicing. Here we describe a novel function for the SR protein ASF/SF2. We used an embryonic chicken cDNA library to screen for differential mRNA expression in the chicken B-cell line DT40-ASF, expressing or not expressing ASF/SF2. Remarkably, out of 3 × 106 clones screened, only one, isolated several times independently, showed ASF/SF2-related differential expression. The isolated cDNA, referred to here as PKCI-r (for PKCI-related), is closely related to the protein kinase C interacting protein (PKCI-1) gene. Transcript levels were increased ∼sixfold in ASF/SF2-depleted cells compared with cells expressing ASF/SF2, indicating a negative role for the SR protein. Strikingly, inhibition of ASF/SF2 expression had no significant effect on PKCI-r splicing, or transcription, but markedly increased the half-life of PKCI-r mRNA (6.6-fold). Similarly, increased mRNA stability was also observed upon expression of exogenous PKCI-r mRNA in cells depleted of ASF/SF2. ASF/SF2 bound to a discrete region containing a purine-rich sequence in the 3′ UTR of the PKCI-r transcript, and deletion of this region eliminated ASF/SF2-mediated regulation of transcript stability. Together these data indicate a novel, direct effect of ASF/SF2 on PKCI-r mRNA stability. Therefore, ASF/SF2, and perhaps other SR proteins, affects gene expression in vertebrate cells through regulation of mRNA stability as well as splicing.
Keywords: ASF/SF2, mRNA stability, PKCI-1 gene
Pre-mRNA splicing plays an important role in gene expression. A multicomponent complex called the spliceosome catalyzes both constitutive and regulated mRNA splicing, and consists of small ribonucleoprotein (snRNP) particles, U1, U2, and U4/U5/U6, as well as many non-snRNP proteins (Guthrie 1991; Sharp 1994). Among these non-snRNP proteins are the SR proteins (Manley and Tacke 1996; Graveley 2000). Members of the SR protein family are the best-characterized mammalian splicing regulators. The SR protein family comprises related factors that share common sequence motifs, containing either one or two N-terminal RNA recognition motifs (RRMs), and a C-terminal region consisting of an arginine/serine-rich (R/S) domain (hence the designation SR proteins; Zahler et al. 1992). To date, at least eight human cDNAs encoding SR proteins have been cloned, including the prototype, ASF/SF2 (Valcarcel and Green 1996).
SR proteins function as both constitutive and alternative splicing factors. They are essential for early steps in spliceosome formation, as they can reconstitute in vitro pre-mRNA splicing when added to cell extracts lacking SR proteins but containing all other necessary splicing factors (Krainer et al. 1990b, 1991; Ge et al. 1991; Fu and Maniatis 1992; Kim et al. 1992; Zahler et al. 1992). In addition to their role in constitutive splicing, SR proteins are also involved in alternative splicing, as they can regulate use of competing splice sites in a concentration-dependent manner in several pre-mRNAs (e.g., Ge and Manley 1990; Krainer et al. 1990a; Fu et al. 1992; Zahler et al. 1993). In contrast to their function as essential splicing factors, SR proteins appear to have a nonredundant function as alternative splicing factors, because individual SR proteins can show differences in selection of alternative splicing sites (Kim et al. 1992; Zahler et al. 1993). SR proteins likely activate splice sites differentially because of different affinity interaction with either 5′ splice sites or exonic splicing enhancers (Kohtz et al. 1994; Zuo and Manley 1994; Tacke and Manley 1995), and may similarly affect 3′ splice site selection (Fu et al. 1992). Recently, SR proteins have also been suggested to play a role in mRNA transport. SRp20 and 9G8 interact specifically with a 22-nt element from the histone H2a gene to promote the nucleocytoplasmic export of intronless RNAs (Huang and Steitz 2001).
The importance of SR proteins in cell function has been emphasized by the targeted disruption of ASF/SF2 in the chicken B-cell line DT40 (Wang et al. 1996). Deletion of both alleles of ASF/SF2 was only possible in the presence of an ASF/SF2-expressing transgene under the control of a tetracycline-repressible promoter. Depletion of ASF/SF2 led to cell death and was associated with a reduced rate of pre-mRNA processing and with changes in alternative splicing of several viral transcripts produced from exogenous transgenes (Wang et al. 1996, 1998). These results indicated the importance of ASF/SF2 in constitutive and alternative splicing, but did not identify endogenous targets regulated by ASF/SF2.
Here, we have investigated potential links between alternative splicing and downstream regulation of gene expression, focusing on the SR protein ASF/SF2. Using retrotranscribed poly(A) mRNAs isolated from the above-described DT40 cells conditionally expressing or not expressing ASF/SF2, we screened for differential gene expression using a chicken cDNA library. Out of 3 × 106 clones screened, four showed ASF/SF2-related differential expression. All of these clones contained the same cDNA, encoding a protein (designated PKCI-r) similar to protein kinase C interacting protein 1 (PKCI-1). Inhibition of ASF/SF2 expression in DT40 cells induced a sixfold increase in PKCI-r gene expression. Analysis of intracellular mechanisms involved in ASF/SF2 down-regulation of PKCI-r expression revealed that ASF/SF2 does not regulate PKCI-r through either a change in PKCI-r pre-mRNA splicing or transcription. Unexpectedly, ASF/SF2 decreased PKCI-r mRNA stability by binding to a purine-rich region element positioned within the 3′ UTR of the mature PKCI-r mRNA. The role of the splicing factor ASF/SF2 in regulating stability of the PKCI-r mRNA highlights a novel function for a SR protein in regulating gene expression.
Results
Isolation of genes regulated by the SR protein ASF/SF2
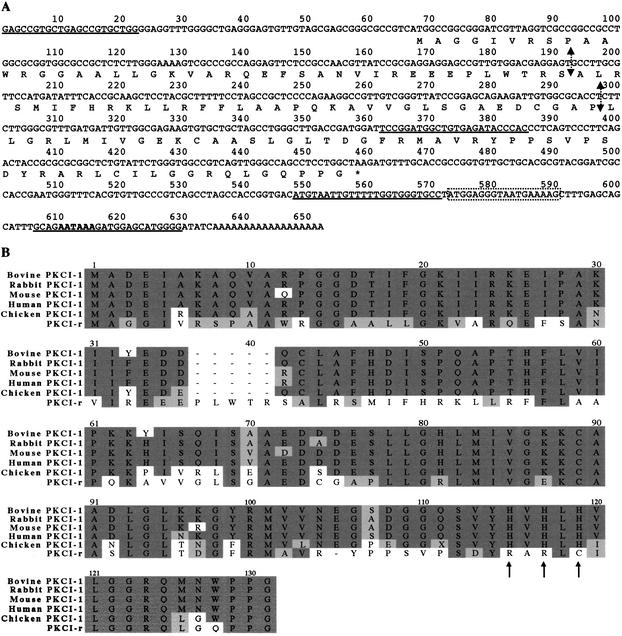
To investigate whether ASF/SF2 regulates expression of specific mRNAs, we developed a strategy to screen for genes differentially expressed in chicken cells expressing or lacking ASF/SF2. For this study we took advantage of the DT40 cell line described above in which the only source of ASF/SF2 is a human ASF/SF2 cDNA controlled by a tetracycline (tet)-repressible promoter (Wang et al. 1996). When this cell line (designated DT40-ASF) is grown in medium containing 1.0 μg/mL tetracycline, the amount of ASF/SF2 decreases between 0 and 30 h of tetracycline treatment, and becomes undetectable after 36 h (Wang et al. 1996). We screened a chicken cDNA library with 33P-labeled retrotranscribed poly(A) mRNAs prepared from DT40-ASF cells either expressing (without tet treatment) or not expressing (with tet treatment) ASF/SF2. To obtain the maximum number of cDNA clones regulated by ASF/SF2, DT40-ASF cells were treated with tetracycline for 48 h. We screened 3 × 106 cDNA clones, and >70 putative positive clones were carried through a secondary screening. On secondary screens only four clones showed ASF/SF2-related differential expression, with apparent down-regulation by ASF/SF2, as the cDNAs were found overexpressed in tetracycline-treated DT40-ASF cells compared with untreated DT40-ASF (Fig. 1A, PKCI-r, tet + vs. tet − DT40-ASF cells). Remarkably, the four clones identified had identical sequences, but showed length differences at their 5′ ends. The longest cDNA was 652-bp long; the truncated ones were 642-bp, 636-bp, and 608-bp long. All of the clones contained a 390-bp open reading frame with an in-frame upstream stop codon, coding for a 130-amino-acid protein. This cDNA was designated PKCI-r (see below). Our screening method did not detect a change in ASF/SF2 expression in tetracycline-treated DT40-ASF, possibly because the exogenous tetracycline-regulated gene is of human origin. Although chicken and human ASF/SF2 share 98% homology at the amino acid level, the DNA sequence is only 78% conserved. This level of homology may not be detected with the high-stringency hybridization protocol used.
Figure 1.
Isolation of PKCI-r, a cDNA down-regulated by ASF/SF2. (A) ASF/SF2-related differential screening of a chicken cDNA library. DT40-ASF cells were treated with tetracycline (tet; 1 μg/μL) for 48 h or left untreated. Total RNA was extracted from ASF/SF2-expressing cells (tet-untreated, designated tet −) and ASF/SF2-nonexpressing cells (tet-treated, designated tet +). A chicken cDNA library was plated on agar plates, double-lifted on nylon transfer membranes. Each of the duplicate lifts was then hybridized with either tet + or tet − DT40-ASF cell 33P-labeled retrotranscribed poly(A) mRNAs. Arrows indicate a clone showing differential expression between tet + and tet − mRNAs. (B) Expression of PKCI-r and ASF/SF2 in DT40-ASF cells. DT40-ASF cells were treated with tet for 48 h (tet +) or left untreated (tet −). Blotted poly(A) mRNAs were hybridized sequentially with 32P-labeled cDNA of mouse PKCI-r and human ASF/SF2. (C) Expression of PKCI-r in DT40-ASF and wild-type DT40 (WT) cells. DT40-ASF and wild-type cells were tet-treated for 48 h (tet +) or left untreated (tet −). Blotted poly(A) mRNAs were probed for PKCI-r as above. (D) Kinetics of ASF/SF2-related PKCI-r induction during tet treatment. DT40-ASF cells were tet-treated (tet +) or left untreated (tet −) for 24, 30, or 36 h, before RNA preparation. Northern blot analysis was then performed for PKCI-r and 18S ribosomal RNA as above. Numbers at bottom indicate PKCI-r induction for each time point on the basis of PhosphorImager analysis (data normalized against 18S rRNA).
To confirm that ASF/SF2 down-regulates expression of PKCI-r mRNA, transcript levels were analyzed by Northern blot using the PKCI-r cDNA as a probe. Tetracycline-induced depletion of ASF/SF2 in DT40-ASF cells was accompanied by up-regulation of a unique 650-bp PKCI-r mRNA, which matched in size the 649-bp PKCI-r cDNA (Fig. 1B, PKCI-r and ASF/SF2, tet + vs. tet + DT40-ASF), indicating that this clone includes all or most of the mature PKCI-r transcript. To exclude the possibility that the tetracycline rather than the ASF/SF2 depletion was stimulating PKCI-r mRNA expression, transcript levels were also analyzed in wild-type (WT) DT40 cells. Whereas tetracycline treatment of DT40-ASF cells induced increased PKCI-r mRNA expression, identical tetracycline treatment of wild-type DT40 cells did not affect PKCI-r mRNA expression (Fig. 1C, WT vs. DT40-ASF cells, tet + vs. tet −).
Because ASF/SF2 levels change over time in tetracycline-treated DT40-ASF cells, we next examined the kinetics of PKCI-r induction. Inhibition of ASF/SF2 expression leads to inhibition of cell growth at ∼36 h and eventually to cell death starting at 60 h of tetracycline treatment. The cause of cell death is not known, but involves an apoptotic pathway (J. Wang, X. Li, and J. Manley, unpubl.). However, the cells grow normally for at least 36 h (Wang et al. 1996), and no change in accumulation of several mRNAs was detected after 48 h (Wang et al. 1998). No change in PKCI-r mRNA levels was found at 24 h of tetracycline treatment (Fig. 1D, PKCI-r, 24 h, tet + vs. tet − DT40-ASF cells), consistent with only partial ASF/SF2 depletion induced in DT40-ASF cells after 24 h of tetracycline treatment (Wang et al. 1996). A 2.3-fold and a maximal 5.7-fold up-regulation of PKCI-r mRNA expression was obtained in DT40-ASF cells at 30 h and 36 h of tetracycline treatment, respectively (Fig. 1D, PKCI-r, 30 and 36 h, tet + vs. tet − DT40-ASF cells). Longer treatment times did not lead to further increased PKCI-r mRNA levels (data not shown).
PKCI-r, a chicken gene homologous to PKCI
The nucleotide sequence of PKCI-r revealed a predicted amino acid sequence highly homologous to PKC interacting protein 1 (PKCI-1), a member of the HINT (histidine triad nucleotide-binding protein) family (Fig. 2B). The PKCI/HINT family along with the fragile histidine triad (FHIT) family comprise the histidine triad (HIT) superfamily, which shows a conserved histidine triad (His-X-His-X-His) sequence motif (Seraphin 1992; Robinson and Aitken 1994). These conserved histidyl residues are prominent features in the active site, unifying the HIT superfamily as nucleotidyl hydrolases, transferases, or both (Lima et al. 1997). In contrast to the divergent FHIT family, which deviates throughout evolution at both N and C termini, the PKCI/HINT family is characterized by a highly conserved C terminus sequence and >94% amino acid sequence identity overall among mammalian homologs (Pearson et al. 1990; Brzoska et al. 1995, 1996; Lima et al. 1996; Brenner et al. 1997; Klein et al. 1998). The predicted chicken PKCI-r protein is more closely related to the chicken PKCI member (50% amino acid homology) than to mammalian PKCI members (∼41% amino acid identity with bovine, rabbit, mouse, or human members; Fig. 2B, dark shaded boxes). A 68% partial amino acid similarity was found between PKCI-r and chicken PKCI (Fig. 2B, light shaded boxes). Importantly, this novel gene product has lost all of the three conserved histidyl residues that mediate biological activity of the HIT proteins (Fig. 2B, arrows below amino acid sequence), indicating a distinct function from other family members. Parallel and simultaneously to us, two other groups also cloned this cDNA in chicken using different screening strategies (GenBank database accession nos. AB02667 and AF148455).
Figure 2.
PKCI-r identity. (A) DNA and deduced amino acid sequence of PKCI-r. The 652-bp PKCI-r cDNA contains a 390-bp open reading frame that encodes a 130-amino-acid protein. The termination codon (*), putative polyadenylation sequence (bold at position 607), and boundaries of exons (vertical arrows) are indicated. The sequences of the primers used for PCR are underlined. The purine-rich region bound by ASF/SF2 (position 573–589) is boxed. (B) Amino acid sequence comparison of PKCI-r with PKCI-1 family members. Dark shaded regions indicate conserved residues in most family members. Light shaded regions indicate partial amino acid homology with any other family member. Arrows show the three conserved histidyl residues that unify the HIT superfamily. The PKCI-1 sequences used for this alignment include Bos taurus (GenBank database accession no. A35350), Oryctolagus cuniculus (Y11175), Mus musculus (U60001), Homo sapiens (U27143), Gallus gallus (AI982310), and Gallus gallus PKCI-r.
ASF/SF2 does not inhibit PKCI-r mRNA expression through alteration of pre-mRNA splicing
Because ASF/SF2 has well-characterized roles in pre-mRNA splicing regulation, we tested whether ASF/SF2 affects PKCI-r transcript levels by changing the pattern or kinetics of PKCI-r splicing. The PKCI-r gene consists of three exons (192, 105, and 355 bp) and two introns (985 and 831 bp; Fig. 3A; Hori et al. 2000). To analyze possible effects of ASF/SF2 on PKCI-r alternative splicing, we performed RT–PCR experiments with DT40-ASF cell mRNAs using primers designed to amplify the entire PKCI-r cDNA sequence (Fig. 2A). Inhibition of ASF/SF2 expression did not induce any detectable changes in the splicing pattern of PKCI-r. A unique ∼630-bp band, consistent with amplification of the mature spliced transcript, was similarly amplified from both cells expressing or not expressing ASF/SF2 (Fig. 3C, tet + vs. tet − DT40-ASF cells). Unspliced primary transcript (∼2468 bp) was not detected. The resulting PCR products were subsequently analyzed by restriction enzyme digestion using MspI. High-resolution gel electrophoresis of digestion products showed an identical digestion pattern for PKCI-r between ASF/SF2-expressing and nonexpressing DT40-ASF cells (Fig. 3D, tet + vs. tet − DT40-ASF cells). Furthermore, sequencing of PKCI-r cDNAs amplified from both cells expressing or not expressing ASF/SF2 showed identical sequence of the full-length PKCI-r mRNA, with the same exon–exon junctions (data not shown). These data indicate that ASF/SF2 does not affect PKCI-r mRNA expression by altering splice site selection.
Figure 3.
ASF/SF2 does not inhibit PKCI-r mRNA expression through alternative splicing. (A) Poly(A) mRNA from DT40-ASF cells tetracycline-treated (tet +) or not treated (tet −) for 36 h were retrotranscribed, and the full-length PKCI-r cDNA was amplified by PCR using primers PKCI-r 1/22 and PKCI-r 606/629 located, respectively, at both the 5′ and 3′ ends of the PKCI-r sequence (see Fig. 2A). PCR products were resolved by agarose gel electrophoresis. (B) Restriction enzyme analysis of PCR-amplified PKCI-r fragments. PCR-amplified PKCI-r fragments from tet + and tet − DT40-ASF cells were digested by MspI, then resolved by high-resolution electrophoresis in a 3% metaphor agarose gel. (M.W.) Molecular weight markers. (C) Exons (E) and introns (I) of the PKCI-r pre-mRNA. DNA fragments amplified in D at exon/intron junctions of the PKCI-r pre-mRNA (arrows) or through the mature spliced mRNA (arrowhead) are shown. (D) Poly(A) mRNAs from DT40-ASF cells treated with tetracycline or left untreated for 36 h were DNase treated, retrotranscribed into cDNAs, and analyzed by real-time PCR for PKCI pre-mRNA containing introns 1 or 2, mature PKCI-r mRNA, or chicken β-actin mRNA. PKCI-r intron 1, intron 2, and mature mRNA levels were normalized to β-actin levels and the fold change between cells expressing (tet −) or depleted (tet +) of ASF/SF2 is shown. Data shown are the average of two assays. Error bars, SEM.
We next tested whether ASF/SF2 might reduce the rate of PKCI-r pre-mRNA splicing, which could conceivably have escaped detection in the above analyses. To this end, we analyzed whether ASF/SF2 depletion changes levels of unspliced PKCI-r pre-mRNAs, by real-time PCR (Morrison et al. 1998) using primers designed to amplify through the junction of either exon 1/intron 1 or exon 2/intron 2 of the PKCI-r pre-mRNA (Fig. 3C). For comparison, completely spliced PKCI-r was amplified using primers bridging both introns. RNAs from DT40-ASF cells, treated with tetracycline or left untreated, were DNase-treated, retrotranscribed, and then analyzed by real-time PCR. Amplification of appropriately sized fragments was confirmed by agarose gel electrophoresis, and assays were normalized to chicken β-actin mRNA expression in parallel assays. RNAs treated the same as the samples but without the addition of reverse transcriptase were amplified in parallel to exclude amplification caused by genomic DNA contamination. PKCI-r pre-mRNAs containing intron 1 were expressed at similar levels in both ASF/SF2-expressing (tet −) and nonexpressing (tet +) cells (Fig. 3D, fold difference tet +/tet − = 1.06). PKCI-r pre-mRNA transcripts containing intron 2 were slightly increased in cells depleted of ASF/SF2 (tet +) compared with cells expressing ASF/SF2 (tet −; Fig. 3D, fold difference tet +/tet − = 1.75). Thus, in the absence of a change in transcription (analyzed below), ASF/SF2 expression appears to modestly facilitate PKCI-r pre-mRNA second intron splicing. If second intron splicing is the rate-limiting step to formation of the mature PKCI-r mRNA, a decreased rate of pre-mRNA splicing caused by ASF/SF2 depletion might lead to a slight decrease in mature PKCI-r mRNA. This contrasts with the large increase in PKCI-r mRNA (∼6.7-fold; Fig. 3D) detected upon ASF/SF2 depletion, indicating that the change in PKCI-r mRNA abundance is not due to a change in splicing.
ASF/SF2 does not inhibit PKCI-r mRNA transcription
Although a role of ASF/SF2 in transcriptional regulation has not been described, many transcription factors are alternatively spliced, raising the possibility that ASF/SF2 depletion might have indirectly affected PKCI-r mRNA transcription by influencing production of a required transcription factor. We investigated the potential involvement of ASF/SF2 in regulation of PKCI-r mRNA transcription by performing nuclear run-on analysis with DT40-ASF cells (see Materials and Methods). Tetracycline-induced inhibition of ASF/SF2 accumulation in DT40-ASF cells did not induce any changes in PKCI-r mRNA transcription, which was found to be identical in both tetracycline-treated and tetracycline-untreated DT40-ASF cells. Data were normalized against 18S rRNA transcription on the basis of densitometric analysis of the radiograph (Fig. 4, tet + vs. tet − DT40-ASF cells; PKCI-r/18S ratios are shown for two cDNA dilution points that were not showing over- or underexposed signals). This result shows that ASF/SF2 does not inhibit PKCI-r mRNA expression through transcriptional regulation.
Figure 4.
ASF/SF2 does not inhibit PKCI-r mRNA transcription. Run-on assays were performed with nuclei isolated from DT40-ASF cells treated with tetracycline (1 μg/μL; tet +) to inhibit ASF/SF2 expression or left untreated (tet −) for 36 h. Nascent transcripts were 32P-labeled in vitro for 30 min, purified, and used to probe filters containing increased concentrations of PKCI-r or 18S ribosome RNA cDNAs. Data were normalized against 18S rRNA on the basis of densitometric analysis of the radiograph; numbers below panels show PKCI-r/18S rRNA ratios.
ASF/SF2 inhibits PKCI-r mRNA expression through alteration of mRNA stability
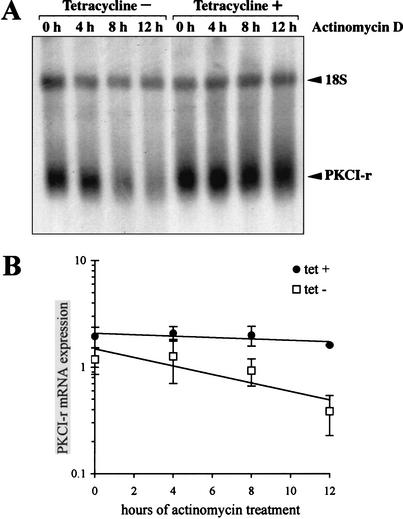
The lack of effect of ASF/SF2 on PKCI-r splicing or transcription suggested that ASF/SF2 might alter PKCI-r stability. To test whether ASF/SF2 regulates PKCI-r transcript stability, 24-h tetracycline-treated or control DT40-ASF cells were treated with actinomycin D for 0, 4, 8, and 12 h to inhibit new RNA synthesis. RNA was then extracted and analyzed by Northern blotting for PKCI-r expression (Fig. 5A). After actinomycin D treatment, PKCI-r mRNA expression decreased 6.6 times more rapidly in cells expressing ASF/SF2 (half-life of 7.0 h) than in cells not expressing ASF/SF2 (half-life of 46.0 h; Fig. 5A,B, tet − vs. tet + DT40-ASF cells). These results indicate that ASF/SF2 down-regulates PKCI-r expression in DT40-ASF cells mainly through alteration of PKCI-r mRNA stability.
Figure 5.
ASF/SF2 inhibits PKCI-r mRNA expression through alteration of mRNA stability. (A) DT40-ASF cells were treated with tetracycline (1 μg/μL; tet +) or left untreated (tet −) for 24 h, and then cells were additionally treated with actinomycin D (10 μg/μL) for 0, 4, 8, and 12 h. RNAs were then extracted, and 5 μg of each RNA sample was electrophoresed on a formaldehyde-agarose gel, blotted onto nylon, and hybridized simultaneously to 32P-labeled chicken PKCI-r and 18S ribosome cDNAs. The radiograph shown is representative of two other independent experiments. (B) PKCI-r mRNA expression is graphed after normalization to control 18S ribosome RNA on the basis of PhosphorImager analysis of the radiograph. Data shown are the average of three independent experiments for each time point. Error bars, SEM.
ASF/SF2 regulates stability of mature PKCI-r mRNA
To confirm that ASF/SF2 down-regulates accumulation of the PKCI-r transcript through alteration of its stability independently of splicing, an exogenous mature PKCI-r mRNA was expressed from a PKCI-r cDNA driven by the RSV promoter following transfection into DT40-ASF cells. Expression of exogenous PKCI-r transcript levels was assessed through RT–PCR. To permit specific amplification of exogenous versus endogenous PKCI-r transcripts, a fragment of the lacZ ORF was cloned upstream of the PKCI-r cDNA. After transfection of DT40-ASF cells with either an expression vector containing the full-length PKCI-r cDNA tagged with the lacZ ORF (pBK–RSV/lacZ–PKCI-r) or the empty expression vector (pBK–RSV), cells were treated with tetracycline (tet +) or left untreated (tet −) for 36 h. lacZ–PKCI-r cDNAs were amplified using 5′ and 3′ oligonucleotides positioned, respectively, within the 3′ regions of the lacZ and the PKCI-r ORFs. Expression of the lacZ–PKCI-r mRNA was normalized by coamplification of β-actin. In DT40-ASF cells transfected with pBK–RSV/lacZ–PKCI-r, inhibition of ASF/DT40-ASF in tetracycline-treated DT40-ASF cells induced a 1.8-fold increase in accumulation of exogenous lacZ–PKCI-r (Fig. 6A, pBK–RSV/lacZ–PKCI-r, tet + vs. tet −). No lacZ–PKCI-r mRNA was detected in DT40-ASF cells transfected with the empty pBK–RSV expression vector (Fig. 6A, pBK–RSV, tet + and tet −). Therefore, ASF/SF2 down-regulates expression of an exogenous PKCI-r mRNA.
Figure 6.
ASF/SF2 regulates the stability of overexpressed PKCI-r mRNA. (A) DT40-ASF cells were transfected with either pBK–RSV/lacZ–PKCI-r or the empty vector pBK–RSV. The next day, cells were treated with tetracycline (1 μg/μL; tet +) or left untreated (tet −) for 36 h, then processed for RNA purification. After retrotranscription of mRNAs into cDNAs, part of the lacZ–PKCI-r cDNA was PCR-amplified using primers lacZ ORF 2701/2730 and PKCI-r 382/359 (see Fig. 2A). β-Actin was coamplified as an internal control. PCR products were resolved by agarose gel electrophoresis. The level of expression of the lacZ–PKCI-r mRNA was quantified after normalization against control β-actin mRNA (see PKCI-r/actin ratio at bottom of figure) on the basis of densitometry. (B) The RNA probe, targeted transcripts, and protected fragments for RNase protection assay of lacZ–PKCI-r RNAs expressed in pBK–RSV/lacZ–PKCI-r transfected DT40-ASF cells. The RNA probe was synthesized in vitro from the T7 promoter using a modified version of pBK–RSV/lacZ–PKCI-r, in which most part of the lacZ cDNA and the 3′ end of the PKCI-r cDNA have been deleted. Hybridization of this RNA probe to endogenous and exogenous PKCI-r transcripts induces protection from RNase digestion of, respectively, 225-bp and 328-bp and 240-bp fragments (light shaded boxes). (C) DT40-ASF cells were transfected with pBK–RSV/lacZ–PKCI-r. The next day, cells were treated with tetracycline (1 μg/μL; tet +) or left untreated (tet −) for 30 h, then additionally treated with actinomycin D for 8 h or left untreated (0 h). Cells were then processed for RNA purification, and RNAs were analyzed by RNase protection, as diagrammed in B. TBP RNA was also processed for RNase protection as an internal control. Protected fragments from untransfected DT40-ASF cells (U) and undigested probe (P) are shown. Numbers on the left of the figure indicate size of sequenced DNA. Numbers on the right side of the figure indicate the expected size of protected fragments. The experiment shown is representative of two other experiments.
To extend this analysis, we next investigated whether ASF/SF2 down-regulates accumulation of the exogenous lacZ–PKCI-r transcript through alteration of its stability. DT40-ASF cells were transfected with pBK–RSV/lacZ–PKCI-r, and then treated with tetracycline (tet +) or left untreated (tet −). After 30 h, cells were treated with actinomycin D for 8 h to inhibit new RNA synthesis or left untreated (0 h). RNAs were then purified and analyzed by RNase protection for PKCI-r RNAs. The PKCI-r RNA probe was designed to detect both exogenous (328- and 240-bp protected fragment) and endogenous (225-bp protected fragment) PKCI-r mRNA (Fig. 6B). After 30 h of tetracycline treatment and before actinomycin D treatment, the lacZ–PKCI-r mRNA transcript was expressed at similar levels in both ASF/SF2-expressing and nonexpressing cells (Fig. 6C, 328- and 240-bp protected fragments, actinomycin D, 0 h, tet − vs. tet +). In contrast, after actinomycin D treatment, the exogenous PKCI-r transcript was present at significantly lower levels in cells expressing ASF/SF2 than in cells depleted of ASF/SF2 (Fig. 6C, 3.7- and 3.2-fold changes for, respectively, 328- and 240-bp protected fragments, actinomycin D, 8 h, tet − vs. tet +). Although the endogenous PKCI-r mRNA was already expressed at slightly lower levels in cells expressing ASF/SF2 than in cells not expressing ASF/SF2 before actinomycin D treatment (Fig. 6C, 225-bp protected fragments, actinomycin D, 0 h, tet − vs. tet +), the difference was markedly enhanced after actinomycin D treatment (Fig. 6C, 2.7-fold change, 225-bp protected fragment, actinomycin D, 8 h, tet − vs. tet +), consistent with the change in half-life of endogenous PKCI-r mRNA seen previously (Fig. 5). The appearance of several PKCI-r mRNA protected bands might be attributable to several factors, such as heterogeneity in probe length, nonoptimal RNase digestion, or degradation of the target RNA. The presence of an ∼550-bp protected band, not seen in the untransfected cells, is due to incomplete digestion of an RNA loop formed by the exogenous PKCI-r transcript with the probe (Fig. 6B, 328 bp + ∼240-bp protected bands). As a control, TBP mRNA levels were analyzed in parallel by RNase protection (Wang et al. 1998). As observed previously, no change in TBP expression was seen upon treatment with tetracycline (Fig. 6B).
ASF/SF2 interacts with a purine-rich region localized in the PKCI-r 3′ UTR
To understand the molecular basis of the effect of ASF/SF2 on PKCI-r RNA stability, we next investigated whether ASF/SF2 binds PKCI-r mRNA. 32P-radiolabeled mRNA fragments of the full-length PKCI-r RNA were synthesized in vitro (Fig. 7B, PKCI-r m1, m2, m3, m4) and tested for interaction with purified recombinant baculovirus-expressed ASF/SF2 by gel shift assay. Intriguingly, ASF/SF2 bound PKCI-r m4 mRNA, which corresponds to the 3′ UTR, and not significantly PKCI-r m1, m2, or m3 (Fig. 7A, PKCI-r m4 vs. PKCI-r m1, m2, or m3). Interaction between ASF/SF2 and PKCI-r m4 occurred in an all or nothing manner, with the extent of the shift increasing with increasing concentration of ASF/SF2, suggesting a highly cooperative interaction. Similar results (data not shown) were obtained with recombinant ASFΔRS, which lacks the highly charged RS domain. The PKCI-r m4 sequence contains an 18-nt purine-rich region (Figs. 2A and 7A, black box, sequence ATGGAGGGTAATGAAAAG), which contains two sequences related to the characterized ASF/SF2-binding site (Tacke and Manley 1995). A deletion encompassing this purine-rich region completely eliminated retardation of the m4 PKCI-r mRNA fragment (Fig. 7B, PKCI-r m4.1). These data indicate that ASF/SF2 binds directly to a region within the 3′ UTR of the mature PKCI-r mRNA, containing a purine-rich element similar to known ASF/SF2-binding sites.
Figure 7.
ASF/SF2 binds a purine-rich element positioned within the 3′ UTR of the mature PKCI-r mRNA. (A) PKCI-r RNA deletion mutants assessed for binding to ASF/SF2 in gel shift assays. Radiolabeled PKCI-r m1, m2, m3, m4, and m4.1 RNAs were synthesized in vitro from the T3 promoter and then gel-purified. A purine-rich region is localized in the 3′ UTR of PKCI-r RNA (black box plus sequence above). (B) Binding of each of the five PKCI-r RNA fragments to ASF/SF2 was then assessed using increasing amounts (0, 17, 50, and 150 ng) of purified baculovirus-expressed ASF/SF2 in gel shift assays. The experiment shown is representative of two other experiments. (C) RNA probe, targeted transcripts, and protected fragments for RNase protection assay of lacZ–PKCI-r RNAs expressed, respectively, in pBK–RSV–PKCI-r m4 and pBK–RSV–PKCI-r m4.1 transfected DT40-ASF cells. A 314-nt PKCI-r RNA probe spanning 338–652 nt of the PKCI-r transcript was synthesized in vitro from a T7 promoter. Hybridization of this RNA probe to exogenous PKCI-r m4 and m4.1 transcripts induces protection from RNase digestion of, respectively, 165-bp and 85-bp fragments (light shaded boxes). (D) DT40-ASF cells were transfected with pBK–RSV–PKCI-r m4 or pBK–RSV–PKCI-r m4.1. The next day, cells were treated with tetracycline (tet +) or left untreated (tet −) for 30 h, then additionally treated with actinomycin D for 8 h or left untreated (0 h). RNAs were purified and analyzed by RNase protection as diagrammed in C. TBP RNA was also analyzed for RNase protection as an internal control. The experiment shown is representative of two other experiments. (E) RNAs from m4 and m4.1 transfected cells as in D were retrotranscribed into cDNAs and analyzed by real-time PCR using a common set of primers for both transcripts (see Fig. 7C, arrows). PKCI-r m4 and m4.1 and chicken β-actin were analyzed in parallel wells. PKCI-r m4 and m4.1 levels were normalized to β-actin mRNA levels and the fold change in PKCI-r m4 or m4.1 mRNA levels between cells nonexpressing (tet +) and expressing (tet −) ASF/SF2 are shown. Data shown are the average of two assays. Error bars, SEM.
The ASF/SF2-binding site mediates instability of the PKCI-r mRNA
To establish a direct link between the interaction of ASF/SF2 with the purine-rich element in the 3′ UTR of the PKCI-r mRNA and the change in PKCI-r mRNA stability, we tested whether ASF/SF2 regulates accumulation of exogenous lacZ–PKCI-r transcript mutants containing or not containing the purine-rich element. To this end, PKCI-r RNA fragments corresponding to m4 or m4.1 (see Fig. 7A,C) were cloned into the pBK—RSV vector and expressed in DT40-ASF cells, and ASF/SF2 was depleted with tetracycline. After 30 h, new RNA synthesis was inhibited and stability of the mutant lacZ–PKCI-r RNAs was analyzed by RNase protection. As an internal control, TBP mRNA levels were analyzed in parallel. Before transcriptional arrest, exogenous lacZ–PKCI-r m4 mRNAs were expressed at similar levels in both ASF/SF2-expressing and nonexpressing cells (Fig. 7D, upper panel, PKCI-r m4 transfection, actinomycin D, 0 h, tet − vs. tet +). Absence of the PKCI-r m4 protected fragment in the lacZ–PKCI-r m4.1 transfected cells confirmed the specificity of the signal. In sharp contrast, 8 h after transcriptional arrest, the lacZ–PKCI-r m4 transcript was undetectable in cells expressing ASF/SF2, but easily detectable in cells depleted of ASF/SF2 (Fig. 7D, upper panel, PKCI-r m4 transfection, actinomycin D, 8 h, tet − vs. tet +). These results indicate that ASF/SF2 modulates mRNA stability through elements within the m4 fragment, which binds ASF/SF2, and that regulation does not depend on the remainder of the PKCI-r mRNA. The greater difference in stability of this mutant RNA compared with the full-length PKCI-r transcript (cf. Figs. 6C and 7D) suggests that other regions within the full-length transcript may, in fact, moderate ASF/SF2-induced instability. RNase protection assays of the m4.1 transcript showed no apparent change in mRNA expression (Fig. 7D, bottom panel, PKCI-r m4.1 transfection, actinomycin D, 8 h, tet − vs. tet +), but high background from partially protected PKCI-r probe fragments resulting from endogenous PKCI-r mRNA expression made accurate quantification difficult (see Fig. 7D, lanes transfected with m4).
To confirm the results on m4 and m4.1 transcript expression, we used real-time PCR with SYBR Green fluorescent detection of amplified products. This technique has shown superior dynamic range compared with other techniques in analyzing RNA stability (Schmittgen et al. 2000). We were able to specifically detect and quantify exogenous lacZ–PKCI-r m4 and m4.1 mRNAs by choosing a 5′ primer in the lacZ promoter region, part of the RNA transcribed from the transfected plasmids (Fig. 7C). All real-time PCR assays were normalized to chicken β-actin mRNA expression in parallel assays. Real-time PCR confirmed the RNase protection results in cells transfected with exogenous PKCI-r m4. Strikingly, ASF/SF2 depletion dramatically stabilized m4 transcripts (25.4-fold increase compared with ASF/SF2-expressing cells, Fig. 7E). In contrast, ASF/SF2 depletion had no significant effect on m4.1 expression (Fig. 7E). It is notable that the m4.1 deletion, although abrogating the response to ASF/SF2 depletion, did not by itself result in high levels of the PKCI-r m4.1 mRNA. This indicates that in addition to removing the ASF/SF2-responsive sequence the deletion must also disrupt an element for stabilizing the transcript. Together these data show that ASF/SF2 regulates the stability of the mature PKCI-r mRNA by binding directly to a purine-rich element positioned within the mRNA 3′ UTR.
Discussion
SR proteins are best characterized for their activities in regulating pre-mRNA splicing. We have shown here that one SR protein, ASF/SF2, can also regulate mRNA stability. This effect is highly selective, because four different PKCI-r cDNAs, and no other differentially regulated clones, were identified upon screening ∼3 × 106 plaques. Further attesting to the specificity of this regulation, we have failed to observe any effect of ASF/SF2 depletion on expression of ∼18,000 genes using low-stringency hybridization to a human gene array filter, and detected only one change in expression of >200 proteins using 2D gel electrophoresis (R. Lemaire and R. Lafyatis, unpubl.). These results suggest that ASF/SF2 does not uniquely control abundance of other mRNAs, or that such regulation is relatively rare. This is, on the one hand, somewhat surprising, given the important role of ASF/SF2 in splicing and the fact that it is essential for DT40 viability. On the other hand, SR proteins are well known to behave redundantly for many splicing-related functions in vitro, and our previous analysis failed to detect changes in the abundance of several mRNAs following ASF/SF2 depletion (Wang et al. 1998). The current assay might not detect mRNAs whose abundance was only modestly altered by loss of ASF/SF2, and would also likely not detect mRNAs that have undergone changes in alternative splicing—even dramatic ones—if this did not quantitatively affect mRNA accumulation. These seem to be the types of splicing events controlled nonredundantly by ASF/SF2 (Wang et al. 1998). Additionally, the modest decrease in PKCI-r mRNA second intron splicing following ASF/SF2 depletion is consistent with a small reduction in the overall rate of pre-mRNA processing described previously (Wang et al. 1996). Nonetheless, the results presented are remarkable because they establish that the only detectable mRNA regulated quantitatively by ASF/SF2 depletion is controlled not at the level of splicing, but by mRNA stability. Our results thus establish a novel function for SR proteins.
Although functions for SR proteins other than in nuclear pre-mRNA splicing were not described originally, recent reports suggest a possible role for some SR proteins in cytoplasmic events. Evidence has been presented that three SR proteins, ASF/SF2, SRp20, and 9G8, are capable of shuttling rapidly and continuously between the nucleus and the cytoplasm (Caceres et al. 1998). The novel effect of ASF/SF2 on mRNA stability that we have documented here suggests a functional importance to SR protein nucleus/cytoplasm shuttling. Our transfection experiments showed that the increased PKCI-r mRNA stability induced by ASF/SF2 depletion is observed with mature, spliced mRNA, and thus is likely mediated at least in part in the cytoplasm. Therefore, it could be that the effect of ASF/SF2 on PKCI-r mRNA stability is related directly or indirectly to transport from nucleus to cytoplasm. For example, perhaps PKCI-r mRNA is naturally transported in a complex with ASF/SF2, and this pathway results in relatively rapid turnover of the mRNA. In the absence of ASF/SF2, another transport complex would assemble, perhaps with SRp20 or 9G8, and this would have the effect of stabilizing the mRNA (Huang and Steitz 2001). Although speculative, this hypothesis suggests that different mRNA transport complexes could influence mRNA stability.
The half-lives of eukaryotic mRNAs vary >1000-fold, from several minutes to as long as several days (Ross 1995). The impact of mRNA stability on overall gene expression is more pronounced in mRNAs with exceptionally long or short half-lives. The short half-lives of labile mRNA, such as c-myc, c-fos, and cytokines like interleukin-2 (IL-2) or granulocyte-macrophage colony-stimulating factor (GM-CSF; Carter and Malter 1991; Beelman and Parker 1995), permit changes in transcription to be rapidly reflected in the level of cytoplasmic mRNA. In contrast, the long half-lives of stable mRNAs, such as collagens (Hamalainen et al. 1985; Maata et al. 1995), crystallins (Li and Beebe 1991), or globins (Russell and Liebhaber 1996; Russell et al. 1998), permit them to accumulate to high levels, providing the cells with a substantial saving in the energy required for their transcription, processing, and transport. The marked stabilization in PKCI-r mRNA associated with inhibition of ASF/SF2 expression suggests this is an important factor regulating PKCI-r expression.
Determinants of mRNA stability are both general and specific, appearing to act through multiple and frequently overlapping pathways (for reviews, see Decker and Parker 1994; Beelman and Parker 1995). The vast majority of eukaryotic mRNAs contain a 5′ cap and a 3′ polyadenylate tail, structures believed to protect mRNA from exonuclease cleavage. One common mRNA decay pathway, characterized in yeast, is initiated by shortening of the 3′ poly(A) tail, which leads to 5′ decapping followed by 5′ to 3′ exonucleolytic degradation of the transcript (Peltz et al. 1991; Decker and Parker 1994; Beelman and Parker 1995). Other less common decay pathways are initiated independently of deadenylation by sequence-specific endonucleolytic cleavage (Brown et al. 1993; Binder et al. 1994). In most cases, these decay pathways appear to be controlled by RNA–protein (RNP) complexes that assemble on the target mRNA (Peltz et al. 1991; Klausner et al. 1993; Ross 1995; Holcik and Liebhaber 1997; Chkeidze et al. 1999; Makeyev et al. 1999). Although SR proteins have not been previously implicated in regulating mRNA stability, other proteins containing RRMs have been shown to affect RNA stability (Deo et al. 1999; Grosset et al. 2000; Park et al. 2000). ASF/SF2 is known to show sequence-specific RNA-binding activities through its two RRMs, which can recognize and bind exonic sequences with purine-rich elements (Sun et al. 1993; Tacke and Manley 1995). We have shown here that that ASF/SF2 binds directly to a region containing such a purine-rich element positioned within the 3′ UTR of the mature PKCI-r mRNA, likely through its RRMs, and that this region is sufficient to bring about ASF/SF2-induced destabilization. These data are consistent with other data showing that cis-elements regulating mRNA stability are generally localized in the 3′ UTR of the mRNA (e.g., Binder et al. 1994; Chen et al. 1994; Decker and Parker 1994; Chen and Shyu 1995; Holcik and Liebhaber 1997; Grosset et al. 2000). Therefore, ASF/SF2 likely destabilizes PKCI-r mRNA by binding to the purine-rich element of the mRNA 3′ UTR. The mechanism responsible for this instability remains to be determined. Perhaps ASF/SF2 directly recruits factors that initiate mRNA decay. Alternatively, it may prevent binding of factors that stabilize mRNA, and rapid turnover is the default pathway. Whatever the mechanism, our results are thus consistent with, and extend, previous studies establishing the binding specificity of ASF/SF2, the ability of the protein to shuttle from nucleus to cytoplasm, and the frequent presence of destabilizing sequences in 3′ UTRs.
How ASF/SF2-mediated regulation of PKCI-r mRNA stability contributes to the physiological role of PKCI-r remains unclear, mostly because the biological function of PKCI-r is still speculative. Several data suggest that PKCI-r may function in female sex determination in birds. First, the PKCI-r gene is located on the female-specific W chromosome, enabling female-specific expression. Indeed, PKCI-r mRNA is actively expressed in the female chicken embryo before the onset of gonad differentiation, at about the same time that its virtual counterpart, PKCI, whose gene is located on the Z chromosome, is expressed (Hori et al. 2000). Second, PKCI-r does not display the same biological function as PKCI because PKCI-r lacks the HIT motif that unifies the HIT superfamily as nucleotidyl hydrolases/transferases (Fig. 2; Lima et al. 1997). Third, three-dimensional analysis performed on the human PKCI indicates that PKCI-r may form a homodimer and/or heterodimer with PKCI through highly conserved regions in the C- and N-terminal parts of the protein (Lima et al. 1996). These data together suggest that PKCI-r may act as a dominant-negative form of PKCI. Binding to PKCI and so interfering with its normal function might subsequently trigger gonadal differentiation. Of note, ASF/SF2 and other SR proteins in association with proteins Transformer (Tra) and Transformer 2 (Tra 2) regulate sex determination by altering splicing of Drosophila double sex (dsx) pre-mRNA (Lynch and Maniatis 1996). Therefore, ASF/SF2 may also be involved in sex determination in birds but, unlike in Drosophila, through a novel function in regulating mRNA stability.
In summary, our study highlights a new activity for a SR protein in controlling gene expression, as we show an unexpected role of the splicing factor ASF/SF2 in regulating stability of the PKCI-r mRNA. Our findings provide new insights into the intracellular mechanisms involved in SR protein regulation of gene expression. Further studies will better delineate the general impact of SR proteins in regulating mRNA stability.
Materials and methods
Cell culture and transfection
The ASF/SF2-knockout DT40 cell line, designated here as DT40-ASF cell line, has been described previously (Wang et al. 1996). DT40-ASF cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 nM L-glutamine, 0.03 mM 2-mercaptoethanol, 10% fetal bovine serum, and 5% chicken serum 5 (Life Technologies). For each transfection, 106 cells were resuspended in 0.5 mL of Opti-MEM mixed with 3 μL of DMRIE-C transfection reagent (Life Technologies) and 2 μg of plasmid DNA. After 4 h, 2 mL of culture medium was added. The next day, cells were treated or left untreated with tetracycline for 48 h, before being processed for RNA extraction and purification.
mRNA differential display
A chicken embryonic cDNA library (3 × 106 plaques; Clontech) was screened for ASF/SF2-related differential gene expression using 33P-labeled retrotranscribed poly(A) mRNAs prepared from cultured DT40-ASF cells either expressing (without tet treatment, tet −) or not expressing (after 48-h tetracycline treatment, tet +) ASF/SF2. Briefly, 1 mg of total RNA was prepared by direct lysis of 108 cells by the addition of 7 mL of RT-lysis buffer and purification according to the procedure for isolation of total RNA described (RNeasy Total RNA Midi Kit, QIAGEN). After annealing to biotinylated oligo(dT), 20 μg of poly(A) mRNA was then purified on streptavidin paramagnetic particles (Promega). Purified poly(A) mRNAs were subsequently labeled with [α−33P]dCTP while retrotranscribed into cDNA. In detail, 1 μg of poly(A) mRNA and 4 μg of oligo(dT)12–18 (Life technologies) were combined in water up to 22 μL, heated to 70°C for 10 min, and cooled on ice for 1 min. To this mixture, 12.4 μL of 5× RT buffer, 2 μL of 100 mM DTT, 3 μL of 20 mM nucleotide triphosphate mix (dATP, dGTP, dTTP, Pharmacia), 20 μL of [α−33P]dCTP, and 3 μL of Superscript II RNase Reverse Transcriptase (200 U/μL, Life Technologies) was added. After a 2-h incubation at 42°C, 33P-labeled retrotranscribed poly(A) mRNAs were purified through a G50 sephadex column (Roche). Meanwhile, double lifts of agar-plated cDNA library onto nylon membranes (Pharmacia) were performed. Each copy of the duplicate filter set was hybridized with either tet + or tet − DT40-ASF cell 33P-labeled retrotranscribed poly(A) mRNA. Prehybridization, hybridization, and washing of the filters were done at 65°C according to the high-stringency protocol of Church (Church and Gilbert 1984).
cDNA preparation and PCR
For preparation of cDNAs, 3 μL of poly(A) mRNA (100 ng), 1 μL of oligo(dT)12–18 (0.5 μg/μL), and 8 μL of water were heated to 70°C for 10 min and cooled on ice for 1 min. To this mixture, 4 μL of 5× RT buffer, 2 μL of 100 mM DTT, 1 μL of 10 mM nucleotide triphosphates, and 1 μL of Superscript II RNase Reverse Transcriptase (200 U) were added. After a 1-h incubation at 42°C, the cDNA was then heated to 95°C for 5 min.
For analysis of the full-length region of the endogenous PKCI-r mRNA, reverse-transcribed cDNAs were PCR-amplified using 5′ and 3′ oligonucleotides positioned, respectively, within terminal 5′ and 3′ regions of the PKCI-r mRNA (see Fig. 2A and oligonucleotide sequences below, PKCI-r 1/22 and PKCI-r 603/626). For PCR analysis of expression of the exogenous lacZ–PKCI-r mRNA, part of the reverse-transcribed lacZ–PKCI-r cDNA was amplified using 5′ and 3′ oligonucleotides positioned, respectively, within the 3′ regions of the lacZ and PKCI-r ORFs (see Fig. 2A and oligonucleotide sequences below). The 25-μL PCR reactions were composed of 2.5 μL of 10× PCR buffer, 25 ng of each oligonucleotide, 2.5 μL of 1.25 mM nucleotide triphosphates, 2 μL of 50 mM MgCl2, 0.2 units of Taq polymerase (Life Technologies), and 1 μL of template cDNA. Amplification conditions were: 96°C for 45 sec; 65°C for 1 min; 72°C for 2 min; 30 cycles.
Plasmid constructs
Plasmid pPKCI-r was constructed by cloning the 652-bp cDNA of the chicken PKCI-r gene isolated by ASF/SF2-related differential screening of a chicken cDNA library into a pBlueScript SK vector as an EcoRI/XhoI fragment. Plasmid pBK–RSV/lacZ–PKCI-r, generating a full-length PKCI-r transcript 5′-tagged with part of the lacZ ORF, was constructed by inserting both the 1119-bp 3′ end of the lacZ ORF as a SacI/EcoRI fragment and the full-length PKCI-r cDNA as an EcoRI/XhoI fragment into the pBK–RSV expression vector (Stratagene). The PKCI-r m1 construct was made from linearization of pPKCI-r by NaeI digestion. The PKCI-r m2 construct was derived from pPKCI-r by EcoRI/NaeI digestion followed by blunt end religation, and linearization by NheI. The PKCI-r m3 construct was derived from pPKCI-r by EcoRI/NheI digestion followed by blunt end religation, and linearization by MluI. The PKCI-r m4 construct was derived from pPKCI-r by EcoRI/MluI digestion followed by blunt end religation, and linearization by XhoI. The PKCI-r m4.1 construct was derived from the PKCI-r m4 construct. The 132-bp XbaI/HindIII fragment of PKCI-r m4.1, containing the 18-nt purine-rich region (Figs. 1A and 7B), was replaced by a PCR-amplified XbaI/HindIII fragment identical to the original 132-bp XbaI/HindIII fragment but missing the purine-rich region. The pBK–RSV–PKCI-r m4 and m4.1 plasmids were constructed by cloning the m4 and m4.1 cDNAs from, respectively, PKCI-r m4 and PKCI-r m4.1 constructs as SpeI/KpnI fragments into the pBK–RSV vector.
Oligonucleotide sequences
Numbers refer to nucleotide positions in the PKCI-r cDNA (Fig. 2). PKCI-r 1/22: 5′-GAGCCGTGCTGAGCCGTGCTGG-3′; PKCI-r 606/629: 5′-CCCCATGCTCCATCTTTATTCTGC-3′; LacZ ORF 2701/2730: 5′-GTTGAAGTGGCGAGCGATACA CCG-3′; PKCI-r 385/362: 5′-GAGGGTATCTCACAGCCATC CGGA-3′; β-actin 5′: 5′-GGGTGTGATGGTTGGTATGGGC CA-3′; β-actin 3′: 5′-AGGTAGTCCGTCAGGTCACGGCCA-3′; PKCI-r 571–548: 5′-GAGTTAGCTCACTCATTAGGCACC-3′; PlacZ 5′: 5′-GAGTTAGCTCACTCATTAGGCACC-3′; E1 5′: 5′-AGGTTTGGGGCTGAGGGAGTGTTG-3′; I1 3′: 5′-ACGG GAGGGCAAGGGAAGAATC-3′; E2 5′: 5′-CCGCAAGCTCC TACGCTTTTTCC-3′; I2 5′: 5′-CCACTTACTGGCACCGTTT CGTG-3′.
Nuclear run-on assay
For the nuclear run-on assay, 107 DT40-ASF cells were harvested and washed twice in cold PBS. Cell pellets were then resuspended in 100 μL of ice-cold lysis buffer (0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-HCl at pH 8.0, 1 mM DTT, 0.5 mM PMSF, 0.5% NP-40). After plasma membrane lysis, cell lysates were centrifuged for 5 min at 500g. Nuclear pellets were then washed twice in 1.5 mL of ice-cold NP-40-free lysis buffer, resuspended in 50 μL of ice-cold storage buffer (50 mM Tris-HCl at pH 8.0, 5 mM MgCl2, 0.1 mM EDTA, 40% glycerol), and stored at −80°C or used immediately. The elongation reaction was carried out in vitro by mixing 25 μL of isolated nuclei with 25 μL of 2× reaction buffer containing 10 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 300 mM KCl, 5 mM DTT, 1 mM each of ATP, CTP, and GTP, 0.3 mM UTP, and 200 μCi of [α−32P]UTP (800–6000 Ci/mmole). After a 30-min incubation at room temperature, nuclear RNA transcripts were extracted and purified according to the procedure described (RNeasy Mini Kit, QIAGEN). Gel-purified PKCI-r and 18S ribosome cDNAs were applied onto a nylon filter using a dot blot apparatus. After UV-cross-linking, nylon-bound cDNAs were denatured for 5 min with 0.5 M NaOH and 1.5 M NaCl, neutralized for 5 min with 1 M Tris-HCl/1.5 M NaCl, then washed for 5 min with 20× SSC. Filters were hybridized with synthesized elongation reaction mRNAs. Prehybridization, hybridization, and washing of the filters were done at 65°C according to the high-stringency protocol of Church (Church and Gilbert 1984).
RNase protection assay
Linearized pBK–RSV expressing various mRNA transcript mutants of PKCI-r was used for in vitro transcription of radioactive RNA probe using [α−32P]UTP (Promega). To monitor exogenous or endogenous PKCI-r RNAs expressed in transfected DT40-ASF cells, 15 μg of each RNA sample and 105 CPM of the RNA probe were hybridized overnight at 45°C. RNase digestion using RNase A and T1 were performed using an RPA III kit (Ambion). Protected fragments were separated on a 6% polyacrylamide-8 M urea gel and detected by autoradiography.
RNA binding assay
32P-radiolabeled RNAs were synthesized in vitro from the T3 promoter and purified on denaturing polyacrylamide gels prior to RNA binding assays. RNA binding assays were performed with 2.5 fmole of radiolabeled RNA. RNA binding buffer contained 10 mM HEPES (pH 7.9), 250 mM KCl, 50 mM NaCl, 20 mM EDTA, 0.025% NP-40, 1 mM DTT, 15 μg/mL BSA, and 10% glycerol. Purified baculovirus His-tagged ASF/SF2 protein and 5 μg of tRNA were added to 25-μL reaction mixtures prior to the labeled probes. Samples were incubated at 30°C for 20 min and analyzed on 5% polyacrylamide (acrylamide/bisacrylamide ratio, 40:1) gels. Gels were electrophoresed in 0.5× TBE buffer at 180 V for 150 min.
Real-time PCR
For real-time PCR analysis of specific mRNA transcripts, RNAs were retrotranscribed as described above. PKCI-r cDNAs containing intron 1 were amplified using oligonucleotides positioned upstream (E1 5′) and downstream (I1 3′) of the junction exon 1/intron 1. PKCI-r cDNAs containing intron 2 were amplified using a set of oligonucleotides positioned upstream (E2 5′) and downstream (I2 3′) of the junction exon 2/intron 2. PKCI-r m4 and m4.1 cDNAs were amplified using a common set of oligonucleotides. The 5′ and 3′ oligonucleotides were chosen, respectively, within the lacZ promoter region of the pBK–RSV vector, the lacZ promoter being transcribed along with the downstream cloned cDNA from the RSV promoter (placZ 5′), and upstream of the ASF/SF2-binding purine-rich region (PKCI-r 571–548; see Fig. 2A and oligonucleotide sequences above). Chicken β-actin cDNA was amplified in parallel (see oligonucleotide sequences above). PCR was performed on the ABI 7700 Sequence Detector (Perkin-Elmer) using SYBR Green fluorescence. The 50-μL PCR reactions were composed of 5 μL of 1:5 diluted cDNA, 25 μL of 2× SYBR Green mix (Perkin-Elmer), 2.5 μL of primer mix (100 nM final concentration), and 17.5 μL of water. Amplifications conditions were: 95°C for 30 sec; 60°C for 1 min; 40 cycles. PCR products were analyzed using the Sequence Detector software. After setting the threshold to compare the fluorescence of samples at identical points along the logarithmic portion of the amplification curve, the relative RNA concentration was calculated.
Acknowledgments
Support for this work was provided by grants to R.L. from the Arthritis Foundation and from the National Institutes of Health (Shannon Award). J.L.M was supported by NIH grant R37 GM 48259. R.L. thanks the Philippe Foundation for his financial support.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL rlafyatis@med-med1.bu.edu; FAX (617) 638-5226.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.939502.
References
- Beelman CA, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–183. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- Binder R, Horowitz JA, Basilion JP, Koeller DM, Klausner RD, Harford JB. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3′ UTR and does not involve poly(A) tail shortening. EMBO J. 1994;13:1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Garrison P, Gilmour J, Peisach D, Ringe D, Petsko GA, Lowenstein JM. Crystal structures of HINT demonstrate that histidine triad proteins are GalT-related nucleotide-binding proteins. Nat Struct Biol. 1997;4:231–238. doi: 10.1038/nsb0397-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Zipkin ID, Harland RM. Sequence-specific endonucleolytic cleavage and protection of mRNA in Xenopus and Drosophila. Genes & Dev. 1993;7:1620–1631. doi: 10.1101/gad.7.8.1620. [DOI] [PubMed] [Google Scholar]
- Brzoska PM, Chen H, Zhu Y, Levin NA, Disatnik MH, Mochly-Rosen D, Murnane JP, Christman MF. The product of the ataxia-telangiectasia group D complementing gene, ATDC, interacts with a protein kinase substrate and inhibitor. Proc Natl Acad Sci. 1995;92:7824–7828. doi: 10.1073/pnas.92.17.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzoska PM, Chen H, Levin NA, Kuo W-L, Collins C, Fu KK, Gray JW, Christman MF. Cloning, mapping, and in vivo localization of a human member of the PKCI-1 protein family (PRKCNH1) Genomics. 1996;36:151–156. doi: 10.1006/geno.1996.0435. [DOI] [PubMed] [Google Scholar]
- Caceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes & Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Malter JS. Regulation of mRNA stability and its relevance to disease. Lab Invest. 1991;65:610. [PubMed] [Google Scholar]
- Chen CY, Shyu AB. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Chen CY, Chen TM, Shyu AB. Interplay of the two functionally and structurally distinct domains of the c-fos AU-rich element specifies its mRNA-destabilizing function. Mol Cell Biol. 1994;14:416–426. doi: 10.1128/mcb.14.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chkeidze AN, Lyakov DL, Makeyev AV, Morales J, Kong J, Liebhaber SA. Assembly of the α-globin mRNA stability complex reflects binary interaction between the pyrimidine-rich 3′ untranslated region determinant and poly(C) binding protein αCP. Mol Cell Biol. 1999;19:4572–4581. doi: 10.1128/mcb.19.7.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Parker R. Mechanisms of mRNA degradation in eukaryotes. Trends Biochem Sci. 1994;19:336–340. doi: 10.1016/0968-0004(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Deo RC, Bonanno JB, Sonenberg N, Burley SK. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell. 1999;98:835–845. doi: 10.1016/s0092-8674(00)81517-2. [DOI] [PubMed] [Google Scholar]
- Fu XD, Maniatis T. The 35-kDa mammalian splicing factor mediates specific interactions between U1 and U2 small nuclear ribonucleoprotein particles at the 3′ splice site. Proc Natl Acad Sci. 1992;89:1725–1729. doi: 10.1073/pnas.89.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XD, Mayeda A, Maniatis T, Krainer AR. General splicing factors SF2 and SC35 have equivalent activities in vitro, and both affect alternative splicing 5′ and 3′ splice site selection. Proc Natl Acad Sci. 1992;89:11224–11228. doi: 10.1073/pnas.89.23.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Manley JL. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990;62:25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- Ge H, Zuo P, Manley JL. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991;66:373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosset C, Chen CY, Xu N, Sonenberg N, Jacquemin-Sablon H, Shuy AB. A mechanism for translationally coupled mRNA turnover: Interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell. 2000;103:29–40. doi: 10.1016/s0092-8674(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: Clues to why the spliceosome is a ribonucleoprotein. Science. 1991;253:157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Hamalainen L, Oikarinen J, Kivirikko K. Synthesis and degradation of type I procollagen mRNA in cultured human skin fibroblasts and the effects of cortisol. J Biol Chem. 1985;260:720–726. [PubMed] [Google Scholar]
- Holcik M, Liebhaber S. Four highly stable eukaryotic mRNAs assemble 3′ untranslated region RNA–protein complexes sharing cis and trans components. Proc Natl Acad Sci. 1997;94:2410–2414. doi: 10.1073/pnas.94.6.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Asakawa S, Itoh Y, Shimizu N, Mizuno S. Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: Implication of its role in female sex determination. Mol Biol Cell. 2000;11:3645–3660. doi: 10.1091/mbc.11.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Steitz JA. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Biol Cell. 2001;7:899–905. doi: 10.1016/s1097-2765(01)00233-7. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Zuo P, Manley JL, Baker BS. The Drosophila RNA-binding protein RBP1 is localized to transcriptionally active sites of chromosomes and shows a functional similarity to human splicing factor ASF/SF2. Genes & Dev. 1992;6:2569–2579. doi: 10.1101/gad.6.12b.2569. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Rouault TA, Harford JB. Regulating the fate of mRNA: The control of cellular iron metabolism. Cell. 1993;72:19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Klein MG, Yao Y, Slosberg ED, Lima CD, Doki Y, Weinstein IB. Characterization of PKCI and comparative studies with FHIT, related members of the HIT protein family. Exp Cell Res. 1998;244:26–32. doi: 10.1006/excr.1998.4153. [DOI] [PubMed] [Google Scholar]
- Kohtz JD, Jamison SF, Will CL, Zuo P, Lurhermann R, Garcia-Blanco MA, Manley JL. Protein–protein interactions and 5′ splice site recognition in mammalian mRNA precursors. Nature. 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- Krainer AR, Conway GC, Kozak D. The essential splicing pre-mRNA splicing factor SF2 influences 5′ splice site selection by activating proximal sites. Cell. 1990a;62:35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- ————— Purification and characterization of pre-mRNA splicing factor SF2 fron HeLa cells. Genes & Dev. 1990b;4:1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- Krainer AR, Mayeda A, Kozak D, Binns G. Functional expression of cloned human splicing factor SF2: Homology to RNA-binding proteins, U1 70k, and Drosophila splicing regulators. Cell. 1991;66:383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Li XA, Beebe DC. Messenger RNA stabilization in chicken development: A reexamination. Dev Biol. 1991;146:239–241. doi: 10.1016/0012-1606(91)90463-d. [DOI] [PubMed] [Google Scholar]
- Lima CD, Klein MG, Weinstein IB, Hendrickson WA. Three-dimensional structure of human protein kinase C interacting protein 1, a member of the HIT family of proteins. Proc Natl Acad Sci. 1996;93:5357–5362. doi: 10.1073/pnas.93.11.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CD, Klein MG, Hendrickson AW. Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science. 1997;278:286–290. doi: 10.1126/science.278.5336.286. [DOI] [PubMed] [Google Scholar]
- Lynch KW, Maniatis T. Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes & Dev. 1996;10:2089–2101. doi: 10.1101/gad.10.16.2089. [DOI] [PubMed] [Google Scholar]
- Maata A, Elkholm E, Penttinen RP. Effect of the untranslated region on the expression levels and mRNA stability of alpha1(1) collagen gene. Biochim Biophys Acta. 1995;1260:294–300. doi: 10.1016/0167-4781(94)00207-j. [DOI] [PubMed] [Google Scholar]
- Makeyev AV, Chkheidze AN, Liebhaber SA. A set of highly conserved RNA-binding proteins, α-CP1 and α-CP2, implicated in mRNA stabilization, are coexpressed from an intronless gene and its intron-containing paralog. J Biol Chem. 1999;274:24849–24857. doi: 10.1074/jbc.274.35.24849. [DOI] [PubMed] [Google Scholar]
- Manley JL, Tacke R. SR proteins and splicing control. Genes & Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954–962. [PubMed] [Google Scholar]
- Park S, Myszka DG, Yu M, Littler SJ, Laird-Offringa I. HuD RNA recognition motifs play distinct roles in the formation of a stable complex with AU-rich RNA. Mol Cell Biol. 2000;13:4765–4772. doi: 10.1128/mcb.20.13.4765-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JD, Dewald DB, Mathews WR, Mozier NM, Zuercher-Neely HA, Heinrikson RL, Morris MA, McDonald WD, Fraser ED, Vogel HJ, et al. Amino acid sequence and characterization of a protein inhibitor protein kinase C. J Biol Chem. 1990;265:4583–4591. [PubMed] [Google Scholar]
- Peltz SW, Brewer G, Bernstein P, Hart PA, Ross J. Regulation of mRNA turnover in eukaryotic cells. Crit Rev Eukaryot Gene Expr. 1991;1:99–126. [PubMed] [Google Scholar]
- Robinson K, Aitken A. Identification of a new protein family which includes bovine protein kinase C inhibitor-1. Biochem J. 1994;304:662–664. doi: 10.1042/bj3040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:429–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JE, Liebhaber SA. The stability of human β-globin mRNA is dependent on structural determinants positioned within its 3′ untranslated region. Blood. 1996;87:5314–5323. [PubMed] [Google Scholar]
- Russell JE, Morales J, Aleksandr V, Makeyvev V, Liebhaber SA. Sequence divergence in the 3′ untranslated regions of human ζ- and α-globin mRNAs mediates a difference in their stabilities and contributes to efficient gene developmental switching. Mol Cell Biol. 1998;18:2173–2183. doi: 10.1128/mcb.18.4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- Seraphin B. The HIT protein family: A new family of proteins present in prokaryotes, yeast and mammals. DNA Seq. 1992;3:177–179. doi: 10.3109/10425179209034013. [DOI] [PubMed] [Google Scholar]
- Sharp PA. Split genes and RNA splicing. Cell. 1994;77:805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Sun Q, Mayeda A, Hampson RK, Krainer AR, Rottman FM. General splicing factor SF2/ASF promotes alternative splicing by binding to an exonic splicing enhancer. Genes & Dev. 1993;7:2598–2608. doi: 10.1101/gad.7.12b.2598. [DOI] [PubMed] [Google Scholar]
- Tacke R, Manley JL. The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities. EMBO J. 1995;14:3540–3551. doi: 10.1002/j.1460-2075.1995.tb07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel J, Green MR. The SR protein family: Pleiotropic functions in pre-mRNA splicing. Trends Biochem Sci. 1996;21:296–301. [PubMed] [Google Scholar]
- Wang J, Takagaki Y, Manley JL. Targeted disruption of an essential vertebrate gene: ASF/SF2 is required for cell viability. Genes & Dev. 1996;10:2588–2599. doi: 10.1101/gad.10.20.2588. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiao S, Manley JM. Genetic analysis of the SR protein ASF/SF2: Interchangeability of RS domains and negative control of splicing. Genes & Dev. 1998;12:2222–2233. doi: 10.1101/gad.12.14.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler AM, Lane WS, Stolk JA, Roth MB. SR proteins: A conserved family of pre-RNA splicing factors. Genes & Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- Zahler AM, Neugebauer KM, Lane WS, Roth MB. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993;260:219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- Zuo P, Manley JL. The human splicing factor ASF/SF2 can specifically recognize pre-mRNA 5′ splice site. Proc Natl Acad Sci. 1994;91:3363–3367. doi: 10.1073/pnas.91.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]