Abstract
In the developing spinal cord, signals from the roof plate are required for the development of three classes of dorsal interneuron: D1, D2, and D3, listed from dorsal to ventral. Here, we demonstrate that absence of Wnt1 and Wnt3a, normally expressed in the roof plate, leads to diminished development of D1 and D2 neurons and a compensatory increase in D3 neuron populations. This occurs without significantly altered expression of BMP and related genes in the roof plate. Moreover, Wnt3a protein induces expression of D1 and D2 markers in the isolated medial region of the chick neural plate, and Noggin does not interfere with this induction. Thus, Wnt signaling plays a critical role in the specification of cell types for dorsal interneurons.
Keywords: Wnt signaling, neuronal specification, interneuron, spinal cord
During development of the vertebrate central nervous system (CNS), highly proliferative cells in the ventricular zone of the neural tube serve as progenitors of the various types of neurons, such as interneurons and motor neurons. In the ventral half of the spinal cord, the secreted signaling molecule Sonic hedgehog (Shh) functions as a gradient signal for the generation of five distinct classes of neurons along the dorsoventral axis. Shh secreted from the notochord and floor plate controls the specification of ventral cell types in a dose-dependent manner (Roelink et al. 1995; Ericson et al. 1996; Tanabe and Jessell 1996; Jessell 2000).
Three subclasses of interneuron, called D1, D2, and D3 positioned from the dorsal side, are indicated by the expression of homeodomain proteins LH2, Islet1, and Lim1/2 in the dorsal half of the spinal cord (Liem et al. 1997). These dorsal interneurons are derived from progenitors in the ventricular zone. These progenitors are also subdivided by expression of the basic helix–loop–helix (bHLH) proteins Math1, Neurogenin1 (Ngn1), and Mash1 (Lee et al. 1998, 2000). It has been established that Math1-expressing cells give rise to LH2+ neurons (Helms and Johnson 1998). Recent studies indicate that the roof plate is the major source of inductive signals controlling the generation of the D1 and D2 classes of dorsal interneuron (Lee and Jessell 1999). Experiments using cultured chick neural plate tissue have demonstrated that signals from the roof plate are sufficient to promote dorsal interneuron differentiation in vitro (Liem et al. 1997). Moreover, absence of D1/D2 class neurons in mouse embryos lacking the roof plate, either by homozygosity of the dreher (dr) allele carrying a loss-of-function mutation in the LIM-homeobox gene Lmx1, or by genetic roof plate ablation, has provided compelling evidence for the roof plate as the determinant source of dorsal interneurons (Lee et al. 2000; Millonig et al. 2000).
Around the time when dorsal interneurons are generated, cells at the dorsal end of the neural tube express secretory proteins belonging to BMP, FGF, and Wnt families (Lee and Jessell 1999). It has been shown in the chick that BMP family proteins mimic the roof plate in the induction of dorsal interneurons in the isolated medial region of the neural plate (Liem et al. 1997; Lee et al. 1998). Thus, BMP family proteins have been considered to be the major signaling molecules originating from the roof plate and defining the dorsal interneurons of the spinal cord.
Gene disruption studies on the function of BMP family members, expressed in the roof plate, have revealed several essential roles of this group of secreted protein. However, studies have not thus far indicated a requirement for these BMP proteins in the development of dorsal interneurons, except in the case of GDF7. In Bmp7-deficient mice, the development of eye and kidney is perturbed (Dudley et al. 1995; Luo et al. 1995), but defects of neurogenesis in the spinal cord have not been reported. In addition, the disruption of Bmp6 has no effect on the developing nervous system (Solloway et al. 1998). Bmp4-deficient embryos show severe defects around the gastrulation stage and do not survive to the stages at which protein function in neuronal development could be analyzed (Winnier et al. 1995). In contrast, in Gdf7-deficient mice, the generation of D1A neurons, the dorsal subclass of D1 class interneurons, is eliminated, whereas the generation of D1B and other identified dorsal interneurons remains unaffected. The limited effect of the deficiency of BMP proteins in the dorsal spinal cord may be accounted for either by functional redundancies among coexpressed BMP family proteins (Dudley and Robertson 1997), or by the contribution of other signaling molecules expressed in the roof plate, such as Wnt proteins.
Wnt proteins constitute a large family of signaling molecules that have important roles in the regulation of embryonic patterning, cell proliferation, and cell determination (Wodarz and Nusse 1998). Wnt1 and Wnt3a are expressed in extensively overlapping regions within the CNS, predominantly along the dorsal midline from the diencephalon to the spinal cord (Wilkinson et al. 1987; Roelink and Nusse 1991; McMahon et al. 1992; Parr et al. 1993). Expression in the roof plate continues throughout the period of neurogenesis. Absence of both genes results in reduction of neural crest cell populations and a deficiency of dorsolateral neural precursors in the developing hindbrain (Ikeya et al. 1997).
This study demonstrates that mouse embryos lacking both Wnt1 and Wnt3a are indeed defective in determination of dorsal interneurons. Generation of D1 and D2 classes of dorsal interneurons is impaired; this loss of the dorsal interneurons is compensated by a dorsal expansion of D3 interneuron populations. Most importantly, expression of BMP family proteins is not significantly affected in these mutant embryos. Moreover, the induction of D1 and D2 class interneurons by Wnt3a protein in the isolated medial region of chick neural plate is demonstrated. Together, these observations clearly indicate that Wnt signaling has a critical role in the generation of D1 and D2 dorsal interneurons.
Results and Discussion
A previous study indicated that in Wnt1−/−; Wnt3a−/− embryos, expression of Pax3 and Pax6 in the dorsal and medioventral regions of the neural tube, respectively, was not significantly affected in the spinal cord, indicating that a basic dorsoventral division was normally established (Ikeya et al. 1997). We extended the analysis to finer dorsal subdivisions employing expression of homeodomain proteins LH2, Islet1, Pax2, and Lim1/2 as markers of neural identity. D1, D2, and D3 subclasses of dorsal interneurons are identified by the combined expression of these proteins; D1 neurons express LH2, D2 neurons express Islet1, and D3 neurons express Pax2 and Lim1/2 (Burrill et al. 1997; Liem et al. 1997). In Wnt1−/−;Wnt3a−/− embryos, only trace cell populations expressing LH2 or Islet1 remained at the dorsal margin of the neural tube. This indicated that D1 and D2 neurons were largely absent (Fig. 1A,D–F,I,J). On the other hand, there was a twofold increase in the population of cells marked by expression of Pax2 and Lim1/2 in the dorsal half of Wnt1−/−;Wnt3a−/− spinal cord (Fig. 1K,N–R). Thus the number of D3 neurons was increased in the dorsal spinal cord, compensating for the absence of D1 and D2 neurons. This observation clearly indicates that the activity of Wnt proteins is required for proper generation of the interneuron subclasses D1 and D2. The loss of D1 and D2 interneurons occurred only in Wnt1−/−;Wnt3a−/− double mutant embryos but not in Wnt1−–/− or Wnt3a−/− single mutants (Fig. 1B,C,E,G,H,J,L,M,O), indicating that expression of one of these Wnt proteins in the roof plate is sufficient to generate normal populations of D1/D2 interneurons.
Figure 1.
Defects of dorsal interneuron classes in Wnt1−/−;Wnt3a−/− embryos. Expression of LH2 (A–D), Islet1 (F–I), Pax2 (K–N) and Lim1/2 (P,Q), in the cervical spinal cord of E10.5 wild-type (A,F,K,P), Wnt1−/− (B,G,L), Wnt3a−/− (C,H,M), and Wnt1−/−;Wnt3a−/− (D,I,N,Q) embryos. D1 neurons expressing LH2 (A–D) and D2 neurons expressing Islet1 (F–I) were significantly reduced (to ∼5%) in Wnt-1−/−;Wnt-3a−/− embryos. D3 neurons expressing Pax2 (K–N) and stained with anti-Lim1/2 antibody (P,Q), normally generated from a medial position, were found in the dorsal third of the spinal cord in Wnt1−/−;Wnt3a−/− embryos. Domains expressing LH2 (A), Islet1 (F), Pax2 (K,N) and Lim1/2 (P,Q) in the dorsal neural tube are indicated by brackets. These results were confirmed by analysis of four embryos of each genotype. Islet1 is also expressed by motor neurons (MN) and dorsal root ganglia (DRG). Pax2 and Lim1/2 are also expressed by ventral interneurons. In Wnt1−/−;Wnt3a−/− embryos, development of the dorsal root ganglion was impaired as already reported (I, arrowheads; Ikeya et al. 1997). (E,J,O,R) Cell count analyses for individual neural markers in the dorsal spinal cord. Averages were taken for four serial sections per embryo over two embryos of a genotype. There was a twofold increase in the population of Pax2- and Lim1/2-expressing cells in Wnt1−/−;Wnt3a−/− dorsal spinal cord compared with normal neural tube (O,R). The population sizes of the interneurons were comparable to wild-type embryos in Wnt1−/− or Wnt3a−/− single mutants.
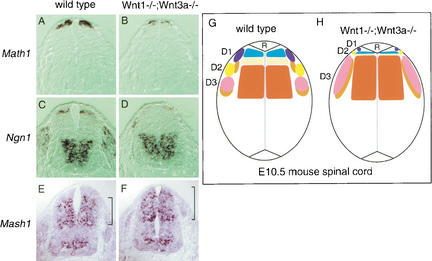
To gain insight into the mechanisms of the loss of D1/D2 interneurons and of dorsal expansion of D3 neurons, the progenitor populations in the ventricular zone were examined. Progenitors of the dorsal interneurons are characterized by the expression of one of the bHLH proteins. These proteins are Math1, Ngn1, and Mash1, from the dorsal toward the ventral aspect, respectively (Helms and Johnson 1998; Lee et al. 1998, 2000). In Wnt1−/−;Wnt3a−/− neural tube, Math1+ cells and Ngn1+ cells are present in the dorsalmost region of the ventricular zone, but are significantly reduced in population size (to ∼15%; Fig. 2A–D), whereas Mash1+ cells expanded their territory dorsally (Fig. 2E,F). Thus it can be seen that a reduction in the potential population of dorsal interneurons proximal to the roof plate has already occurred at the progenitor stage, and this situation is exacerbated as the neurons differentiate (Fig. 2G,H).
Figure 2.
Defects of dorsal neural progenitors in Wnt1−/−;Wnt3a−/− embryos. Expression of Math1 (A,B), Ngn1 (C,D) and Mash1 (E,F) in the cervical spinal cord of E10.5 wild-type (A,C,E) and Wnt1−/−;Wnt3a−/− (B,D,F) embryos. In Wnt1−/−;Wnt3a−/− embryos, dorsal neural progenitors expressing Math1 or Ngn1 were greatly reduced in number (to ∼15%). Ngn1-expressing cells were located more dorsally than normal in Wnt1−/−;Wnt3a−/− neural tube (D). Mash1+ progenitors, normally excluded from the dorsal neural tube, occupied the dorsal area of the spinal cord in Wnt1−/−;Wnt3a−/− embryos (F). These results were confirmed by analysis of four embryos of each genotype. (G,H) Summary of the phenotype of dorsal interneuron development in Wnt1−/−;Wnt3a−/− embryos. D1, D2, and D3 subclasses of the dorsal interneuron are indicated by the expression of homeodomain proteins LH2 (blue), Islet1 (yellow), Pax2 (pink), and Lim1/2 (orange), respectively. The progenitors in the ventricular zone are also subdivided by the expression of bHLH proteins, Math1 (pale blue), Ngn1 (light yellow), and Mash1 (deep orange). Absence of Wnt1 and Wnt3a led to diminished development of D1 and D2 neurons and to a compensatory increase of D3 neurons. Math1+ progenitors and Ngn1+ progenitors are present in the dorsalmost region of the ventricular zone, but are significantly reduced in population sizes, whereas Mash1+ progenitors have increased and expanded their territory dorsally (H).
Given the indication that the ability of the roof plate to induce dorsal characteristics in the spinal cord involves BMP proteins (Liem et al. 1997; Lee et al. 1998), it is important to determine whether the loss of D1 and D2 interneurons in Wnt1−/−;Wnt3a−/− neural tube is a function of altered BMP protein expression, or a more direct consequence of Wnt protein deficiency. Therefore, the expression of Bmp4, Bmp6, Bmp7, Gdf7, and Noggin in the roof plate was examined. Expression of these BMP genes and the BMP-antagonist gene in the roof plate was indistinguishable between wild-type and Wnt1−/−; Wnt3a−/− embryos (Fig. 3A–D; data not shown). Moreover, the expression of Msx1 and Msx2, which respond to BMP activity, was not altered in Wnt1−/−;Wnt3a−/− embryos (Fig. 3E,F; data not shown). Thus, the reduction in D1/D2 neurons and compensatory expansion of D3 neurons in Wnt1−/−;Wnt3a−/− mutants occurred without alteration of the BMP signaling system. This result implies that Wnt proteins are direct regulators in the determination of D1 and D2 interneurons.
Figure 3.

The expression of BMP signal-related molecules in roof plate was not affected in Wnt1−/−;Wnt3a−/− embryos. Expression of Bmp4 (A,B), Gdf7 (C,D) and Msx1 (E,F) in the cervical spinal cord of E10.5 wild-type (A,C,E) and Wnt1−/−;Wnt3a−/− (B,D,F) embryos. All of these genes were expressed normally by roof plate cells in wild-type and Wnt1−/−;Wnt3a−/− embryos. These results were confirmed by analysis of at least two embryos of each genotype.
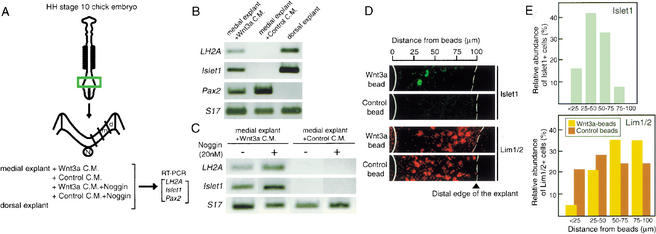
In a previous study, the potential of the roof plate to induce dorsal interneurons of the D1/D2 subclasses was demonstrated by coculturing of roof plate fragments with explants from the medial region of avian neural plate (Liem et al. 1997). If Wnt proteins account for the major component of D1/D2-inducing activity, Wnt protein added to the medial neural plate will exert an effect mimicking the cocultured roof plate. Therefore, Wnt3a protein was added to cultures of medial neural plate explant, in the form of a conditioned medium of Wnt3a-expressing L cells (Fig. 4A). Expression of LH2A (D1 marker), Islet1 (D2 marker), and Pax2 (D3 marker) in the explants was compared with that in control L cell-conditioned medium. Analysis by RT–PCR indicates that expression of LH2A and Islet1 was clearly activated by Wnt3a in the explants, but was not detectable in the control explants. Pax2 expression was significantly reduced by Wnt3a protein (Fig. 4B). Furthermore, activation of LH2A and Islet1 expression by Wnt3a-conditioned medium was not affected by BMP antagonist Noggin at 20 nM (Fig. 4C), which caused an eightfold reduction of the Msx1 expression when applied to the dorsal neural plate (data not shown).
Figure 4.
Wnt3a protein induces D1 and D2 interneurons in the medial neural plates of chick embryos. (A) Experimental procedure. Neural plate tissue at the caudal region of HH stage 10 chick embryo was isolated, dissected into medial (m) and dorsal (d) regions, and cultured as explants under various conditions: with Wnt3a-conditioned medium (Wnt3a C.M.), with control conditioned medium (Control C.M.), with Wnt3a-conditioned medium plus Noggin (20 nM), or with control conditioned medium plus Noggin. RNA was extracted from the explants and analyzed by RT–PCR. (N) Notochord. (B) RT–PCR analysis of Wnt3a-induced gene expression in chick neural plate explants. Analysis of medial region explants cultured for 48 h with Wnt3a-conditioned medium and of those cultured with control conditioned medium in comparison with dorsal region explants. Wnt3a treatment induced expression of both LH2A and Islet1 in medial region explants (n = 4). In contrast, expression of Pax2 in the medial region explant with Wnt3a was weaker than in control explants. Chick S17 ribosomal protein RNA was used to control the amount of RNA used for the RT–PCR analysis. S17 PCR products were in proportion to the amount of template RNA as confirmed by serial dilutions of the input RNA (data not shown). (C) Effect of Noggin on Wnt3a-induced gene expression in chick neural plate explants. Induction of LH2A and Islet1 was not affected by addition of 20 nM Noggin protein (n = 4). The activity of Noggin protein was confirmed by an eightfold decrease of Msx1 expression in dorsal region explants (n = 3). (D) Culture of medial region explants with Wnt3a-beads or control beads. Wnt3a-beads activated Islet1 expression and repressed Lim1/2 expression in cells located in regions surrounding the Wnt3a-beads. In contrast, no Islet1+ cells were observed in explants in contact with control beads. Edges of beads and distal ends of explants are indicated by solid and broken white lines, respectively. (E) Distribution of Islet1+ or Lim1/2+ cells in relation to the distance from the beads. The cell populations expressing Islet1 or Lim1/2 and located in specific areas (indicated by the distance from the beads) are shown as the fraction of cells among those expressing the marker proteins.
To examine how the effect of exogenous Wnt3a is spatially organized, an explant of medial neural plate was cultured in contact with a bead soaked with Wnt3a protein, and expression of Islet1 and Lim1/2 was examined by immunohistology. Expression of Islet1 was induced in cells surrounding the bead with the most frequent occurrence in the region 25–50 μm away from the edge of the neural plate tissue in contact with the Wnt3a beads, whereas the number of Lim1/2-positive cells decreased in areas within 50 μm of the Wnt3a beads (Fig. 4D,E). These results indicate that Wnt3a proteins can organize a pattern of interneurons in the medial neural plate resembling that formed in the dorsal spinal cord. Taken together, the in vitro experiments with explants of the avian neural plate indicate that Wnt3a protein has the activity to induce D1/D2 neurons without dependence on BMP signaling, and that D1/D2 neurons are generated at the expense of D3 neurons by the action of Wnt3a protein. Thus, Wnt signals appear to be directly involved in the specification of dorsal interneurons. This role defines a newly described function of Wnt proteins in neurogenesis.
The specification of the dorsal subclasses of interneurons appears to involve at least two steps of signaling cascades. BMP proteins secreted from the surface ectoderm induce the roof plate in the neural tube, and the roof plate then secretes signaling molecules required for the generation of D1 and D2 interneurons (Liem et al. 1995, 1997; Lee et al. 2000). As shown in this study, concomitant loss of Wnt1 and Wnt3a activities results in deficiency of D1 and D2 dorsal interneurons. This defect resembles that observed in mouse embryos missing the roof plate (Lee et al. 2000; Millonig et al. 2000), providing evidence for the hypothesis that Wnt proteins are the major component of the signal from the roof plate leading to dorsal interneuron specification.
On the other hand, previous in vitro studies have indicated that members of the BMP family, for example, BMP4, induce the expression of D1 and D2 markers in chick neural plate explants. In addition, in vitro assays using chick neural plate explants have shown that the inductive activity of the roof plate is inhibited by Noggin and Follistatin, antagonists of BMP-related molecules (Liem et al. 1997). Thus, BMP family proteins are considered to be another group of signaling molecules emanating from the roof plate and regulating development of dorsal interneurons. However, by use of mouse studies with targeted mutations in BMP family members expressed by roof plate cells have not yet clarified the role of BMP proteins in the development of dorsal interneurons, except for the case of Gdf7-deficient mice. Although ocular and renal development is disrupted in the Bmp7-deficient mouse (Dudley et al. 1995; Luo et al. 1995), and ossification is delayed in the Bmp6 mutant mouse (Solloway et al. 1998), no neuronal defects in the spinal cord have been reported. The Bmp4-deficient mouse dies around the gastrulation stage, and therefore, it is uncertain whether this gene is required for spinal cord development (Winnier et al. 1995). The only evidence for involvement of BMP proteins in the generation of dorsal interneurons is the case of Gdf7-deficient embryos lacking the most dorsal subclass of the interneurons, D1A (Lee et al. 1998).
Evidence indicates that Wnt signals directly control specification of dorsal interneurons without modulating BMP signaling. However, it is still possible that Wnt and BMP signals from the roof plate act in coordination on specification of dorsal interneurons. Although the cells expressing Math1 and Ngn1, representing progenitors of dorsal spinal cord neural cells, were severely decreased in Wnt1−/−;Wnt3a−/− embryos, small cell populations located in the most dorsal regions of the neural tube still expressed these markers of dorsal neural tube (Fig. 2B,D). This is in contrast with embryos lacking the roof plate, where expression of Math1 and Ngn1 is completely missing (Lee et al. 2000). Thus, some signals remaining in the roof plate of Wnt1−/−;Wnt3a−/− embryos, including BMP-related proteins, seem to have a subsidiary role in the development of the dorsal subclasses of interneurons. In fact, expression of Gdf7 appeared normal in the Wnt1−/−;Wnt3a−/− embryo (Fig. 3H) and Wnt1 is expressed normally in the Gdf7 mutant embryo, whereas loss of either GDF7 or of Wnt1/3a affects D1A neurons. This observation may suggest that Wnt1/3a and GDF7 signals in normal circumstances act coordinately to specify dorsal interneurons.
Another possible mechanism is that BMP signals are involved in regulation of the dorsal interneurons by inducing expression of Wnt1/Wnt3a in the roof plate. It has been shown that a constitutively active form of BMP receptor I induces ectopic expression of Wnt1 in the neural tube (Panchision et al. 2001). Thus, BMP proteins secreted from the surface ectoderm and/or from the roof plate probably induce Wnt1/Wnt3a expression in the roof plate. Then, the induced Wnt proteins act to generate D1 and D2 interneurons. Induction of the D1/D2 subtypes of the dorsal interneurons by exogenous BMPs in previous reports may partly be explained by this activity of inducing Wnt protein expression.
Previous studies have shown that Wnt1 and Wnt3a have activities that promote cell proliferation in the neural tube (Dickinson et al. 1994; Ikeya et al. 1997). A slight decrease in the size of the dorsal spinal cord observed in Wnt1−/−;Wnt3a−/− embryos (e.g., Fig. 1D) may be accounted for by the loss of Wnt proteins as proliferative agents. However, this work has clearly shown another important activity of the Wnt proteins, namely, specification of neuron types. The dual functions of Wnt signals thus underscore the importance of Wnt proteins in neurogenesis.
Materials and methods
Wnt1−/−;Wnt3a−/− doubly homozygous mutant embryos
Compound heterozygotes of Wnt1+/− and Wnt3a+/− were produced by crosses between heterozygous mice carrying a null allele of Wnt1 or Wnt3a, and maintained by backcrossing to C57/Bl6 (McMahon and Bradley 1990; Takada et al. 1994). Doubly homozygous mutants were identified among embryos derived from matings between compound heterozygotes.
In situ hybridization of embryos
In situ hybridization on frozen sections (16 μm thickness) was carried out as described previously (Tomita et al. 2000) by use of digoxigenin-labeled probes.
Immunohistochemistry
Immunohistochemistry for neural plate explants and frozen sections was performed as described (Yamada et al. 1993; Tomita et al. 2000). Islet1 was detected with mouse monoclonal antibody 39.4D5 (from Developmental Studies Hybridoma Bank) and Lim1/2 with monoclonal antibody 4F2 (from Developmental Studies Hybridoma Bank).
Neural plate explants
The medial one third of the neural plate between dorsal and ventral thirds was isolated in the form of a strip from stage 10 chick embryos (Hamburger and Hamilton 1951) as described (Yamada et al. 1993; Liu and Jessell, 1998). A group of four isolated strips were explanted in collagen matrix (Vitrogen) with F12 medium containing penicillin/streptomycin and Mito+ Serum Extender (Collaborative Biomedical Products).
Wnt3a protein and conditioned media
Mouse Wnt3a cDNA was transfected into mouse L-cells and expressed under the PGK promoter. Wnt3a-conditioned medium was prepared as described previously (Shibamoto et al. 1998). Transfected cells were cultured in DMEM/F12 with 0.5% fetal bovine serum for 3 d and in serum-free DMEM/F12 for a further 24 h. Wnt3a-containing conditioned medium was then harvested. The concentration of Wnt3a protein in the conditioned medium was estimated as 400 ng/mL. Heparin acrylic beads (H5263, Sigma) of 0.1 mm in diameter, were soaked in Wnt3a-conditioned medium or control medium for 2 h at 4°C. Then, Wnt3a-soaked beads were placed into the medial region of a stage 10 chick neural plate in a collagen matrix. Binding of Wnt3a proteins to heparin beads was confirmed by Western blotting with an anti-Wnt3a monoclonal antibody. Noggin proteins were purchased from R&D systems.
RT–PCR
A group of four explants was collected in 400 μL Trizol reagent (GIBCO BRL) and RNA was prepared according to the manufacturer's protocol. Total RNA was reverse-transcribed (GIBCO BRL) by use of random hexamer primers (Takara) in a reaction volume of 20 μL. The reverse transcripts were amplified by PCR for 35 cycles, each consisting of reactions at 94°C for 30 sec, at 60°C–65°C for 30 sec, and 72°C for 2 min. PCR products were analyzed on a 1.5% agarose gel.
Primer pairs used to amplify chick LH2A, Islet1, Pax2, and Msx1 sequences were as follows: LH2A 5′, 5′-GCATTTCAATCACTCGGATG TAGCTGC-3′; LH2A 3′, 5′-GCTTGGAGTGTTGAATCTGAAGTC-3′; Islet1 5′, 5′-GAGCAACTGGTAGAAATGACTGGCCTCAGT-3′; Islet1 3′, 5′-TCGATCCTCCTCAAGATCATTGAGTAGC-3′; Pax2 5′, 5′-GA CAAAGGATAGTGGAGCTGGC-3′; Pax2 3′, 5′-ACGCTCAAAAA CTCGATCTAAAGC-3′; Msx1 5′, 5′-GAGATATTCGCCTCCTCCAA GACAC-3′; Msx1 3′, 5′-ACACCCAGCCTGTTGCATACAGGATG-3′. Sequences for S17 primers were adopted from a previous publication (Trueb et al. 1988).
Acknowledgments
We thank R. Kageyama, Se-Jin Lee, B.L.M. Hogan, A.P. McMahon, Y. Takahashi, M. Takeichi, and P. Gruss for gifts of probes. We also thank the Developmental Studies Hybridoma Bank for monoclonal antibodies. We are grateful to Y. Tanabe and J.P. Liu for expert help in explant cultures; Y. Yamaguchi, H. Hijikata, and R. Takada for preparing Wnt3a-expressing L cells; and T. Ohtsuka, H. Hirata, K. Tomita, and R. Ohsawa for technical advice. We thank members of the Takada laboratory, the Kondoh laboratory, and the Takeichi laboratory for helpful discussions, especially from S. Koshida and Y. Kishimoto. This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Culture, and Sports of Japan and grants from the Japan Science and Technology Corporation, Japan Society for the Promotion of Science, Kato Memorial Science Foundation, and Takeda Science Foundation to S.T.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL stakada@take.biophys.kyoto-u.ac.jp; FAX 81-75-753-4265.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.937102.
References
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Dickinson ME, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes & Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of chick embryos. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: A requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes & Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liu JP, Jessell TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development. 1998;125:5055–5067. doi: 10.1242/dev.125.24.5055. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes & Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1−/Wnt-1− mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes & Dev. 2001;15:2094–2110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Roelink H, Nusse R. Expression of two members of the Wnt family during mouse development—Restricted temporal and spatial patterns in the developing neural tube. Genes & Dev. 1991;5:381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes & Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueb B, Schreier T, Winterhalter KH, Strehler EE. Sequence of a cDNA clone encoding chicken ribosomal protein S17. Nucleic Acids Res. 1988;16:4723. doi: 10.1093/nar/16.10.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Bailes JA, McMahon AP. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987;50:79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes & Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: Motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]