Abstract
New Zealand mice spontaneously develop antibodies to double-stranded RNA and DNA. The appearance of these antibodies is accelerated by the administration of the synthetic double-stranded RNA, polyinosinic [unk] polycytidylic acid (poly I [unk] poly C). Since poly I [unk] poly C is known to act both as a nucleic acid antigen and as an adjuvant, the mechanism of nucleic acid antibody stimulation was studied to determine which property of poly I [unk] poly C was operative.
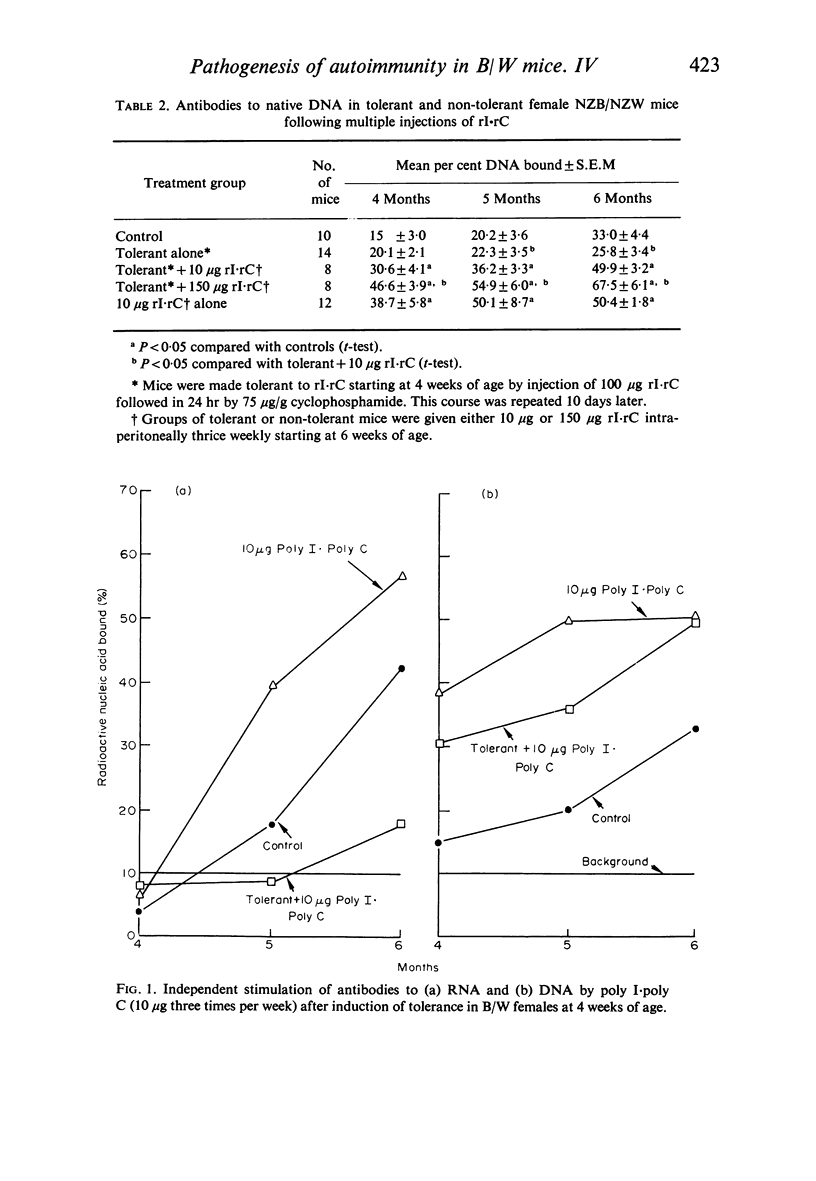
NZB/NZW mice were made tolerant to poly I [unk] poly C as an antigen with cyclophosphamide. They were then given 10 μg or 150 μg of poly I [unk] poly C three times per week. Tolerant mice given 10 μg of poly I [unk] poly C had acceleration of antibodies to DNA but not RNA. In this case the stimulation of antibodies to DNA appeared to represent the adjuvant property of poly I [unk] poly C. Since non-tolerant but not tolerant mice had stimulation of antibodies to RNA with 10 μg poly I [unk] poly C, this anti-RNA acceleration appears to represent antigenic stimulation. All animals given 150 μg poly I [unk] poly C had stimulation of antibodies to both RNA and DNA, suggesting a predominently adjuvant mechanism.
The possible role of antigenic and adjuvant properties of nucleic acids in the natural disease of New Zealand mice is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron S., Buckler C. E., Levy H. B., Friedman R. M. Some factors affecting the interferon-induced antiviral state. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1320–1326. doi: 10.3181/00379727-125-32347. [DOI] [PubMed] [Google Scholar]
- Carpenter D. F., Steinberg A. D., Schur P. H., Talal N. The pathogenesis of autoimmunity in New Zealand mice. II. Acceleration of glomerulonephritis by polyinosinic-polycytidylic acid. Lab Invest. 1970 Dec;23(6):628–634. [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Voller A. Suppression of autoimmune disease in New Zealand mice associated with infection with malaria. I. (NZBxNZW) F1 hybrid mice. Clin Exp Immunol. 1970 Dec;7(6):793–803. [PMC free article] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963 Jan 12;197:197–197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- Jacobs M. E., Gordon J. K., Talal N. Inability of the NZB-NZW F 1 thymus to transfer cyclophosphamide-induced tolerance to sheep erythrocytes. J Immunol. 1971 Aug;107(2):359–364. [PubMed] [Google Scholar]
- Jacobs M. E., Steinberg A. D., Gordon J. K., Talal N. Adjuvant effects on poly I poly C in New Zealand mice. Arthritis Rheum. 1972 Mar-Apr;15(2):201–207. doi: 10.1002/art.1780150211. [DOI] [PubMed] [Google Scholar]
- Many A., Schwartz R. S. On the mechanism of immunological tolerance in cyclophosphamide-treated mice. Clin Exp Immunol. 1970 Jan;6(1):87–99. [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples P. J., Talal N. Relative inability to induce tolerance in adult NZB and NZB-NZW F1 mice. J Exp Med. 1969 Jan 1;129(1):123–139. doi: 10.1084/jem.129.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A. D., Baron S., Talal N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1102–1107. doi: 10.1073/pnas.63.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A. D., Chused T. M., Jacobs M. E., Talal N. Cyclophosphamide tolerance as a test for antigenicity of nucleic acids. Nature. 1970 Dec 12;228(5276):1090–1090. doi: 10.1038/2281090a0. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Daley G. G., Talal N. Tolerance to polyinosinic-polycytidylic acid in NZB-NZW mice. Science. 1970 Feb 6;167(3919):870–871. doi: 10.1126/science.167.3919.870. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Pincus T., Talal N. DNA-binding assay for detection of anti-DNA antibodies in NZB-NZW F1 mice. J Immunol. 1969 Mar;102(3):788–790. [PubMed] [Google Scholar]
- Steinberg A. D., Pincus T., Talal N. The pathogenesis of autoimmunity in New Zealand mice. 3. Factors influencing the formation of anti-nucleic acid antibodies. Immunology. 1971 Apr;20(4):523–531. [PMC free article] [PubMed] [Google Scholar]
- Talal N., Steinberg A. D., Gazdar A. F. Specific immunosuppression, polyinosinic--polycytidylic acid, and viruses in New Zealand mice. Fed Proc. 1971 Nov-Dec;30(6):1842–1845. [PubMed] [Google Scholar]
- Turner W., Chan S. P., Chirigos M. A. Stimulation of humoral and cellular antibody formation in mice by poly Ir:Cr. Proc Soc Exp Biol Med. 1970 Jan;133(1):334–338. doi: 10.3181/00379727-133-34469. [DOI] [PubMed] [Google Scholar]


