Abstract
Vibrio cholerae neuraminidase (VCN) renders mouse lymphoid cells highly susceptible to the cytolytic effects of alloantibody and complement (C). This increased susceptibility to lysis is not due to unmasking of alloantigens since no increase in alloantibody-binding capacity of VCN-treated cells could be detected. However, VCN-treated cells can be lysed by normal rabbit serum, human serum, and guinea-pig serum even if specific antibody is not added to the incubation mixture. VCN, therefore, while not unmasking strong H-2 histocompatibility antigens, may be capable of unmasking other antigens with which heterologous sera can react.
The increased susceptibility to immune cytolysis of VCN-treated cells appears to be related, at least partially, to its extreme susceptibility to C. Complement inactivation by heat totally abrogated the lytic effect, as did C inactivation by ammonium hydroxide, viscarin, and zymosan. In addition, activation of the autologous serum C within the fluid phase by cobra venom factor produced cytolysis only in VCN-treated cells. Thus, VCN renders nucleated cells highly susceptible to lysis by C.
Full text
PDF





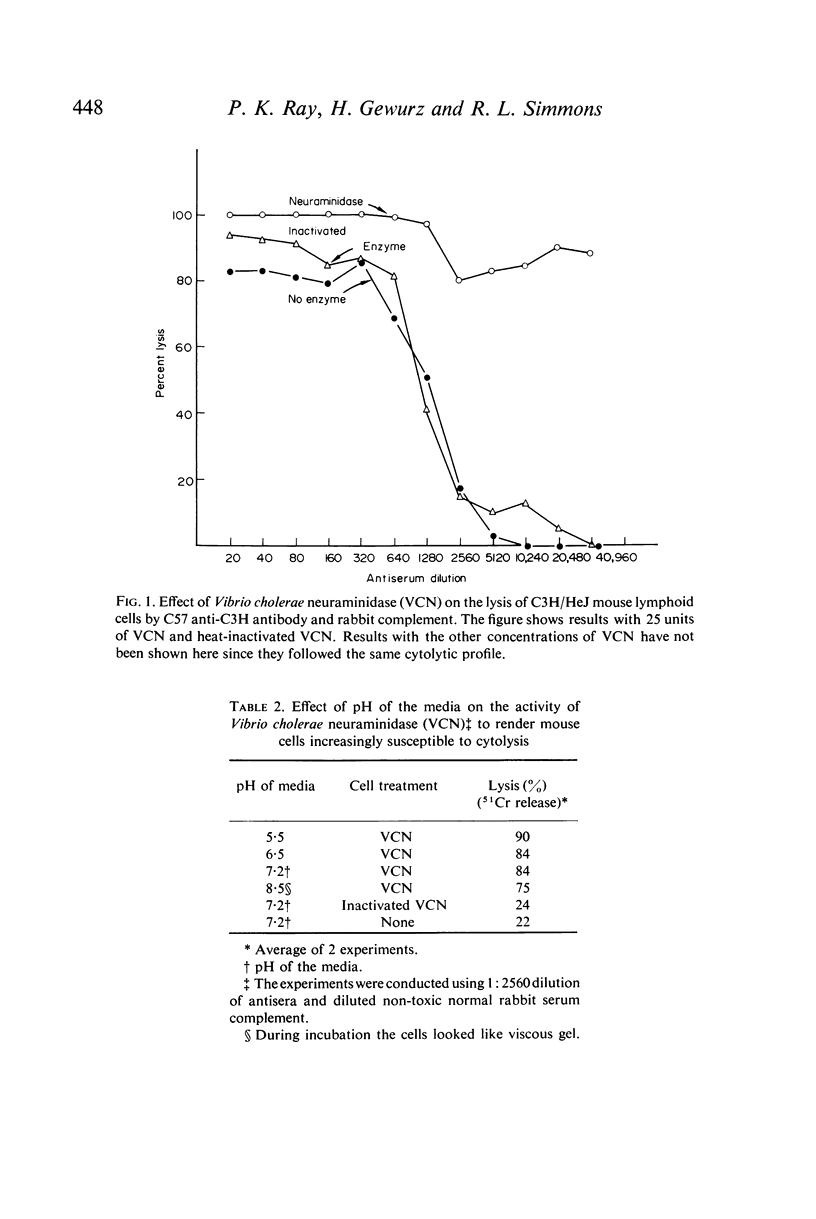
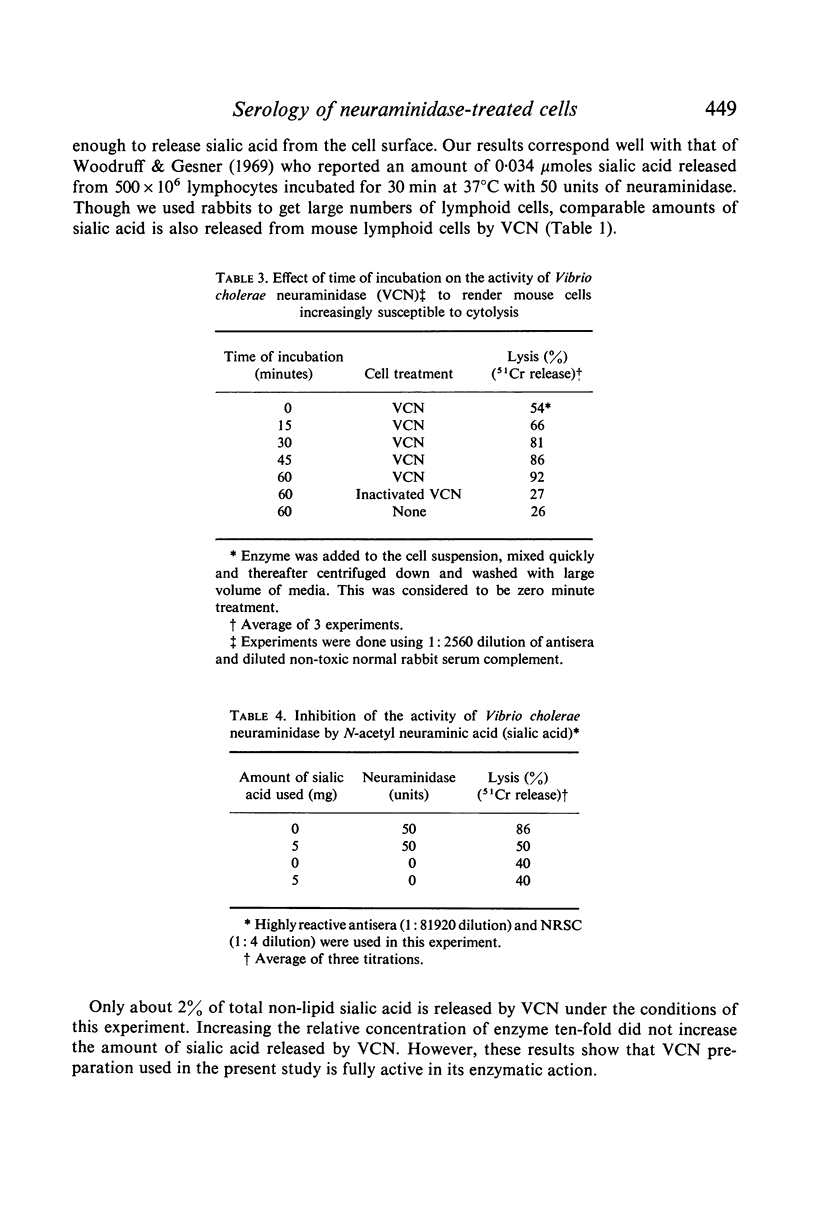
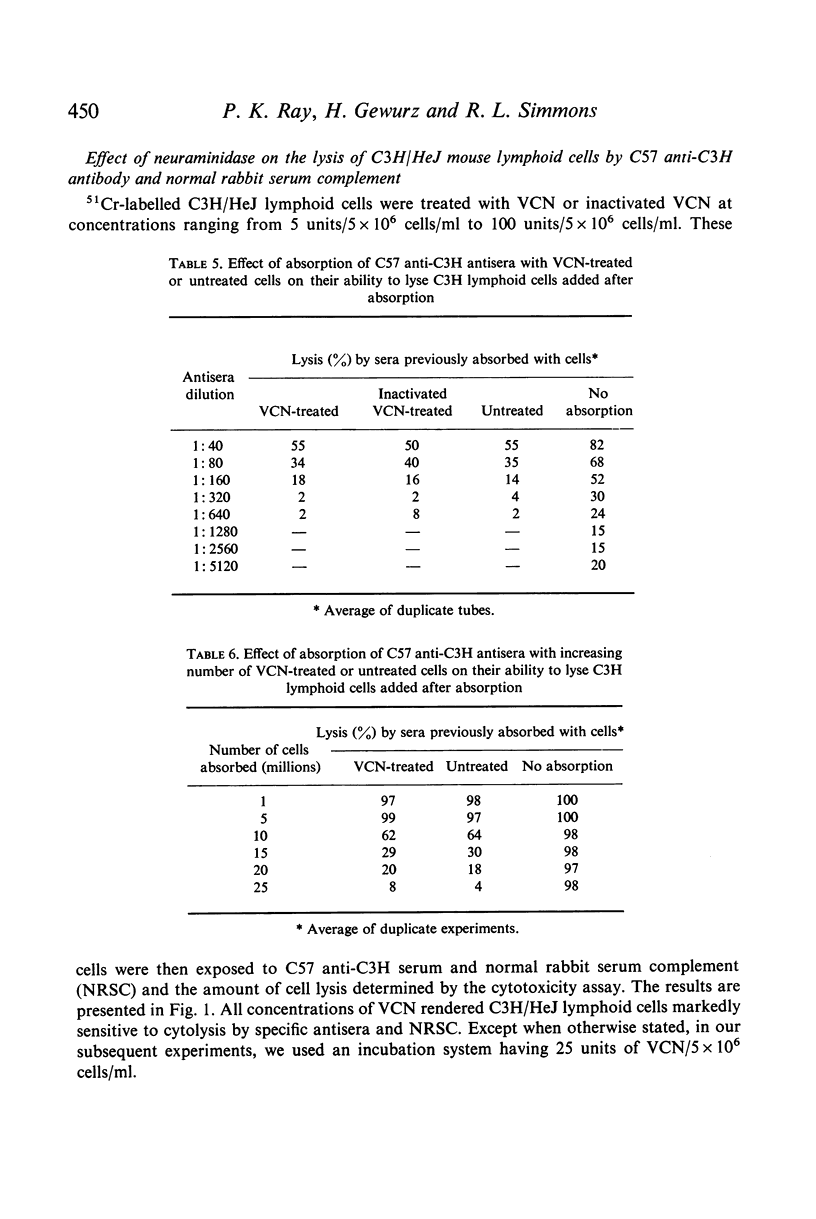
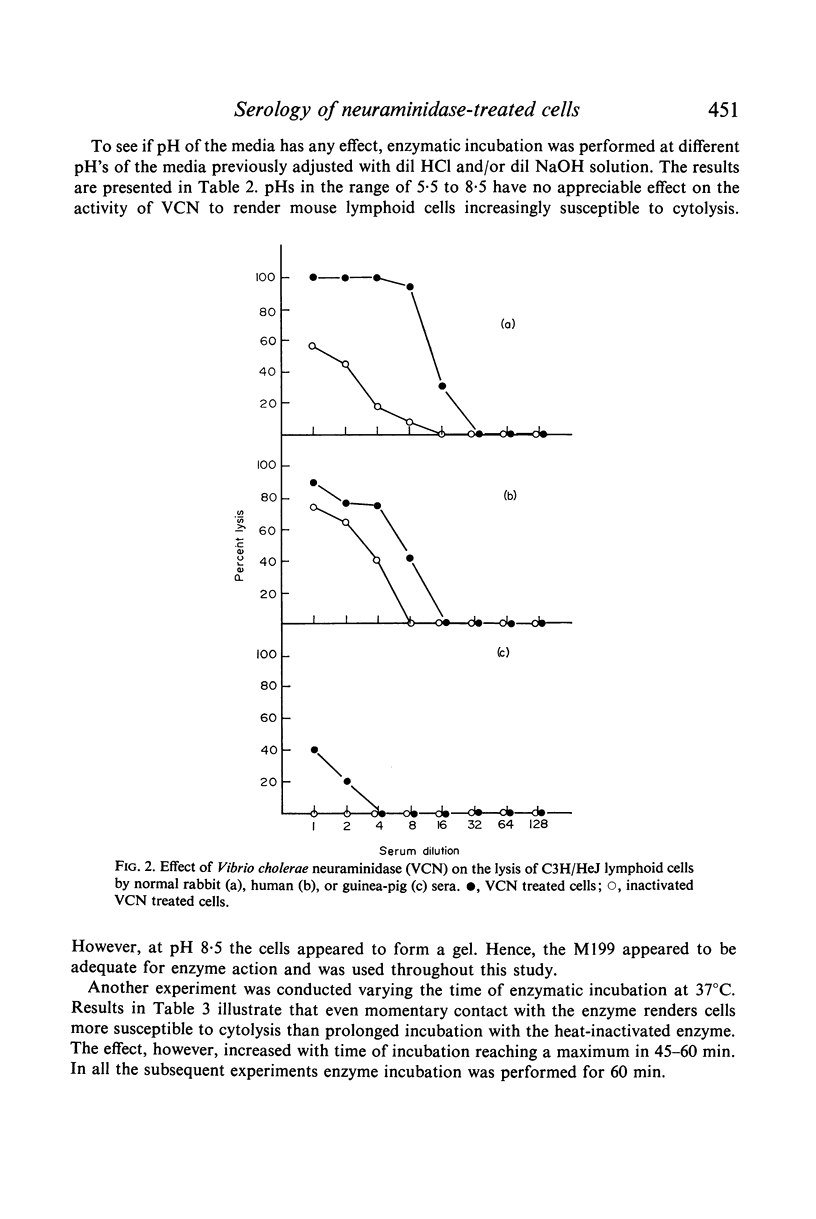
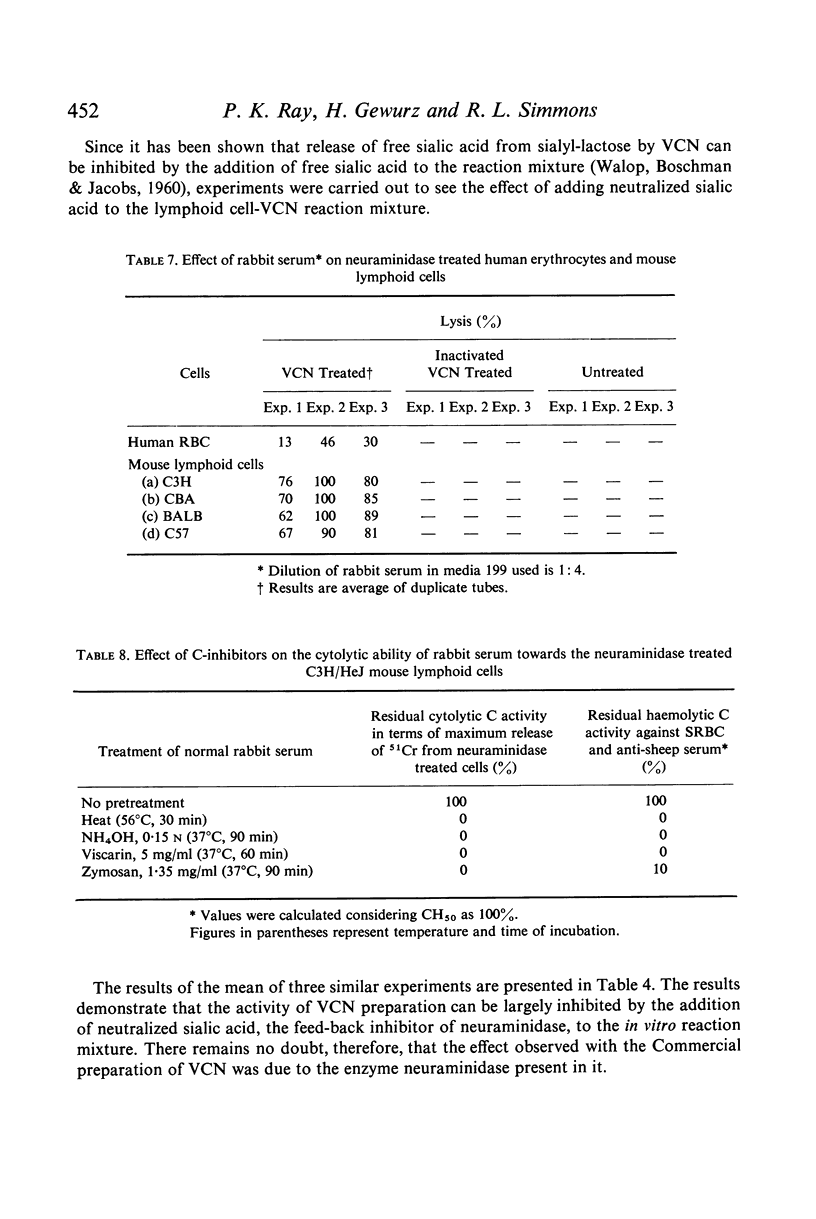
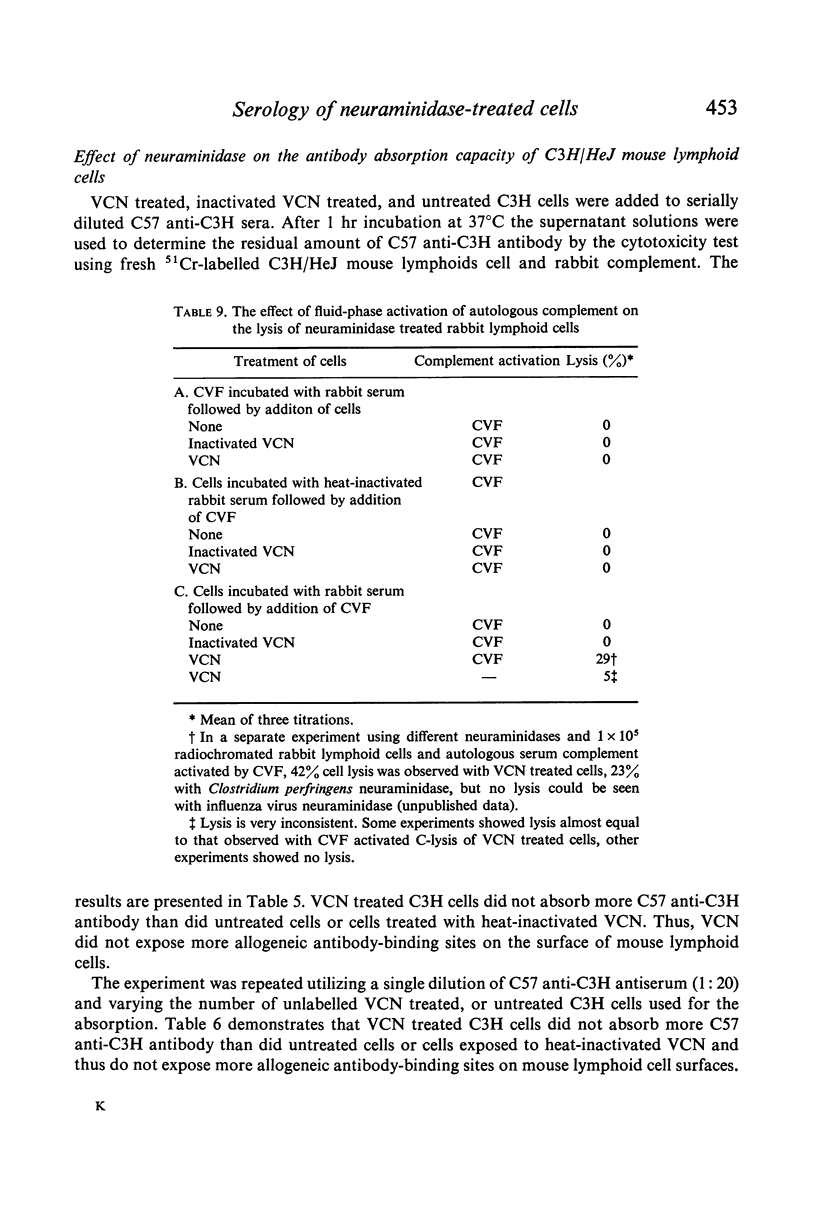







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
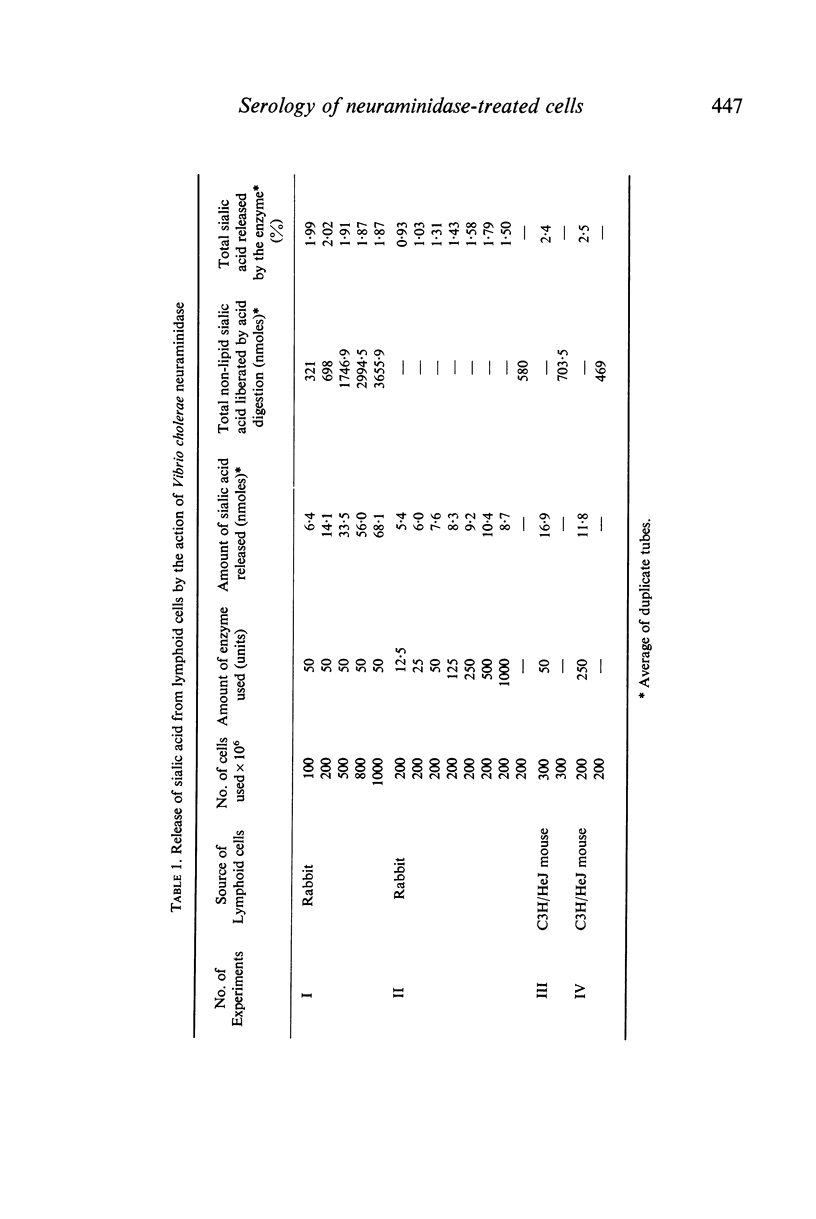
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose E. J. Electrophoretic behaviour of cells. Prog Biophys Mol Biol. 1966;16:241–265. doi: 10.1016/0079-6107(66)90008-3. [DOI] [PubMed] [Google Scholar]
- Apffel C. A., Peters J. H. Regulation of antigenic expression. J Theor Biol. 1970 Jan;26(1):47–59. doi: 10.1016/s0022-5193(70)80031-5. [DOI] [PubMed] [Google Scholar]
- Bagshawe K. D., Currie G. A. Immunogenicity of L 1210 murine leukaemia cells after treatment with neuraminidase. Nature. 1968 Jun 29;218(5148):1254–1255. doi: 10.1038/2181254a0. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Emmelot P. Studies on plasma membranes. IV. The ultrastructural localization and content of sialic acid in plasma membranes isolated from rat liver and hepatoma. J Cell Sci. 1967 Dec;2(4):499–512. doi: 10.1242/jcs.2.4.499. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Michael A. F. Effect of neuraminidase on the accumulation of alpha-aminoisobutyric acid in HeLa cells. Proc Soc Exp Biol Med. 1969 Jun;131(2):568–570. doi: 10.3181/00379727-131-33927. [DOI] [PubMed] [Google Scholar]
- Codington J. F., Sanford B. H., Jeanloz R. W. Glycoprotein coat of the TA 3 cell. I. Removal of carbohydrate and protein material from viable cells. J Natl Cancer Inst. 1970 Oct;45(4):637–647. [PubMed] [Google Scholar]
- Colley D. G., Waksman B. H. Cytotoxic effect of normal rabbit serum on rat lymphoid cells. Transplantation. 1970 Apr;9(4):395–404. doi: 10.1097/00007890-197004000-00005. [DOI] [PubMed] [Google Scholar]
- Cormack D. Effect of enzymatic removal of cell surface sialic acid on the adherence of Walker 256 tumor cells to mesothelial membrane. Cancer Res. 1970 May;30(5):1459–1466. [PubMed] [Google Scholar]
- Currie G. A., Bagshawe K. D. The effect of neuraminidase on the immunogenicity of the Landschütz ascites tumour: site and mode of action. Br J Cancer. 1968 Sep;22(3):588–594. doi: 10.1038/bjc.1968.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A., Bagshawe K. D. The masking of antigens on trophoblast and cancer cells. Lancet. 1967 Apr 1;1(7492):708–710. doi: 10.1016/s0140-6736(67)92183-6. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Bagshawe K. D. The role of sialic acid in antigenic expression: further studies of the Landschütz ascites tumour. Br J Cancer. 1968 Dec;22(4):843–853. doi: 10.1038/bjc.1968.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A., Bagshawe K. D. Tumour specific immunogenicity of methylcholanthrene-induced sarcoma cells after incubation in neuraminidase. Br J Cancer. 1969 Mar;23(1):141–149. doi: 10.1038/bjc.1969.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A., Van Doorninck W., Bagshawe K. D. Effect of neuraminidase on the immunogenicity of early mouse trophoblast. Nature. 1968 Jul 13;219(5150):191–192. doi: 10.1038/219191a0. [DOI] [PubMed] [Google Scholar]
- DALMASSO A. P., MUELLER-EBERHARD H. J. INTERACTION OF AUTOLOGOUS COMPLEMENT WITH RED CELLS IN THE ABSENCE OF ANTIBODY. Proc Soc Exp Biol Med. 1964 Dec;117:643–650. doi: 10.3181/00379727-117-29658. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- GASIC G., GASIC T. Removal of sialic acid from the cell coat in tumor cells and vascular endothelium, and its effects on metastasis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1172–1177. doi: 10.1073/pnas.48.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK A. Correlation between composition, structure, shape and function of a salivary mucoprotein. Nature. 1960 Jun 18;186:949–951. doi: 10.1038/186949a0. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957 Mar;23(3):645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- Gewurz H. Interaction between endotoxic lipopolysaccharides (LPS) and the complement (C) system: solubilization of C-consuming substances during brief absorbtions at 0 degrees. Proc Soc Exp Biol Med. 1971 Feb;136(2):561–564. doi: 10.3181/00379727-136-35311. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassulke J. T., Stutman O., Yunis E. J. Blood-group isoantigens in leukemic cells: reversibility of isoantigenic changes by neuraminidase. J Natl Cancer Inst. 1971 Jun;46(6):1201–1208. [PubMed] [Google Scholar]
- Kemp R. B. Effect of the removal of cell surface sialic acids on cell aggregation in vitro. Nature. 1968 Jun 29;218(5148):1255–1256. doi: 10.1038/2181255a0. [DOI] [PubMed] [Google Scholar]
- Kinne D. W., Simmons R. L. Passive serologic transfer of allograft immunity in immunologically suppressed mice. I. Immunosuppression by heterologous anti-lymphocyte serum. J Immunol. 1967 Feb;98(2):251–255. [PubMed] [Google Scholar]
- Lee A. Effect of neuraminidase on the phagocytosis of heterologous red cells by mouse peritoneal macrophages. Proc Soc Exp Biol Med. 1968 Jul;128(3):891–894. doi: 10.3181/00379727-128-33150. [DOI] [PubMed] [Google Scholar]
- Lundgren G., Simmons R. L. Effect of neuraminidase on the stimulatory capacity of cells in human mixed lymphocyte cultures. Clin Exp Immunol. 1971 Dec;9(6):915–926. [PMC free article] [PubMed] [Google Scholar]
- Pensky J., Schwick H. G. Human serum inhibitor of C'1 esterase: identity with alpha-2-neuraminoglycoprotein. Science. 1969 Feb 14;163(3868):698–699. doi: 10.1126/science.163.3868.698. [DOI] [PubMed] [Google Scholar]
- Pickering R. J., Wolfson M. R., Good R. A., Gewurz H. Passive hemolysis by serum and cobra venom factor: a new mechanism inducing membrane damage by complement. Proc Natl Acad Sci U S A. 1969 Feb;62(2):521–527. doi: 10.1073/pnas.62.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H. Some properties of an esterase derived from preparations of the first component of complement. J Exp Med. 1957 Aug 1;106(2):327–343. doi: 10.1084/jem.106.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios A., Simmons R. L. Immunosuppressive effects of macrophage lysis. Surg Forum. 1970;21:272–273. [PubMed] [Google Scholar]
- SEAMAN G. V., UHLENBRUCK G. The surface structure of erythrocytes from some animal sources. Arch Biochem Biophys. 1963 Mar;100:493–502. doi: 10.1016/0003-9861(63)90117-6. [DOI] [PubMed] [Google Scholar]
- Sanford B. H. An alteration in tumor histocompatibility induced by neuraminidase. Transplantation. 1967 Sep 5;5(5):1273–1279. doi: 10.1097/00007890-196709000-00005. [DOI] [PubMed] [Google Scholar]
- Sanford B. H., Codington J. F. Further studies on the effect of neuraminidase on tumor cell transplantability. Tissue Antigens. 1971;1(4):153–161. doi: 10.1111/j.1399-0039.1971.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M., Amos B. D. Effect of neuraminidase on serological properties of murine lymphoid cells. Transplant Proc. 1971 Mar;3(1):895–897. [PubMed] [Google Scholar]
- Shin H. S., Gewurz H., Snyderman R. Reaction of a cobra venom factor with guinea pig complement and generation of an activity chemotactic for polymorphonuclear leukocytes. Proc Soc Exp Biol Med. 1969 May;131(1):203–207. doi: 10.3181/00379727-131-33840. [DOI] [PubMed] [Google Scholar]
- Simmons R. L., Rios A. Immunotherapy of cancer: immunospecific rejection of tumors in recipients of neuraminidase-treated tumor cells plus BCG. Science. 1971 Nov 5;174(4009):591–593. doi: 10.1126/science.174.4009.591. [DOI] [PubMed] [Google Scholar]
- Simmons R. L., Rios A., Lundgren G., Ray P. K., McKhann C. F., Haywood G. R. Immunospecific regression of methylcholanthrene fibrosarcoma with the use of neuraminidase. Surgery. 1971 Jul;70(1):38–46. [PubMed] [Google Scholar]
- Simmons R. L., Rios A., Ray P. K. Immunogenicity and antigenicity of lymphoid cells treated with neuraminidase. Nat New Biol. 1971 Jun 9;231(23):179–181. doi: 10.1038/newbio231179a0. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., EYLAR E. H. Sialic acid in the cellular membranes of Ehrlich ascites-carcinoma cells. Biochim Biophys Acta. 1961 Sep 30;52:594–596. doi: 10.1016/0006-3002(61)90424-3. [DOI] [PubMed] [Google Scholar]
- WALOP J. N., BOSCHMAN T. A., JACOBS J. Affinity of N-acetylneuraminic acid for influenza virus neuraminidase. Biochim Biophys Acta. 1960 Oct 21;44:185–186. doi: 10.1016/0006-3002(60)91544-4. [DOI] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Weiss L., Mayhew E., Ulrich K. The effect of neuraminidase on the phagocytic process in human monocytes. Lab Invest. 1966 Aug;15(8):1304–1309. [PubMed] [Google Scholar]
- Woodruff J. J., Gesner B. M. The effect of neuraminidase on the fate of transfused lymphocytes. J Exp Med. 1969 Mar 1;129(3):551–567. doi: 10.1084/jem.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell M. M., Ambrose E. J. Studies of tumour invasion in organ culture. II. Effects of enzyme treatment. Eur J Cancer. 1969 Jun;5(3):265–269. doi: 10.1016/0014-2964(69)90076-0. [DOI] [PubMed] [Google Scholar]


