Abstract
Prior work on the potentiation of phytohaemagglutinin-induced human lymphocyte transformation by autologous red blood cells (RBC) and platelets prompted the present report.
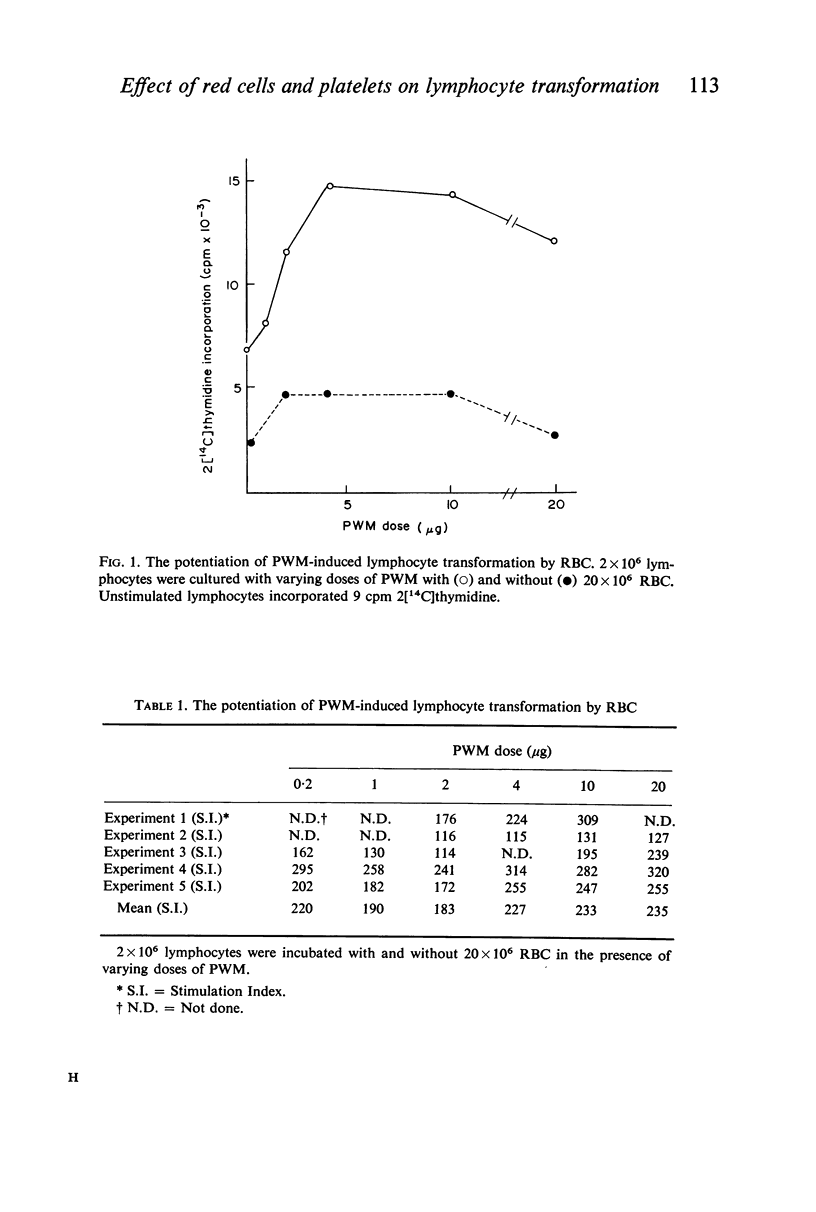
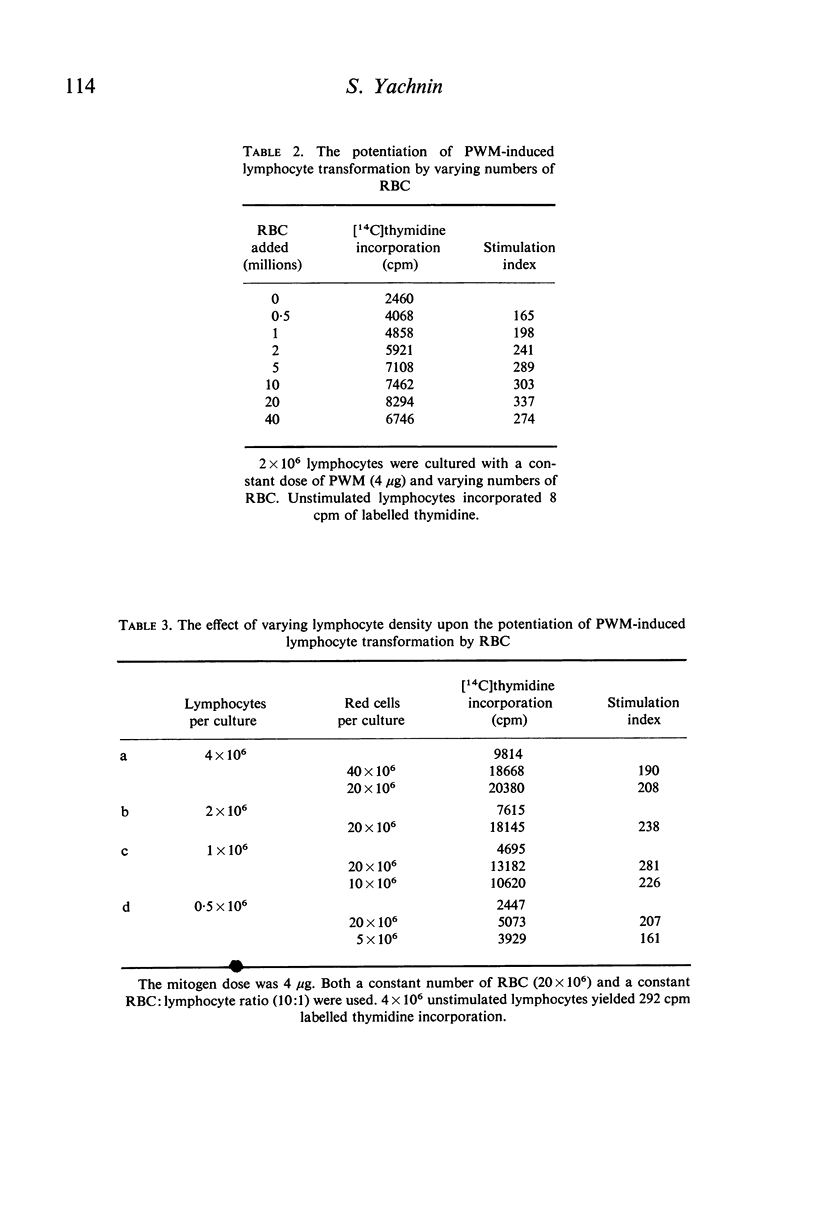
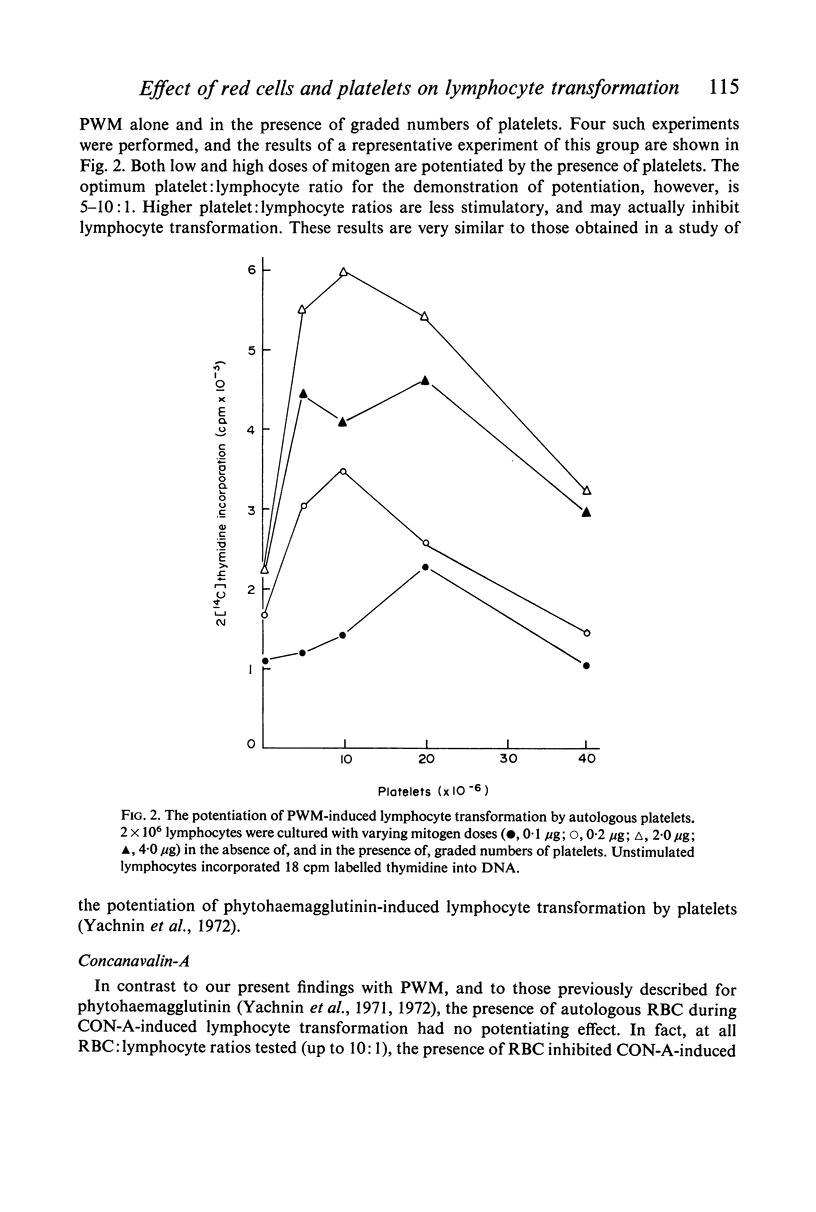
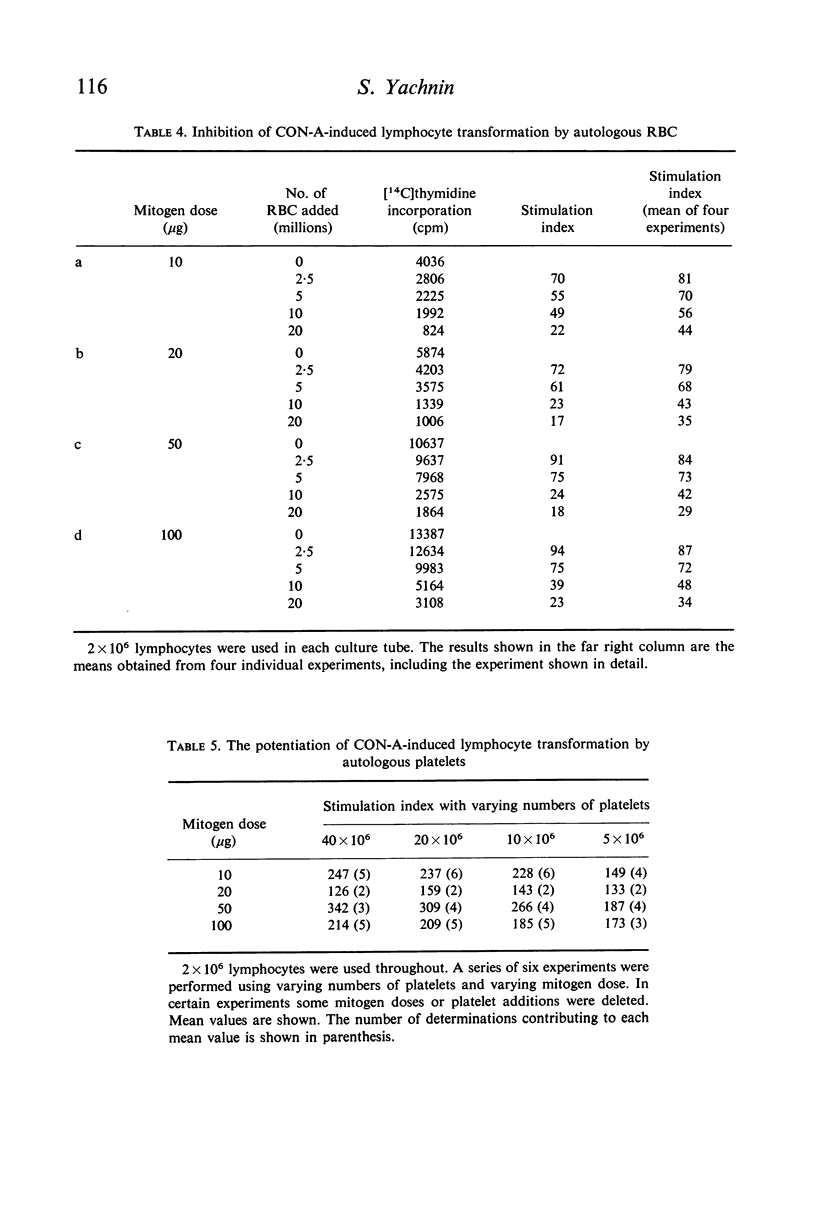
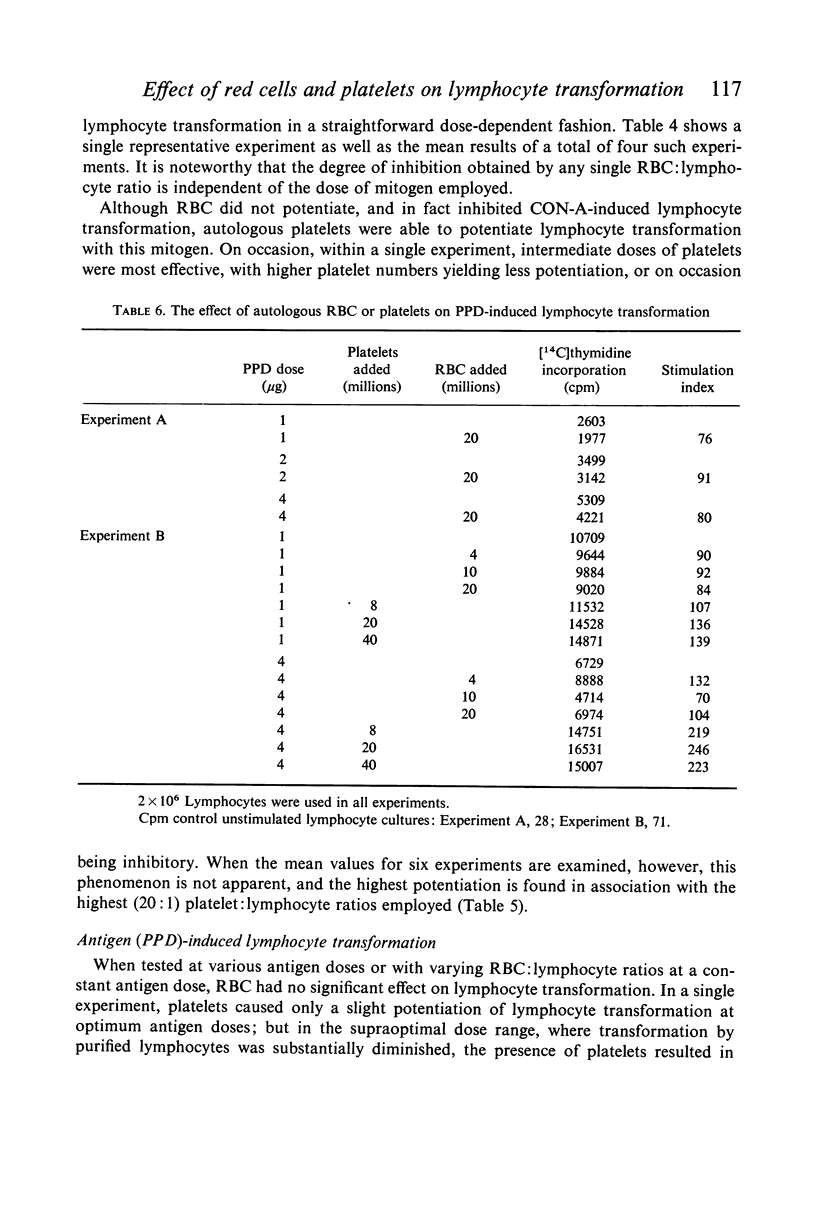
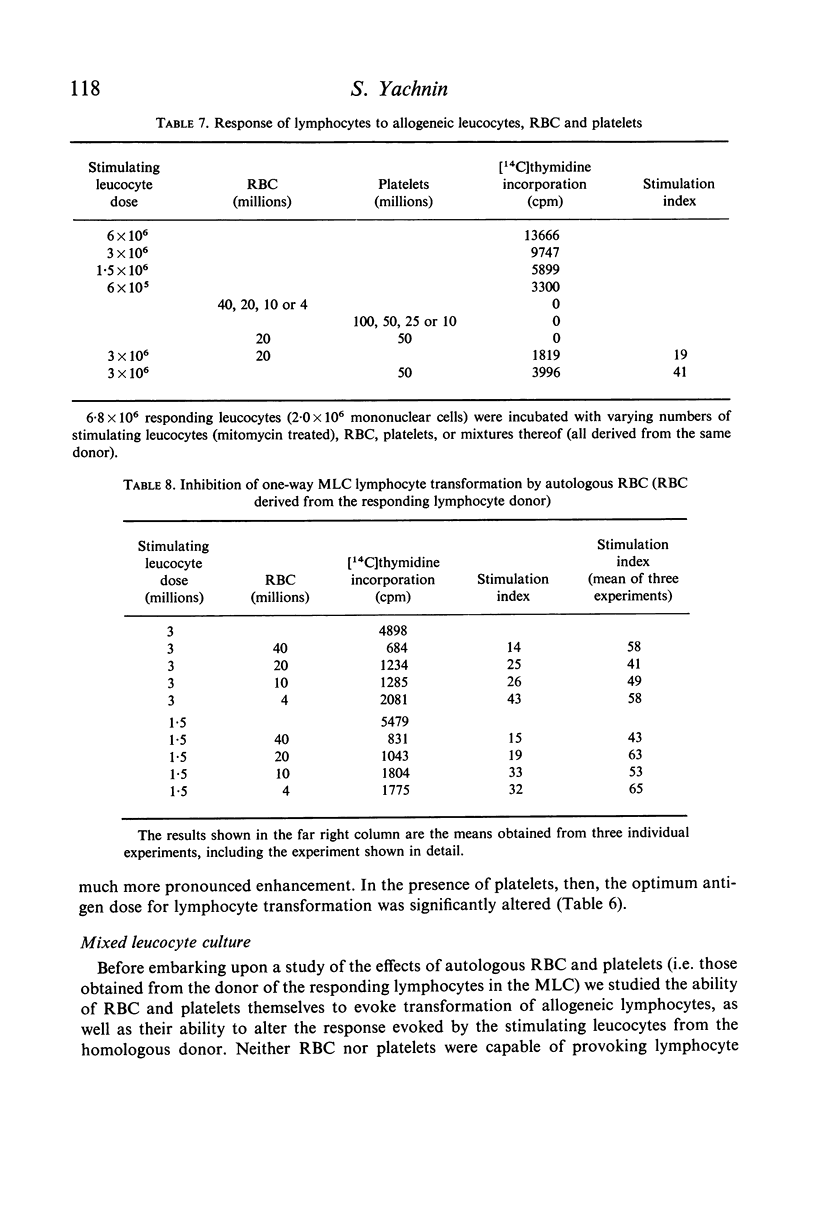
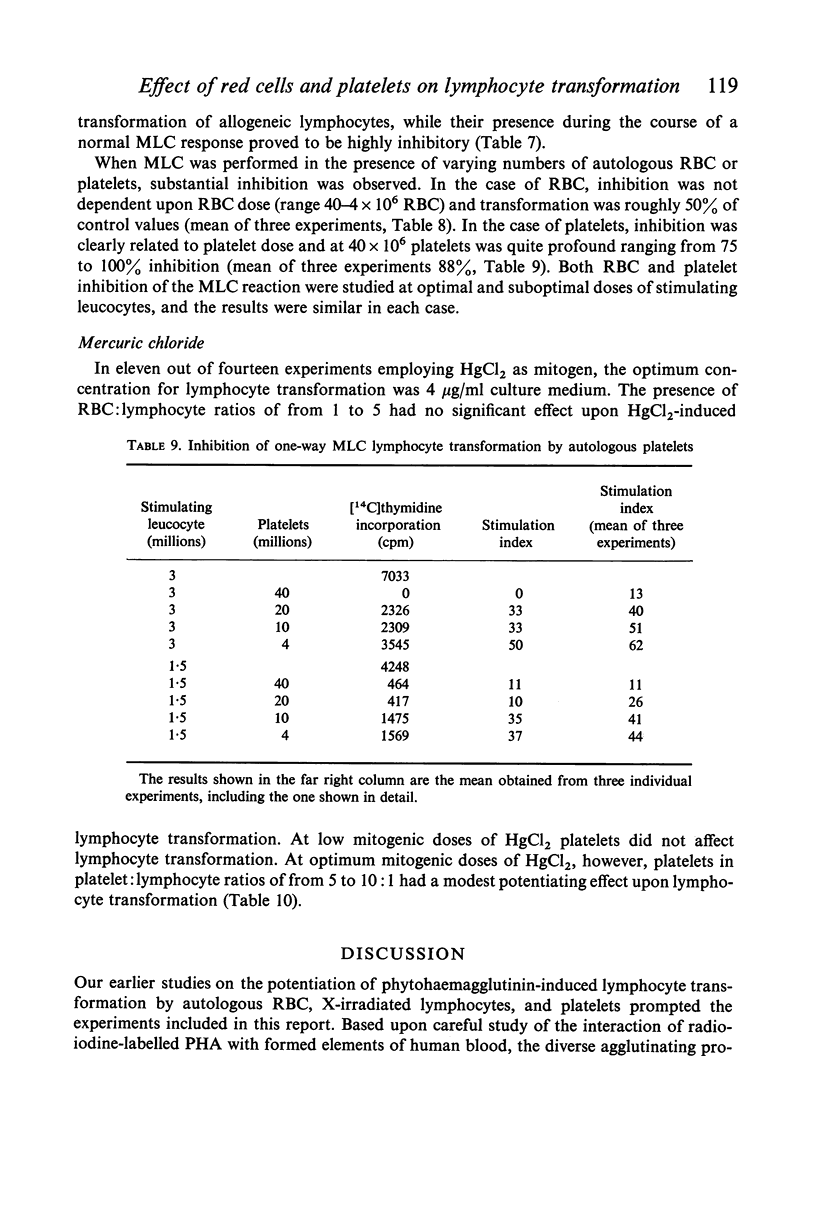
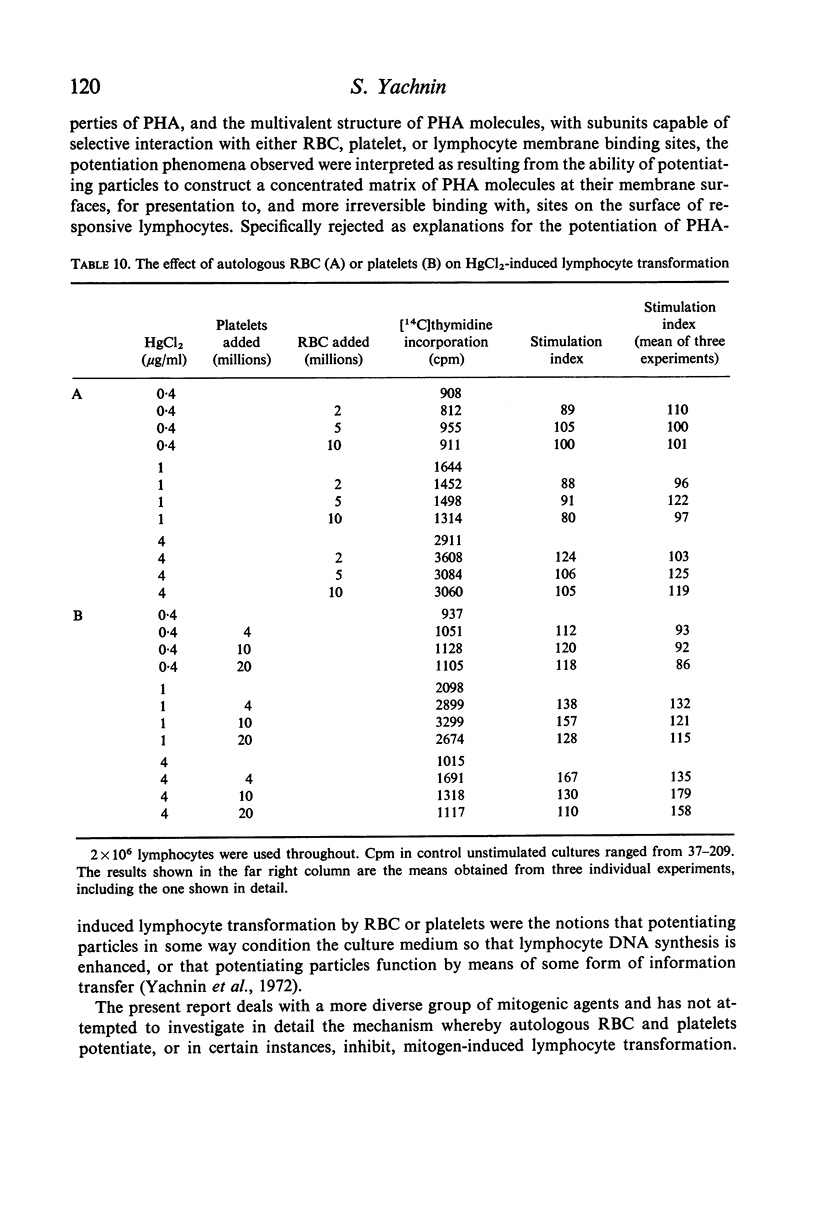
Studies were performed on the effects of autologous RBC and platelets upon lymphocyte transformation induced by a variety of mitogenic stimuli, utilizing human peripheral blood lymphocytes free of RBC and platelets as the starting cell preparation. The mitogenic activity of pokeweed mitogen (PWM) is potentiated to maximum of 300% by RBC: lymphocyte ratios of 0·5–20:1. Potentiation of lymphocyte transformation induced by concanavalin A (CON-A) does not occur, but >50% inhibition is found at RBC: lymphocyte ratios of 5–10:1. The mitogenic activities of both PWM and CON-A are potentiated 200–400% by the presence of platelets in a platelet: lymphocyte ratio of 3–10:1; higher doses of platelets are consistently (PWM) or occasionally (CON-A) less stimulatory and may actually cause inhibition of lymphocyte transformation. In contrast to these phytomitogens, lymphocyte transformation induced by antigen (PPD) in optimum mitogenic doses is not potentiated by either RBC or platelets. RBC do not alter HgCl2-induced lymphocyte transformation, but platelets cause modest potentiation. In mixed leucocyte cultures the presence of either autologous or allogeneic RBC or platelets markedly inhibits transformation (platelets > RBC).
These observations demonstrate that the presence of mitotically inert particles such as autologous RBC or platelets may profoundly affect lymphocyte transformation induced by a variety of mitogenic stimuli. Possible explanations for the mechanism of these effects are discussed. Since many methods of lymphocyte collection and preparation yield populations of lymphocytes significantly contaminated with RBC and/or platelets, comparative studies of human lymphocyte transformation in health and disease which do not remove these cellular contaminants or control their number are subject to the most tentative interpretation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. W., Svenson R. H., Yachnin S. Purification of mitogenic proteins derived from Phaseolus vulgaris: isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc Natl Acad Sci U S A. 1969 Jun;63(2):334–341. doi: 10.1073/pnas.63.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos D. B., Bach F. H. Phenotypic expressions of the major histocompatibility locus in man (HL-A): leukocyte antigens and mixed leukocyte culture reactivity. J Exp Med. 1968 Oct 1;128(4):623–637. doi: 10.1084/jem.128.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIN B., VAS M. R., LOWENSTEIN L. THE DEVELOPMENT OF LARGE IMMATURE MONONUCLEAR CELLS IN MIXED LEUKOCYTE CULTURES. Blood. 1964 Jan;23:108–116. [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron G. A., Poutala S., Provost T. T. Lymphocyte transformation induced by inorganic and organic mercury. Int Arch Allergy Appl Immunol. 1970;37(1):76–87. doi: 10.1159/000230222. [DOI] [PubMed] [Google Scholar]
- Chessin L. N., Börjeson J., Welsh P. D., Douglas S. D., Cooper H. L. Studies on human peripheral blood lymphocytes in vitro. II. Morphological and biochemical studies on the transformation of lymphocytes by pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):873–884. doi: 10.1084/jem.124.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J., Rapaport F. T. Transplantation antigen activity of human blood platelets. Transplantation. 1966 Mar;4(2):182–193. doi: 10.1097/00007890-196603000-00008. [DOI] [PubMed] [Google Scholar]
- Harris R., Zervas J. D. Reticulocyte HL-A antigens. Nature. 1969 Mar 15;221(5185):1062–1063. doi: 10.1038/2211062a0. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L. The agglutination and sensitization of red cells by metallic cations: interactions between multivalent metals and the red-cell membrane. Br J Haematol. 1957 Jan;3(1):19–38. doi: 10.1111/j.1365-2141.1957.tb05768.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Kourilsky F. M., Silvestre D., Levy J. P., Dausset J., Nicolai M. G., Senik A. Immunoferritin study of the distribution of HL-A antigens on human blood cells. J Immunol. 1971 Feb;106(2):454–466. [PubMed] [Google Scholar]
- Main R. K., Cochrum K. C., Jones M. J., Kountz S. L. Immunological potential of the in vitro mixed skin cell-leukocyte reaction. Nat New Biol. 1971 Jan 20;229(3):89–91. doi: 10.1038/newbio229089a0. [DOI] [PubMed] [Google Scholar]
- Marshall W. H., Valentine F. T., Lawrence H. S. Cellular immunity in vitro. Clonal proliferation of antigen-stimulated lymphocytes. J Exp Med. 1969 Aug 1;130(2):327–343. doi: 10.1084/jem.130.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Immunocyte triggering. Cell Immunol. 1970 Dec;1(6):573–582. doi: 10.1016/0008-8749(70)90023-7. [DOI] [PubMed] [Google Scholar]
- Nisbet N. W., Simonsen M., Zaleski M. The frequency of antigen-sensitive cells in tissue transplantation. A commentary on clonal selection. J Exp Med. 1969 Mar 1;129(3):459–467. doi: 10.1084/jem.129.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Penttinen K., Vaheri A., Myllylä G. Detection and characterization of immune complexes by the platelet aggregation test. I. Complexes formed in vitro. Clin Exp Immunol. 1971 Mar;8(3):389–397. [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Börjeson J., Chessin L. N., Small P. A., Jr Isolation and characterization of a mitogen from pokeweek (Phytolacca americana). Proc Natl Acad Sci U S A. 1967 Nov;58(5):2020–2027. doi: 10.1073/pnas.58.5.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHULMAN N. R., MARDER V. J., HILLER M. C., COLLIER E. M. PLATELET AND LEUKOCYTE ISOANTIGENS AND THEIR ANTIBODIES: SEROLOGIC PHYSIOLOGIC AND CLINICAL STUDIES. Prog Hematol. 1964;4:222–304. [PubMed] [Google Scholar]
- Schöpf E., Schulz K. H., Isensee I. Untersuchungen über den Lymphocytentransformations-test bei Quecksilber-Allergie. Unspezifische Transformation durch Hg-Verbindungen. Arch Klin Exp Dermatol. 1969;234(4):420–433. [PubMed] [Google Scholar]
- Twomey J. J., Sharkey O., Jr, Brown J. A., Laughter A. H., Jordan P. H., Jr Cellular requirements for the mitotic response in allogeneic mixed leukocyte cultures. J Immunol. 1970 Apr;104(4):845–853. [PubMed] [Google Scholar]
- Wilson D. B., Nowell P. C. Quantitative studies on the mixed lymphocyte interaction in rats. IV. Immunologic potentiality of the responding cells. J Exp Med. 1970 Feb;131(2):391–407. doi: 10.1084/jem.131.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]



