Abstract
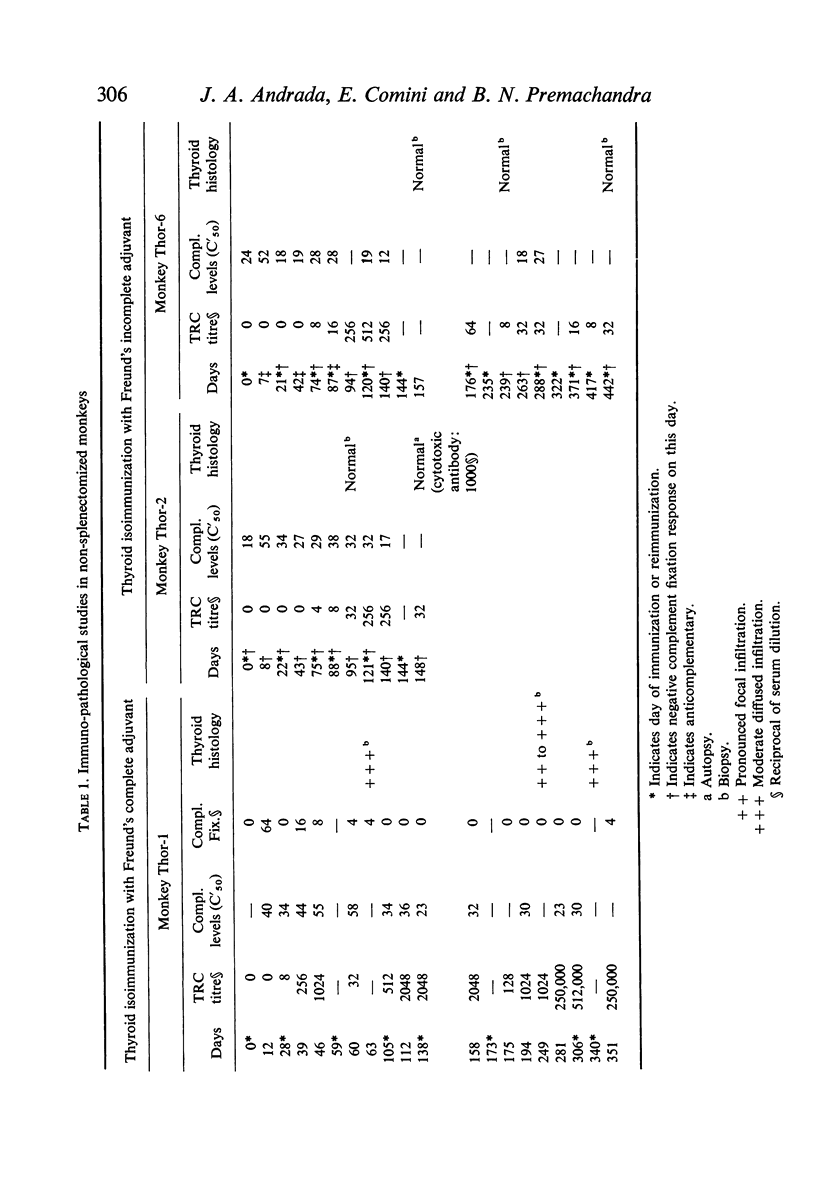
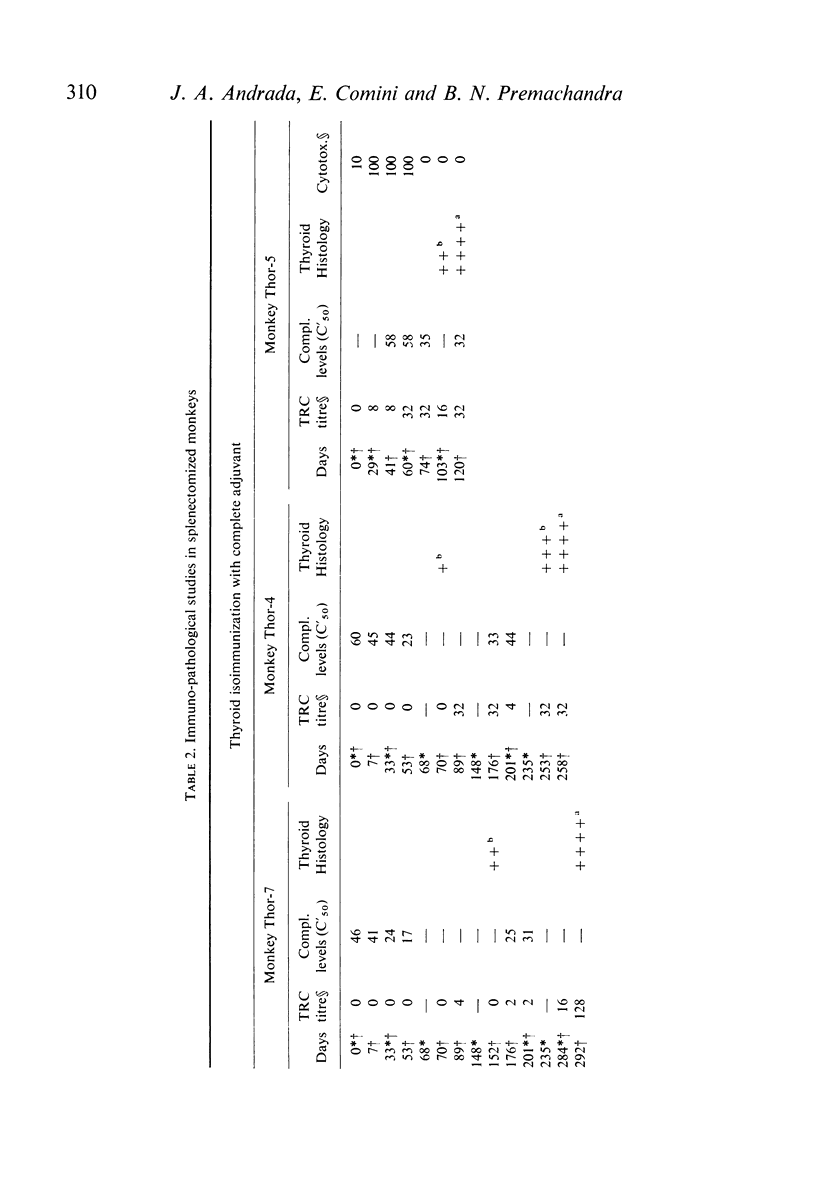
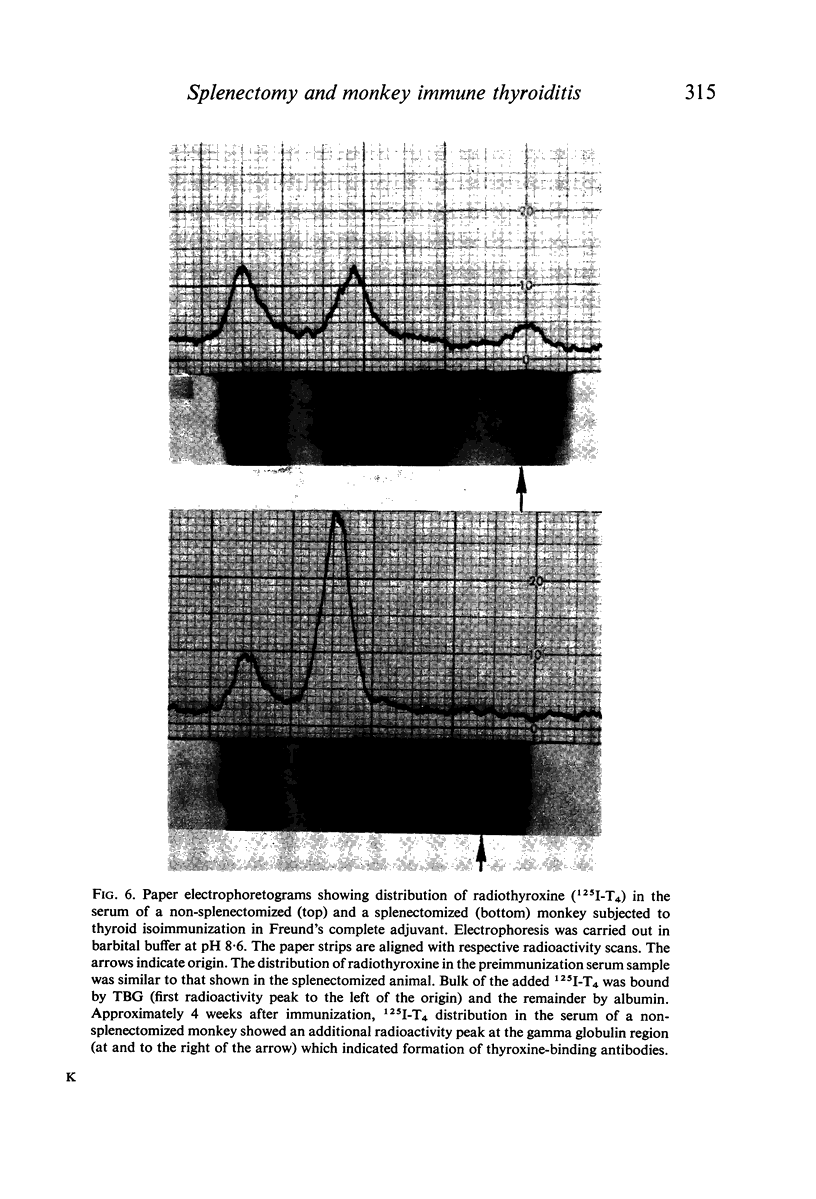
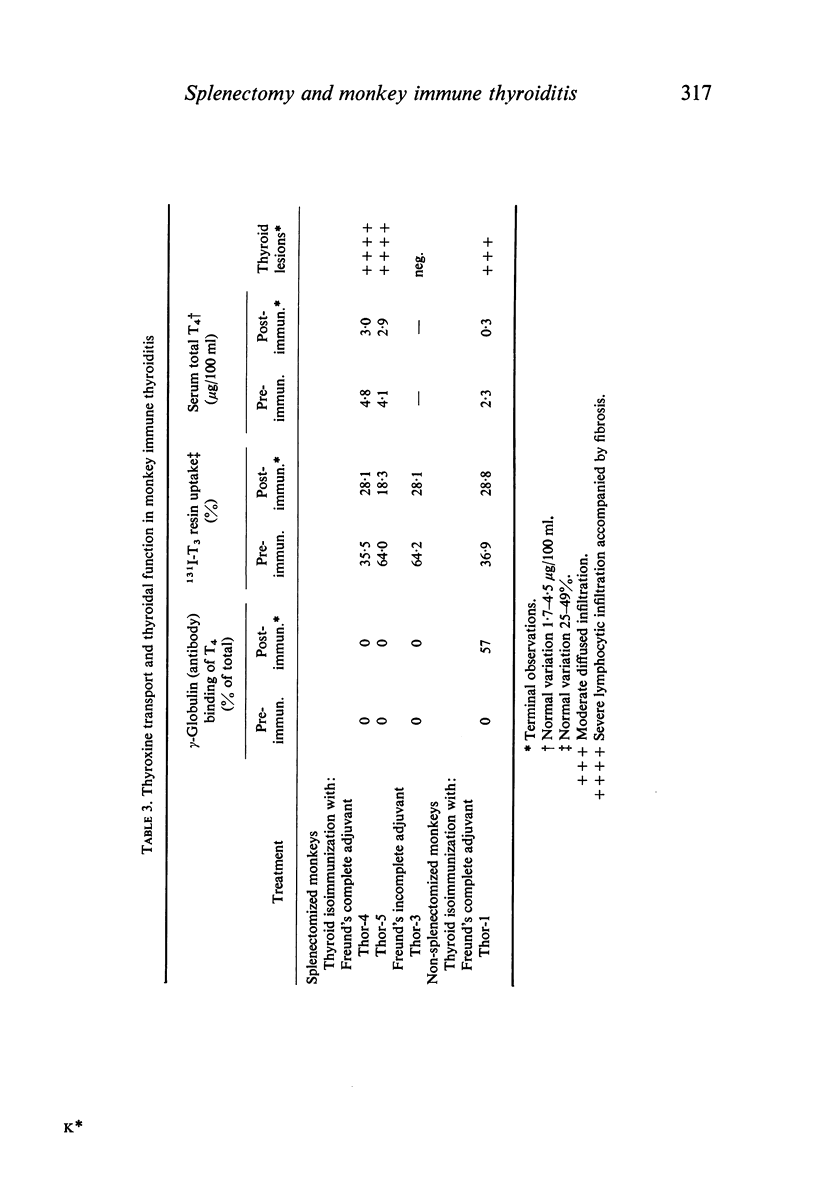
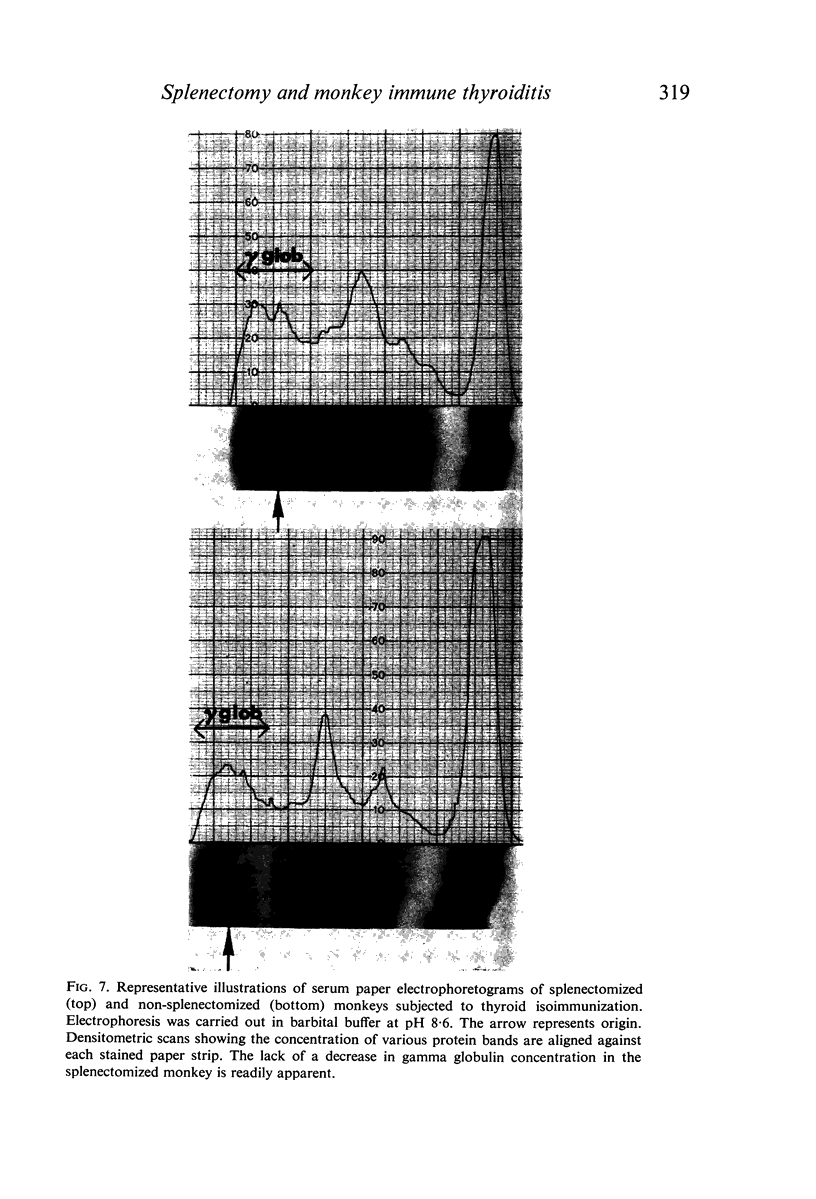
The role of the spleen in humoral antibody formation and in the pathogenesis of immune thyroiditis was studied by splenectomizing four monkeys (Macaca mulatta) prior to thyroid isoimmunization. Splenectomized animals, as well as intact controls, were subjected to sequential immunizations and the course of the immune disease was followed by periodic thyroid biopsies and frequent humoral antibody measurements over a period exceeding 1 yr. Extirpation of the secondary lymphoid organ markedly inhibited agglutinating antibody response, prevented formation of complement-fixing antibodies, but had no effect on thyrocytotoxic antibodies. In animals subjected to immunization in complete adjuvant a trend towards a decrease in serum complement levels was evident at the terminal stages of the experiments. Despite the inhibitory effects on some immunological parameters, splenectomy in monkeys prior to thyroid isoimmunization did not interfere with the initiation and progression of pathological processes in the thyroid. Indeed in all splenectomized animals immunized with thyroid plus complete adjuvant, fibrotic thyroid lesions (4+) with virtual obliteration of thyroid follicles were evident, in some as early as 120 days after primary immunization; in contrast, non-fibrotic and less severe lesions were noted in the intact animal despite being repeatedly subjected to similar immunization procedures over a period of 340 days. Immunization in the absence of complete adjuvant did not induce thyroid lesions in the presence or absence of spleen. In all animals with severe thyroid lesions, thyroid function decreased as revealed by T4 and 131I-T3 resin uptake measurements. Paper electrophoresis of serum specimens from a monkey subjected to thyroid isoimmunization in complete adjuvant (and after equilibration with 125I-T4) showed a pronounced retention of 125I-T4 radioactivity at the gamma globulin region indicating formation of antibodies to thyroxine. On the other hand, in sera with a low thyroid antibody titre as in splenectomized monkeys or in those animals immunized within complete adjuvant, T4-binding antibodies were not evident. It is concluded that removal of the spleen prior to thyroid isoimmunization in monkeys, rather than inhibiting the severity of the disease may even aid and abet immunopathogenic events destructive to the thyroid.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZAR H. A., NAUJOKS G., WILLIAMS J. ROLE OF THE ADULT THYMUS IN IMMUNE REACTIONS. I. OBSERVATIONS ON LYMPHOID ORGANS, CIRCULATING LYMPHOCYTES AND SERUM PROTEIN FRACTIONS OF THYMECTOMIZED OR SPLENECTOMIZED ADULT MICE. Am J Pathol. 1963 Aug;43:213–225. [PMC free article] [PubMed] [Google Scholar]
- Andrada J. A., Rose N. R., Kite J. H., Jr Experimental thyroiditis in the rhesus monkey. IV. The role of thyroglobulin and cellular antigens. Clin Exp Immunol. 1968 Feb;3(2):133–151. [PMC free article] [PubMed] [Google Scholar]
- Beall G. N., Chopra I. J., Solomon D. H. Comparative effects of immunization of rabbits with human thyroglobulin and human and rabbit thyroid microsomal fractions. Clin Exp Immunol. 1971 Apr;8(4):647–656. [PMC free article] [PubMed] [Google Scholar]
- Beall G. N., Solomon D. H. Thyroid-stimulating activity in the serum of rabbits immunized with thyroid microsomes. J Clin Endocrinol Metab. 1968 Apr;28(4):503–510. doi: 10.1210/jcem-28-4-503. [DOI] [PubMed] [Google Scholar]
- Bednarík T., Cajthamlová H. Formation of antibodies to immunoglobulins after splenectomy during intravenous and depot immunization. Physiol Bohemoslov. 1970;19(1):135–138. [PubMed] [Google Scholar]
- Bednarík T., Cajthamlová H. Formation of precipitins to albumin in normal and splenectomized rabbits during intravenous immunization. Physiol Bohemoslov. 1971;20(1):39–42. [PubMed] [Google Scholar]
- Biggar W. D., Bogart D., Holmes B., Good R. A. Impaired phagocytosis of pneumococcus type 3 in splenectomized rats. Proc Soc Exp Biol Med. 1972 Mar;139(3):903–908. doi: 10.3181/00379727-139-36263. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Glynn L. E., Holborow E. J. The dual necessity for delayed hypersensitivity and circulating antibody in the pathogenesis of experimental allergic orchitis in guinea-pigs. Immunology. 1967 Sep;13(3):307–314. [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A., La Via M. F. Effect of splenectomy on primary and secondary response to sheep erythrocytes in rats. Proc Soc Exp Biol Med. 1967 Feb;124(2):571–573. doi: 10.3181/00379727-124-31794. [DOI] [PubMed] [Google Scholar]
- Cole R. K., Kite J. H., Jr, Witebsky E. Hereditary autoimmune thyroiditis in the fowl. Science. 1968 Jun 21;160(3834):1357–1358. doi: 10.1126/science.160.3834.1357. [DOI] [PubMed] [Google Scholar]
- De Carvalho I. F., Borel Y., Miescher P. A. Influence of splenectomy in rats on the formation of 19S and 7S antibodies. Immunology. 1967 May;12(5):505–515. [PMC free article] [PubMed] [Google Scholar]
- FITCH F. W., WINEBRIGHT J. Antibody formation in the rat. II. Agglutinin response to soluble flagellin from salmonella typhosa. J Immunol. 1962 Dec;89:900–905. [PubMed] [Google Scholar]
- FORBES I. J., ROITT I. M., DONIACH D., SOLOMON I. L. The thyroid cytotoxic autoantibody. J Clin Invest. 1962 May;41:996–1006. doi: 10.1172/JCI104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florsheim W. H., Williams A. D., Schönbaum E. On the mechanism of the McKenzie bioassay. Endocrinology. 1970 Nov;87(5):881–888. doi: 10.1210/endo-87-5-881. [DOI] [PubMed] [Google Scholar]
- GOUDIE R. B., McCALLUM H. M. Loss of tissue-specific autoantigens in thyroid tumours. Lancet. 1962 Feb 17;1(7225):348–352. doi: 10.1016/s0140-6736(62)91302-8. [DOI] [PubMed] [Google Scholar]
- HALBERG P. CYTOTOXIC FACTOR AND COMPLEMENT FIXING ANTIBODY IN THYROID DISEASE. Acta Med Scand. 1964 May;175:599–608. doi: 10.1111/j.0954-6820.1964.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Irvine W. J. Studies on the Cytotoxic Factor in Thyroid Disease. Br Med J. 1962 May 26;1(5290):1444–1442.4. doi: 10.1136/bmj.1.5290.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kite J. H., Jr, Argue H., Rose N. R. Experimental thyroiditis in the rhesus monkey. I. Cytotoxic, mixed-agglutinating and complement-fixing antibodies. Clin Exp Immunol. 1966 Apr;1(2):139–157. [PMC free article] [PubMed] [Google Scholar]
- Kite J. H., Jr, Rose N. R., Kano K., Witebsky E. Cytotoxicity of human thyroid autoantibodies. Ann N Y Acad Sci. 1965 Jun 30;124(2):626–643. doi: 10.1111/j.1749-6632.1965.tb18991.x. [DOI] [PubMed] [Google Scholar]
- McKenzie J. M., Haibach H. Increased total thyroxine with normal free thyroxine in antithyroid antiserum. Endocrinology. 1967 Jun;80(6):1097–1100. doi: 10.1210/endo-80-6-1097. [DOI] [PubMed] [Google Scholar]
- McMaster P. R., Lerner E. M., 2nd The transfer of allergic thyroiditis in histocompatible guinea pigs by lymph node cells. J Immunol. 1967 Jul;99(1):208–213. [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Passive transfer of experimental autoimmune thyroiditis from donor rabbits injected with soluble thyroglobulin without adjuvant. Int Arch Allergy Appl Immunol. 1967;32(5):506–520. doi: 10.1159/000229962. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Transfer of experimental autoimmune thyroiditis by serum from thyroidectomized donors. J Exp Med. 1969 Aug 1;130(2):263–285. doi: 10.1084/jem.130.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. A., Rose N. R., Witebsky E. Spontaneous thyroiditis in the obese strain of chickens. VI. Thyroxine-binding antibodies. J Immunol. 1971 Oct;107(4):997–1003. [PubMed] [Google Scholar]
- Nilsson O. Studies on antibody-containing cells in lymph nodes and the lymph of rabbits immunized with human immunoglobulin G according to various schedules. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):534–538. doi: 10.1111/j.1699-0463.1971.tb03807.x. [DOI] [PubMed] [Google Scholar]
- Pierce C. W. The effects of endotoxin on the immune response in the rat. II. Antibodies formed against bovine gamma-globulin in the splenectomized rat. Lab Invest. 1967 May;16(5):782–794. [PubMed] [Google Scholar]
- Pogoriler G., van Maanen J. H., Sellers E. A. Thyroxine binding to antithyroid antibodies: effects of altered metabolic status. J Endocrinol. 1971 Aug;50(4):547–560. doi: 10.1677/joe.0.0500547. [DOI] [PubMed] [Google Scholar]
- Premachandra B. N. Antibody binding of thyroxine in the primate subjected to hetero- and isoimmunization against components of the thyroid gland. Endocrinology. 1970 Mar;86(3):703–706. doi: 10.1210/endo-86-3-703. [DOI] [PubMed] [Google Scholar]
- Premachandra B. N., Berns A. W., Blumenthal H. T. Physiological aspects of thyroglobulin immunity. 3. Studies of localization of antibodies in vascular tissue and abnormal plasma thyroxine binding in the guinea pig. J Lab Clin Med. 1965 Dec;66(6):893–905. [PubMed] [Google Scholar]
- Premachandra B. N., Blumenthal H. T. Abnormal binding of thyroid hormone in sera from patients with Hashimoto's disease. J Clin Endocrinol Metab. 1967 Jul;27(7):931–936. doi: 10.1210/jcem-27-7-931. [DOI] [PubMed] [Google Scholar]
- Premachandra B. N., Perlstein I. B., Blumenthal H. T. Studies on obesity. II. Slow-moving thyroxine binding globulin in the sera of normal and obese subjects. J Clin Endocrinol Metab. 1970 Jun;30(6):752–762. doi: 10.1210/jcem-30-6-752. [DOI] [PubMed] [Google Scholar]
- ROSE N. R., WITEBSKY E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956 Jun;76(6):417–427. [PubMed] [Google Scholar]
- ROSENQUIST G. L., WOLFE H. R. Effect of splenectomy at different ages on precipitin production in chickens. Immunology. 1962 Mar;5:211–221. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- ROWLEY D. A. The formation of circulating antibody in the splenectomized human being following intravenous injection of heterologous erythrocytes. J Immunol. 1950 Nov;65(5):515–521. [PubMed] [Google Scholar]
- Ringertz B., Wasserman J., Packalén T., Perlmann P. Cellular and humoral immune responses in experimental autoimmune thyroiditis. Int Arch Allergy Appl Immunol. 1971;40(6):917–927. doi: 10.1159/000230473. [DOI] [PubMed] [Google Scholar]
- Roitt I. M., Doniach D. Delayed hypersensitivity in auto-immune disease. Br Med Bull. 1967 Jan;23(1):66–71. doi: 10.1093/oxfordjournals.bmb.a070519. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Kite J. H., Jr, Doebbler T. K., Spier R., Skelton F. R., Witebsky E. Studies on experimental thyroiditis. Ann N Y Acad Sci. 1965 Jun 30;124(1):201–230. doi: 10.1111/j.1749-6632.1965.tb18957.x. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Skelton F. R., Kite J. H., Jr, Witebsky E. Experimental thyroiditis in the rhesus monkey. 3. Course of the disease. Clin Exp Immunol. 1966 Apr;1(2):171–188. [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Witebsky E. Thyroid autoantibodies in thyroid disease. Adv Metab Disord. 1968;3:231–277. doi: 10.1016/b978-1-4831-9943-6.50013-x. [DOI] [PubMed] [Google Scholar]
- SASLAW S., BOURONCLE B. A., WALL R. L., DOAN C. A. Studies on the antibody response in splenectomized persons. N Engl J Med. 1959 Jul 16;261(3):120–125. doi: 10.1056/NEJM195907162610303. [DOI] [PubMed] [Google Scholar]
- SASLAW S., CARLISLE H. N. ANTIBODY RESPONSE IN SPLENECTOMIZED MONKEYS. Proc Soc Exp Biol Med. 1964 Jul;116:738–742. doi: 10.3181/00379727-116-29360. [DOI] [PubMed] [Google Scholar]
- SASLAW S., CARLISLE H. N. STREPTOCOCCAL INFECTION IN NORMAL AND SPLENECTOMIZED MONKEYS. Proc Soc Exp Biol Med. 1964 Nov;117:420–425. doi: 10.3181/00379727-117-29598. [DOI] [PubMed] [Google Scholar]
- Shimazaki M. An experimental study on thyroiditis with special reference to the cytotoxic factor in Hashimoto sera. Acta Pathol Jpn. 1968 Nov;18(4):497–500. doi: 10.1111/j.1440-1827.1968.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Solomon D. H., Beall G. N., Chopra I. J. Stimulation of release of thyroid iodine by serum of thyroid-immunized rabbits. J Clin Endocrinol Metab. 1970 Nov;31(5):603–606. doi: 10.1210/jcem-31-5-603. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. The dynamics of hemolysin formation in intact and splenectomized rabbits. J Infect Dis. 1950 Jul-Aug;87(1):37–62. doi: 10.1093/infdis/87.1.37. [DOI] [PubMed] [Google Scholar]
- Themann H., Andrada J. A., Rose N. R., Andrada E. C., Witebsky E. Experimental thyroiditis in the rhesus monkey. V. Electron microscopic investigations. Clin Exp Immunol. 1968 Jul;3(6):491–508. [PMC free article] [PubMed] [Google Scholar]
- Twarog F. J., Rose N. R. Transfer of autoimmune thyroiditis of the rat with lymph node cells. J Immunol. 1970 Jun;104(6):1467–1475. [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Transfer of experimental autoimmune thyroiditis of the mouse by serum. J Immunol. 1971 Apr;106(4):1139–1142. [PubMed] [Google Scholar]
- WHEELER J. R., WHITE W. F., CALNE R. Y. SELECTIVE LYMPHOPENIA BY USE OF INTRALYMPHATIC 198-AU AND SPLENECTOMY. IMMUNOSUPPRESSIVE ACTION ON REJECTION OF CANINE RENAL HOMOGRAFTS. Br Med J. 1965 Aug 7;2(5457):339–342. doi: 10.1136/bmj.2.5457.339. [DOI] [PMC free article] [PubMed] [Google Scholar]