Abstract
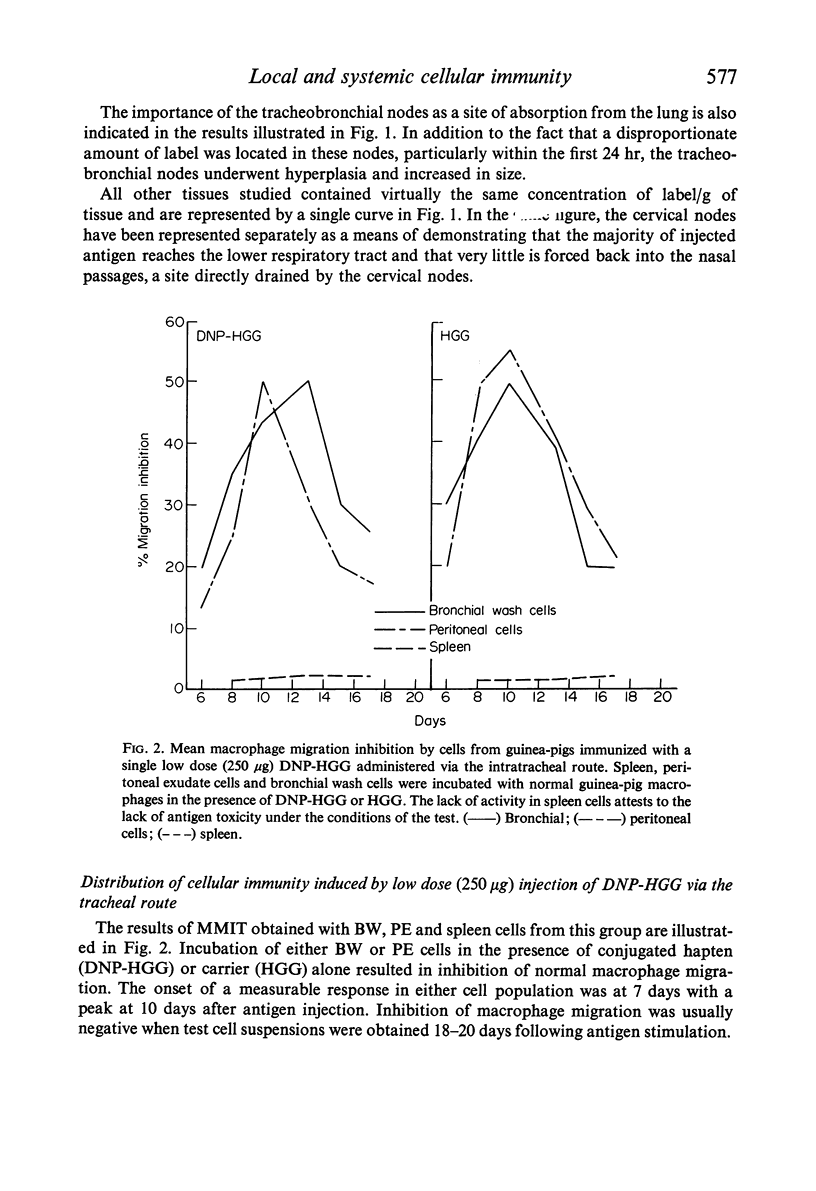
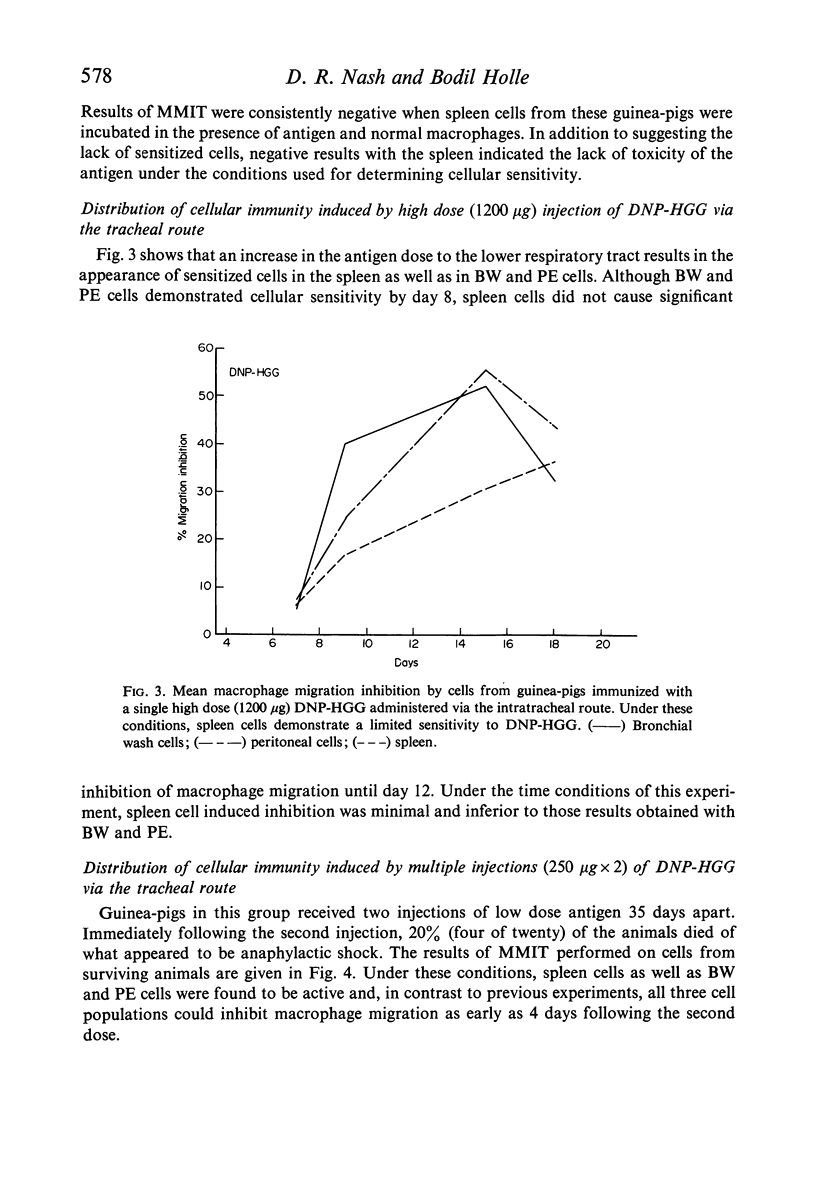
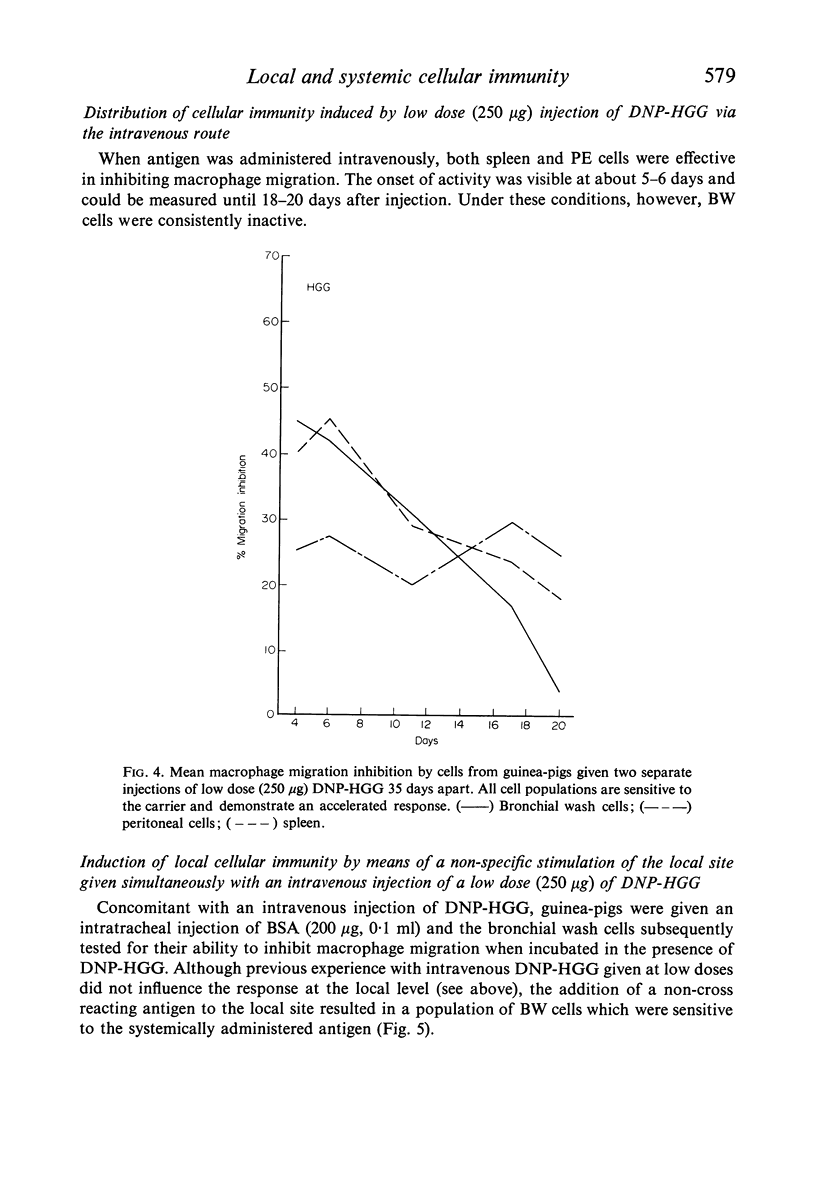
Guinea-pigs were immunized with DNP-HGG either by direct injection into the lower respiratory tract or intravenously. The distribution of cellular sensitivity was assessed by means of the macrophage migration inhibition test applied to bronchial wash cells (local) and to peritoneal exudate and spleen cell suspensions (systemic). A single low antigen dose given locally via the intratracheal route was sufficient for stimulation of cellular immunity in bronchial wash cells and oil induced peritoneal exudate cells but not in cells obtained from the spleen. Alternatively, a low antigen dose given intravenously resulted in splenic and peritoneal exudate cell sensitivity but not by cells obtained from the bronchus. Under these latter conditions, however, the bronchial wash cell population could be rendered sensitive to the systemic antigen providing sensitization was accompanied by a non-specific local stimulation—such as injection of a non-cross-reacting protein. Although a local low antigen dose does not normally result in systemic cellular immunity as judged by spleen cell assays, cellular sensitivity was induced systemic-ally by increasing the amount of locally administered antigen given as a single injection or increasing the number of low dose injections. Furthermore, under conditions of multiple injection, the onset of a measurable response at any site was accelerated.
Experiments to determine the fate of locally injected antigen revealed that proteins do escape from the lung into the general circulation as intact molecules. In addition to other parameters affecting the distribution of cellular immunity, the implication of these findings with special regard to respiratory tract prophylaxis are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- EISEN H. N., CARSTEN M. E., BELMAN S. Studies of hypersensitivity to low molecular weight substances. III. The 2,4-dinitrophenyl group as a determinant in the preciptin reaction. J Immunol. 1954 Nov;73(5):296–308. [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N. Migratory response of granulomatous alveolar cells from BCG-sensitized rabbits. J Immunol. 1970 Jul;105(1):227–237. [PubMed] [Google Scholar]
- HAHN P. F., CAROTHERS E. L. Lymphatic drainage following intrabronchial instillation of silver-coated radioactive gold colloids in therapeutic quantities. J Thorac Surg. 1953 Mar;25(3):265–279. [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Meyer E. C., Dominguez E. A., Bensch K. G. Pulmonary lymphatic and blood absorption of albumin from alveoli. A quantitative comparison. Lab Invest. 1969 Jan;20(1):1–8. [PubMed] [Google Scholar]
- PRENDERGAST R. A. CELLULAR SPECIFICITY IN THE HOMOGRAFT REACTION. J Exp Med. 1964 Mar 1;119:377–388. doi: 10.1084/jem.119.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ A. L., GRISMER J. T., GRANDE F. Absorption of radioactive albumin from the lungs of normal dogs. J Lab Clin Med. 1963 Mar;61:494–500. [PubMed] [Google Scholar]
- SCHULTZ A. L., GRISMER J. T., WADA S., GRANDE F. ABSORPTION OF ALBUMIN FROM ALVEOLI OF PERFUSED DOG LUNG. Am J Physiol. 1964 Dec;207:1300–1304. doi: 10.1152/ajplegacy.1964.207.6.1300. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]


