Abstract
An essential component of type III secretion systems (TTSS) is a supramolecular structure termed the needle complex. In Salmonella enterica, at least four proteins make up this structure: InvG, PrgH, PrgK, and PrgI. Another protein, PrgJ, is thought to play a role in the assembly of this structure, but its function is poorly understood. We have analyzed the expression and localization of PrgJ and the needle protein PrgI in different S. enterica serovar Typhimurium mutant strains. We found that the levels of PrgI and PrgJ were significantly reduced in a TTSS-deficient invA mutant strain and that the decreased levels were due to protein instability. In addition, we found that PrgJ, although associated with the needle complex in wild-type S. enterica serovar Typhimurium, was absent from needle complexes obtained from an invJ mutant strain, which exhibits very long needle substructures. We suggest that PrgJ is involved in capping the needle substructure of the needle complex.
The ability of Salmonella enterica to interact with intestinal epithelial cells is dependent on the presence of a type III secretion system (TTSS) encoded in Salmonella pathogenicity island 1 (SPI-1) (4). Essential for the function of this system is a membrane-spanning protein structure known as the needle complex (7). This structure is composed of at least four proteins: InvG, a member of the secretin family of proteins, which is homologous to components of both type II secretion and pilus assembly systems, and the products of the prg operon, PrgH, PrgI, and PrgK. InvG, PrgH, and PrgK have been shown to form the membrane-localized base substructure of the needle complex (7), while PrgI forms the needle portion of the complex (8). Another, putative component of the needle complex is PrgJ, which is also encoded within the prg operon (1). The location of PrgJ in the needle complex, its potential relationship with other components of this structure such as PrgI, and its putative role in the assembly of the needle complex are unknown. In order to gain a better understanding of these issues, we examined the expression and localization of PrgI and PrgJ in the wild type as well as selected S. enterica serovar Typhimurium mutant strains.
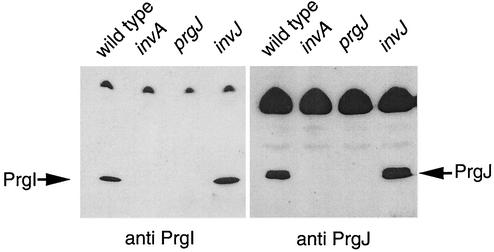
We have previously shown that the needle portion of the needle complex is absent in a number of mutant strains, including an invA and a prgJ mutant (11). Conversely, an invJ mutant was shown to produce extraordinarily long needles (8). We were interested in determining if the expression of PrgI and PrgJ was affected in these mutant strains. We examined the levels of PrgI and PrgJ in the invA, invJ, and prgJ mutant strains and compared them to those in the wild type. Bacteria were grown under SPI-1-inducing conditions (3), 1-ml samples were removed, and proteins were precipitated by the addition of trichloroacetic acid (TCA). Immunoblot analysis of the protein pellets revealed that PrgI was significantly reduced in the invA mutant and could not be detected in the prgJ mutant (Fig. 1). In contrast, the amount of PrgI produced by the invJ mutant strain was slightly increased in comparison to that in the wild type (Fig. 1), a finding that is consistent with the fact that this strain produces abnormally long needles (8). PrgJ could not be detected in the invA mutant (Fig. 1). In contrast, the amount of PrgJ detected in the invJ mutant was somewhat higher than that in the wild type (Fig. 1). The fact that the level of PrgJ is elevated in an invJ mutant (which has extra-long needles) and absent in an invA mutant (which lacks needles) suggests that this protein may be involved in the assembly of the needle portion of the S. enterica serovar Typhimurium SPI-1 needle complex.
FIG. 1.
Expression of PrgI and PrgJ in Salmonella mutant strains. Cultures of wild-type, invA, prgJ, and invJ strains were grown in LB broth containing 0.3 M NaCl at 37°C to an OD600 of 0.8. Aliquots of 1 ml were removed, and TCA was added to a final volume of 10%. Cultures were placed on ice for 30 min and then pelleted by spinning at 10,000 × g for 20 min. The pellets were washed with ice-cold acetone and dried under a vacuum. Pellets were resuspended in loading buffer and loaded onto sodium dodecyl sulfate-12% polyacrylamide gels. After electrophoresis, the gels were transferred to nitrocellulose. The blots were incubated with either anti-PrgI or anti-PrgJ rabbit serum followed by horseradish peroxidase-conjugated goat anti-mouse antiserum. The blots were developed, and bands were detected by chemiluminescence.
In order to determine if the differences seen in PrgI and PrgJ protein levels in the different SPI-1 mutant strains were due to differential transcriptional regulation, the transcription of the prgI and prgJ genes was monitored with the use of xylE reporter gene fusions. A xylE reporter gene cassette lacking a transcription terminator was inserted into either prgI or prgJ, and the fusions were recombined into the chromosome of the wild type as well as the invA, invJ, and prgJ mutant strains as previously described (5). Introduction of this cassette does not lead to polar effects on downstream genes (5). The expression of the different reporter gene fusions was monitored in the resulting strains grown under SPI-1-inducing conditions by assaying catechol-2,3-dioxygenase activity in bacterial lysates (5). Although differences in the levels of catechol-2,3-dioxygenase activity between the wild type and some of the mutant strains carrying the prgI or prgJ reporter fusions were detected (Table 1), these differences were opposite to what would be expected based on the protein expression experiments (Fig. 1). For example, the transcription of both the prgI-xylE and the prgJ-xylE fusions was higher in the invA mutant background and lower in the invJ mutant strain than in the wild type, which does not strictly correlate with the levels of these proteins as determined by Western blotting (Fig. 1). Equivalent results were obtained when merodiploid reporter strains were utilized in these experiments, ruling out potential feedback regulatory mechanisms (data not shown). In addition, equivalent results were also obtained with plasmid-borne reporter fusions or in the presence of a plasmid-borne wild-type copy of the respective genes (data not shown). While these experiments indicate that there may be slight differences in the abilities of these strains to transcribe prgI and prgJ, these differences cannot explain the changes in protein expression observed among these different strains.
TABLE 1.
Analysis of prgI and prgJ transcriptiona
| Strain genotype | XyIE activity (U)
|
|
|---|---|---|
| prgI-xyIE | prgJ-xylE | |
| Wild type | 1,061 ± 31 | 1,345 ± 12 |
| ΔinvA | 1,808 ± 70 | 2,806 ± 31 |
| ΔinvJ | 474 ± 38 | 897 ± 34 |
| ΔprgJ | 1,167 ± 62 | ND |
The levels of transcription of the prgI and prgJ reporter gene fusions in the different strains were monitored by assaying the levels of catechol-2,3-dioxygenase activity in bacterial lysates. Cells were grown under inducing conditions to an OD600 of 0.8 and lysed by sonication, and the levels of catechol-2,3-dioxygenase activity were determined as previously described (5). Values are means and standard deviations of one experiment performed with triplicate samples. Equivalent results were obtained in several repetitions of this experiment. ND, not determined.
Since the changes in the levels of PrgI and PrgJ observed in the various mutant strains could not be explained by the differences in transcription, we wanted to determine if they were due to differences in mRNA translation. We therefore constructed in-frame fusions of prgI and prgJ to lacZ to monitor translation. Single-copy plasmids containing either the prgI-lacZ or the prgJ-lacZ gene fusion were electroporated into the wild type and the invA, invJ, and prgJ mutant strains, and their translation was monitored by measuring the levels of β-galactosidase activity. The expression of the different fusion proteins was under the control of their native promoters. As seen in Table 2, the translation of both the prgI-lacZ and the prgJ-lacZ gene fusions, as measured by β-galactosidase activity, did not significantly vary regardless of which strain background they were placed in. Interestingly, the levels of β-galactosidase activity in the strains carrying the prgI-lacZ fusion were approximately 10 times higher than those found in the strains carrying the prgJ-lacZ fusion. Since both prgI and prgJ were under the control of the same promoter, this finding would indicate that the translation of prgI is more efficient than the translation of prgJ, which is consistent with the fact that PrgI is the main (or only) subunit of the needle substructure (8). Alternatively, it is also possible that this difference reflects different stabilities of the PrgI and PrgJ reporter fusions.
TABLE 2.
Analysis of prgI and prgJ translationa
| Strain genotype | LacZ activity (Miller units)
|
|
|---|---|---|
| prgI-lacZ | prgJ-lacZ | |
| Wild type | 5,355 ± 335 | 490 ± 16 |
| ΔinvA | 5,412 ± 145 | 565 ± 15 |
| ΔinvJ | 5,901 ± 214 | 468 ± 31 |
| ΔprgI | 6,067 ± 325 | 511 ± 17 |
| ΔprgJ | 5,474 ± 162 | 467 ± 7 |
prgI and prgJ translational fusions to lacZ were constructed as follows by using the translational fusion vector plasmid pRS414 (10). DNA fragments containing prgI or prgJ were amplified by PCR and inserted into the EcoRI and BamHI sites of pRS414 so as to create in-frame fusions to lacZ. In either case, the 5′ primer started 370 bp upstream of the prgH gene and therefore contained the native promoter that drives the expression of prgI and prgJ (6). The resulting fusions contained 50 and 60 amino acids of PrgI and PrgJ, respectively, fused to LacZ. The levels of translation of the prgI and prgJ reporter gene fusions in the different strains were monitored by assaying the levels of β-galactosidase activity. Cells were grown under inducing conditions to an OD600 of 0.8, and β-galactosidase activity was assayed as described previously (9). Values are means and standard deviations of one experiment performed with triplicate samples. Equivalent results were obtained in several repetitions of this experiment.
Since the reduction of PrgI and PrgJ levels in the invA mutant did not appear to be due to differences in transcription or translation, we examined the possibility that the differences in protein levels could be due to differences in protein stability. Given the small size of these proteins and their low cysteine-methionine content, a pulse-chase experiment using 35S-labeled amino acids was not feasible. We therefore used an alternative strategy to examine the stability of these proteins. Both the wild type and the invA mutant were transformed with a plasmid containing the prgJ gene under control of an arabinose-inducible promoter. The bacteria were grown under SPI-1-inducing conditions in media containing arabinose. At an optical density at 600 nm (OD600) of 0.8, chloramphenicol was added to halt de novo protein synthesis, and the levels of PrgJ at different times after addition of chloramphenicol were determined by immunoblot analysis. As seen in Fig. 2, the amount of PrgJ in the wild-type strain remained consistent over the course of the experiment, whereas the amount of PrgJ in the invA mutant decreased significantly. These findings indicate that the reduced level of PrgJ in the invA mutant is due to protein instability. We were unable to overexpress PrgI; therefore, we could not perform similar experiments to determine the stability of PrgI in the invA mutant. However, it is likely that the lower level of PrgI seen in this mutant is also due to protein instability.
FIG. 2.
Stability of PrgJ in wild-type and type III secretion-deficient strains. Cultures of the wild type and the invA mutant containing a plasmid carrying prgJ under control of an arabinose-inducible promoter were grown in Luria-Bertani broth (0.3 M NaCl) containing 0.02% arabinose to an OD600 of 0.8. Chloramphenicol was added to a final concentration of 60 μg/ml, and 1-ml aliquots were removed and precipitated by the addition of TCA as described for Fig. 1. Pellets were loaded on sodium dodecyl sulfate-12% polyacrylamide gels, transferred to nitrocellulose, and incubated with anti-PrgJ serum followed by anti-rabbit goat horseradish peroxidase-conjugated antibody. Bands were detected by chemiluminescence. Aliquots were withdrawn 0, 5, 10, 15, 30, and 60 min after the addition of chloramphenicol.
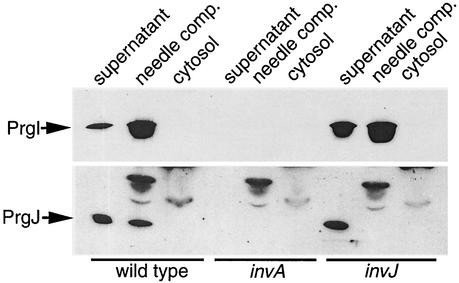
To further investigate the relationship between PrgI and PrgJ, we separated bacterial cultures of the wild type and the invA and invJ mutants into supernatants, needle complexes, and cytosolic components in order to determine the location of these two proteins in these strains. Cultures were grown under SPI-1 TTSS-inducing conditions and separated into the three different components. Western blot analysis revealed that in the wild type, the majority of PrgI was located in the culture supernatant and the needle complex fraction (Fig. 3). Only a very small amount of PrgI could be detected in the cytosol when the blot was overexposed (data not shown). When we examined the invJ mutant we found that the localization of PrgI was similar to that seen in the wild type: PrgI was detected predominantly in the culture supernatant and the needle complex fractions. We also found that in the wild type, the majority of PrgJ was located in the culture supernatant, with a smaller amount located in the needle complex fraction (note that for the PrgJ localization analysis, 5 ml of both the membrane and the cytosolic fractions was loaded, in comparison to 2 ml of culture supernatant fraction; see the legend to Fig. 3). No PrgJ could be detected in the cytosol. As expected, we did not detect PrgI and PrgJ in any fraction of the invA mutant strain (Fig. 3). In contrast to the wild type, we found that in the invJ mutant strain PrgJ could be detected only in the culture supernatant. No PrgJ could be detected in the needle complex fraction isolated from this strain. This finding is surprising given that this mutant not only is capable of expressing and secreting PrgJ but does so in amounts greater than those in the wild-type strain. The absence of PrgJ in the needle complex of the invJ mutant, which assembles abnormally long needle substructures, suggests a role for PrgJ in the termination of needle assembly in conjunction with InvJ. These data also suggest that PrgJ may function as a capper of the needle substructure.
FIG. 3.
Localization of PrgI and PrgJ. Cultures of wild-type, invA, and invJ strains were grown under SPI-1 TTSS-inducing conditions (3). Cells were pelleted by spinning at 100,000 × g. This high centrifugal force ensured that any broken needles would be removed from the culture supernatant. The supernatants were subjected to TCA precipitation as described for Fig. 1. The bacterial pellets were resuspended in 0.5 M sucrose-0.15 M Tris (pH 8.0). Lysozyme (100 μg/ml) and EDTA (1 mM) were added, and after a 30-min incubation on ice followed by a 15-min incubation at 37°C, the cells were lysed by the addition of 1% n-lauryl diethylamine oxide. The lysates were then subjected to centrifugation at 100,000 × g for 1 h. The supernatants were removed and subjected to TCA precipitation. The resulting pellets and the cell lysate supernatants along with the lysate pellets were loaded onto sodium dodecyl sulfate-12% polyacrylamide gels, electrophoresed, and transferred to nitrocellulose membranes. The membranes were reacted with either anti-PrgI or anti-PrgJ antibodies followed by horseradish peroxidase-conjugated secondary antibodies, and bands were detected by chemiluminescence. For the PrgI analysis, the equivalent of 2 ml of each fraction was loaded. In the case of the PrgJ analysis, 2 ml of supernatant and 5 ml of both the membrane and cytosolic fractions were loaded in order to ensure detection of PrgJ in the membrane fraction.
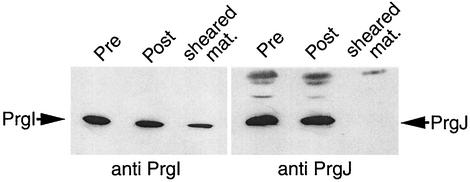
It has recently been proposed that MxiI, the Shigella homolog of PrgJ, may be located at the tip of the closely related needle complex of the Shigella TTSS (2), although no direct evidence for this hypothesis was provided. In order to determine if PrgJ is located at the tip of the Salmonella needle complex, we performed an experiment in which the needle portion of the complex was sheared from the surface of whole cells and examined for its protein content. Wild-type bacteria were grown under inducing conditions, harvested, washed, and passed through a 25-gauge needle 20 times in order to shear off the needle substructure. The amount of PrgI and PrgJ found in the sheared fraction was compared to that found in the cell envelope. A sample of membrane-associated protein from an unsheared sample was included as a control. As seen in Fig. 4, a significant proportion of PrgI is clearly detectable in the sheared fraction, a finding that is in keeping with its surface localization (8). In contrast, no PrgJ could be detected in the sheared fraction. Given the small amount of PrgJ found in the needle complex compared to PrgI, these findings do not rule out the possibility that PrgJ may be part of the needle substructure. However, it seems unlikely that PrgJ is located at the tip of the needle, since it is this region that would be the most likely to break off during the shearing procedure. These results are also in keeping with our inability to detect PrgJ at the tip of the needle substructure by immunoelectron microscopy (data not shown). It is possible that PrgJ may be located at the base of the needle substructure, where it may exert its capping and needle assembly termination functions in conjunction with InvJ. This hypothesis would imply that the needle assembly may occur by subunit addition at the base of the substructure rather than at the tip. Alternatively, it is also possible that PrgJ may serve as an “adaptor” molecule that mediates the anchoring of the PrgI needle filament to the base structure. More experiments will be required to substantiate this hypothesis.
FIG. 4.
Analysis of the needle substructure protein content. Cultures of wild-type bacteria were grown under inducing conditions and pelleted by low-speed centrifugation (10,000 × g). Pellets were washed once with phosphate-buffered saline (PBS), repelleted, and resuspended in PBS. Cells were then passed through a 20-gauge needle 20 times in order to shear off the needle portion of the needle complex. Cells were pelleted and the supernatant was removed and subjected to TCA precipitation. The cell pellet was lysed as described for Fig. 3. Lysate pellets of both unsheared control cells and the sheared cells along with the pellet from the TCA-precipitated supernatant were analyzed by immunoblotting using anti-PrgI or anti-PrgJ antibodies as described above.
In summary, we have shown that (i) in comparison to the levels in the wild-type strain, the levels of PrgI and PrgJ are reduced in an invA mutant and are increased in an invJ mutant; (ii) the lower levels of PrgI and PrgJ in the invA mutant are likely due to protein instability; (iii) PrgJ is not associated with the needle complex of the invJ mutant, suggesting a role for this protein in the capping of the needle complex; and (iv) PrgJ does not appear to be located at the tip of the needle. Further studies will be required to precisely ascertain the function of PrgJ in the control of needle assembly.
Acknowledgments
We thank members of the Galán laboratory for critical review of the manuscript.
This work was supported by Public Health Service grant AI30492 from the National Institutes of Health.
REFERENCES
- 1.Behlau, I., and S. J. Miller. 1993. A Pho-P-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J. Bacteriol. 175:4475-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 3.Eichelberg, K., and J. E. Galán. 1999. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect. Immun. 67:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galán, J. E. 2001. Salmonella interaction with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 5.Kaniga, K., J. C. Bossio, and J. E. Galán. 1994. The Salmonella typhimurium invasion genes invF and invG encode homologues to the PulD and AraC family of proteins. Mol. Microbiol. 13:555-568. [DOI] [PubMed] [Google Scholar]
- 6.Klein, J. R., T. F. Fahlen, and B. D. Jones. 2000. Transcriptional organization and function of invasion genes within Salmonella enterica serovar Typhimurium pathogenicity island 1, including the prgH, prgI, prgJ, prgK, orgA, orgB, and orgC genes. Infect. Immun. 68:3368-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galán, and S.-I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 8.Kubori, T., A. Sukhan, S. -I. Aizawa, and J. E. Galán. 2000. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl. Acad. Sci. USA 97:10225-10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 11.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galán. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]