Abstract
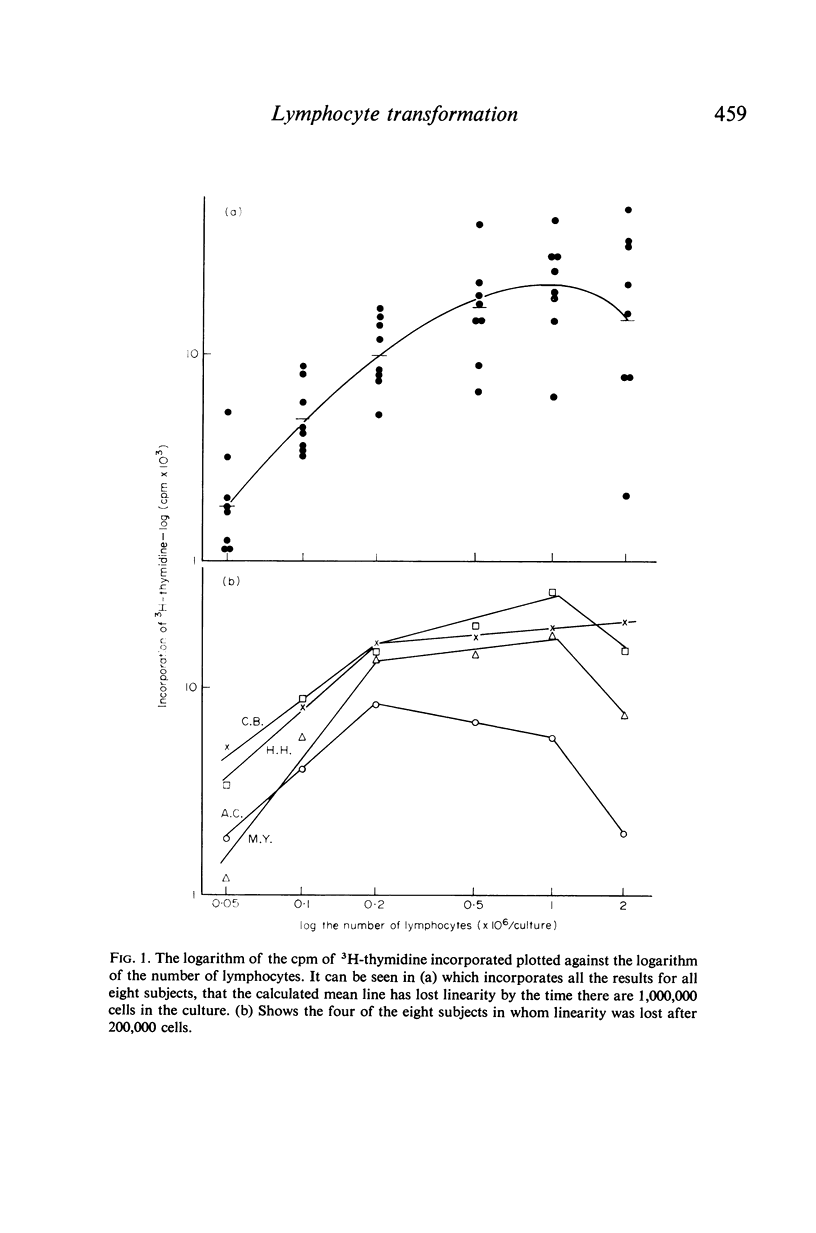
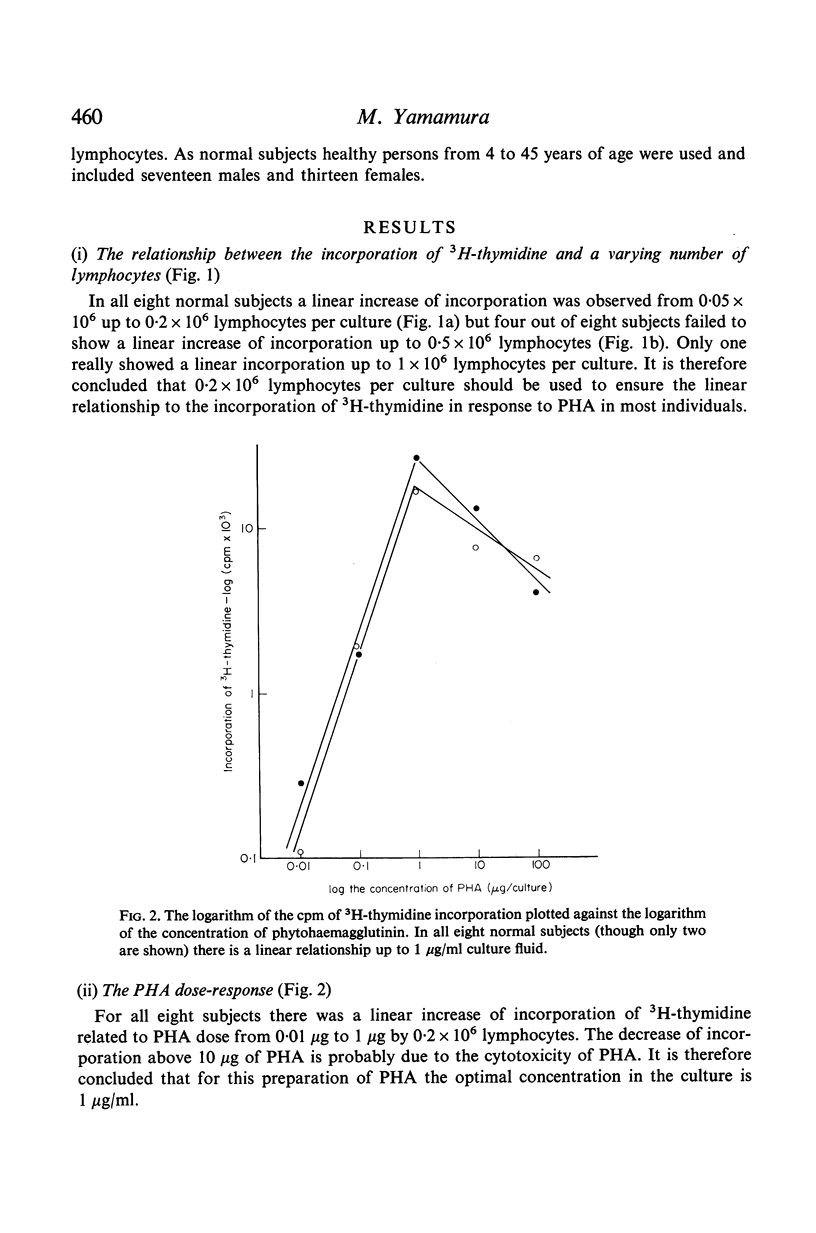
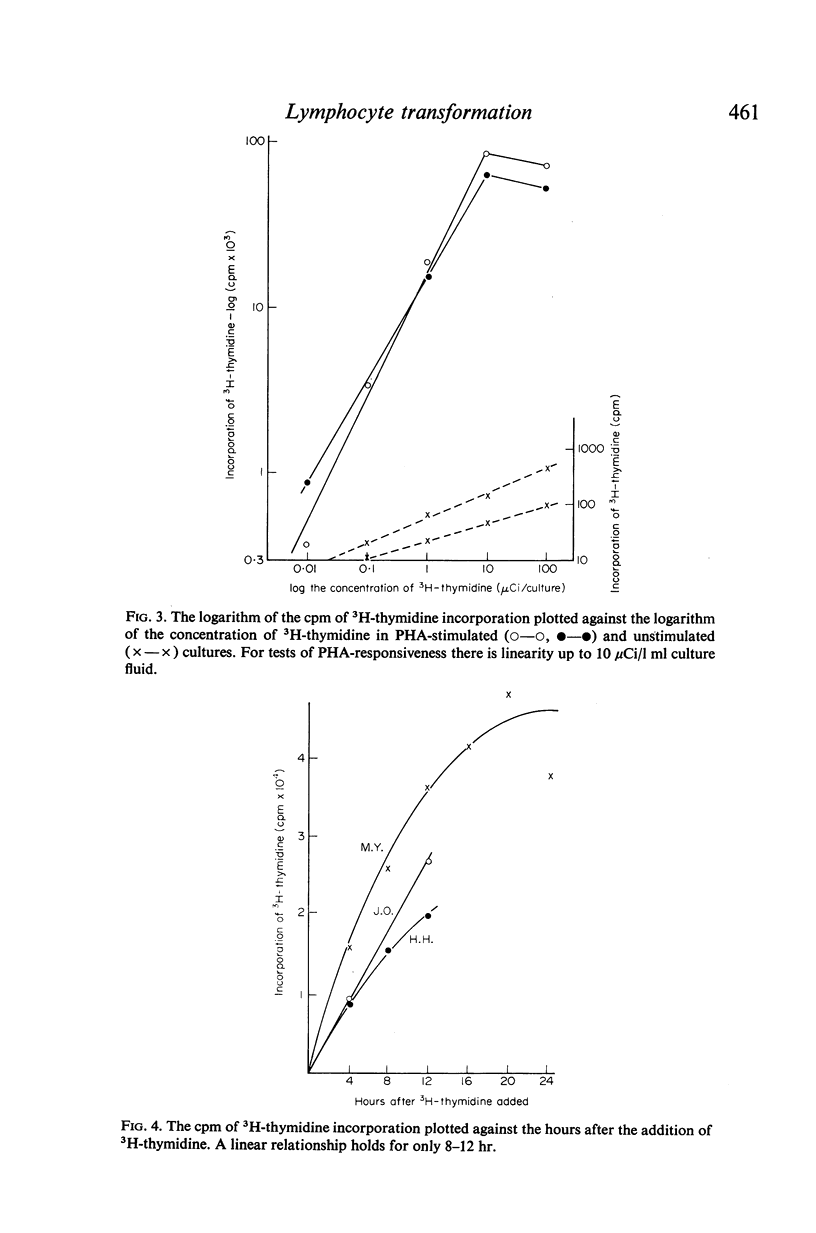
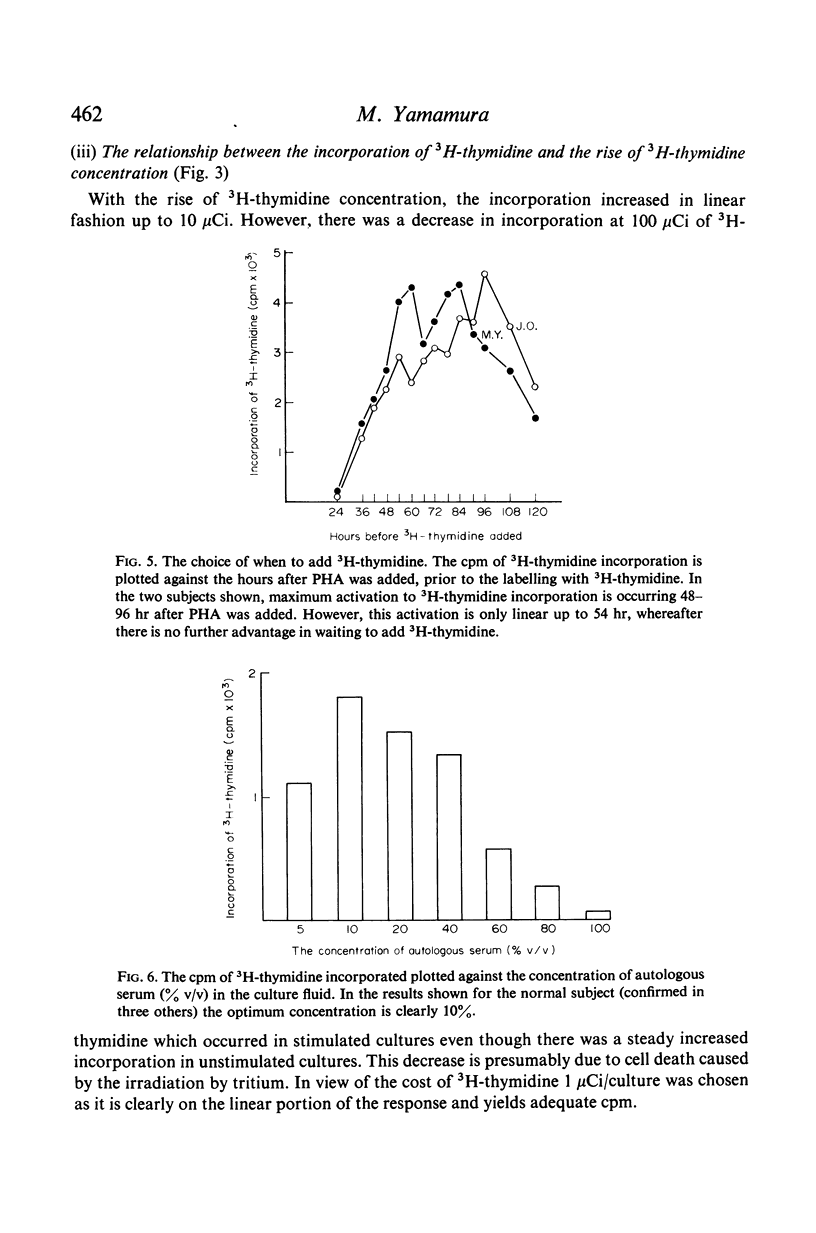
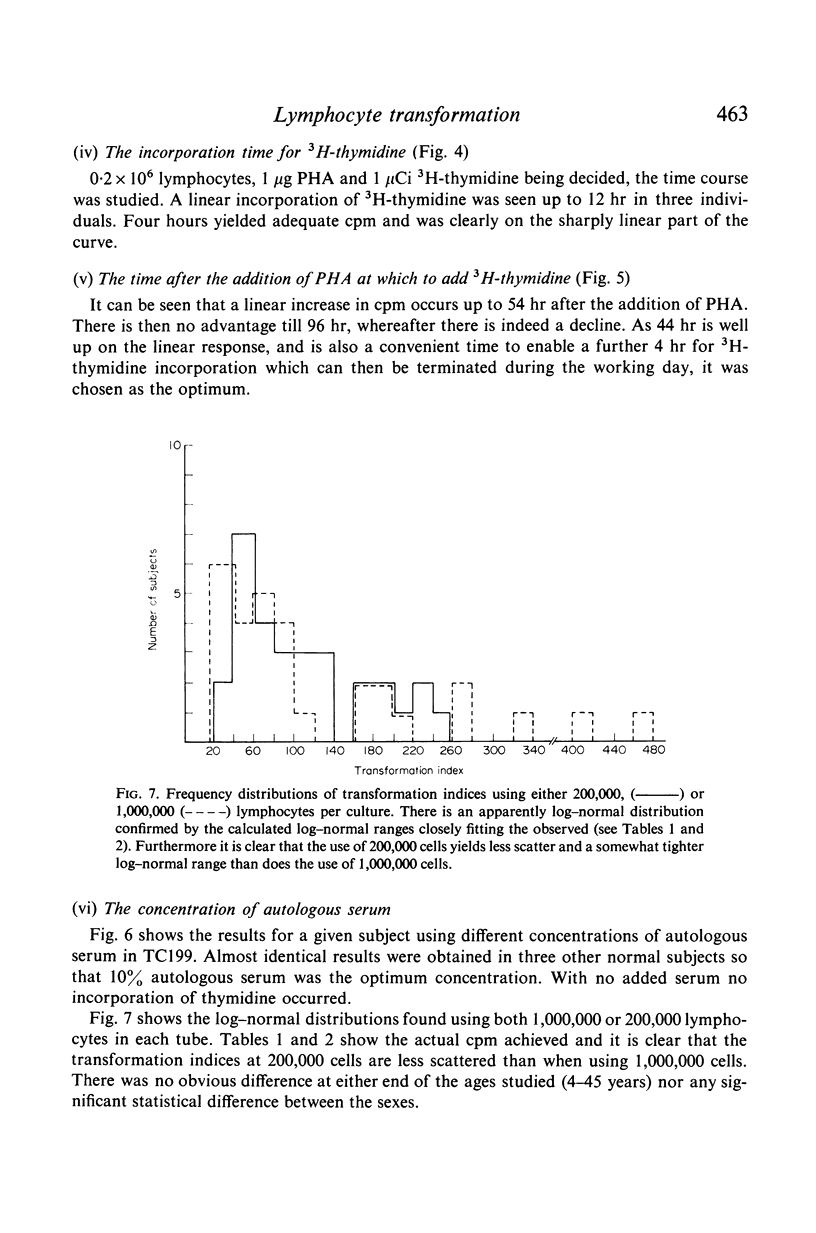
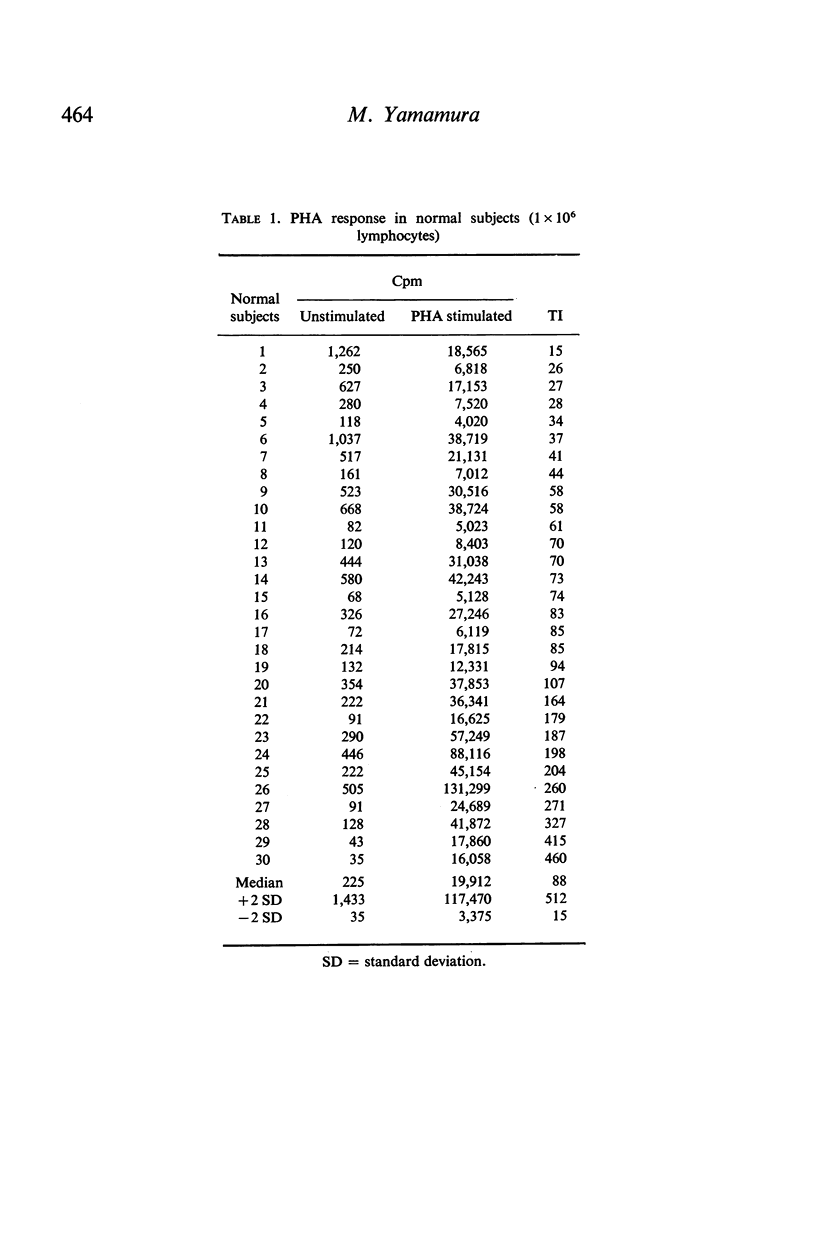
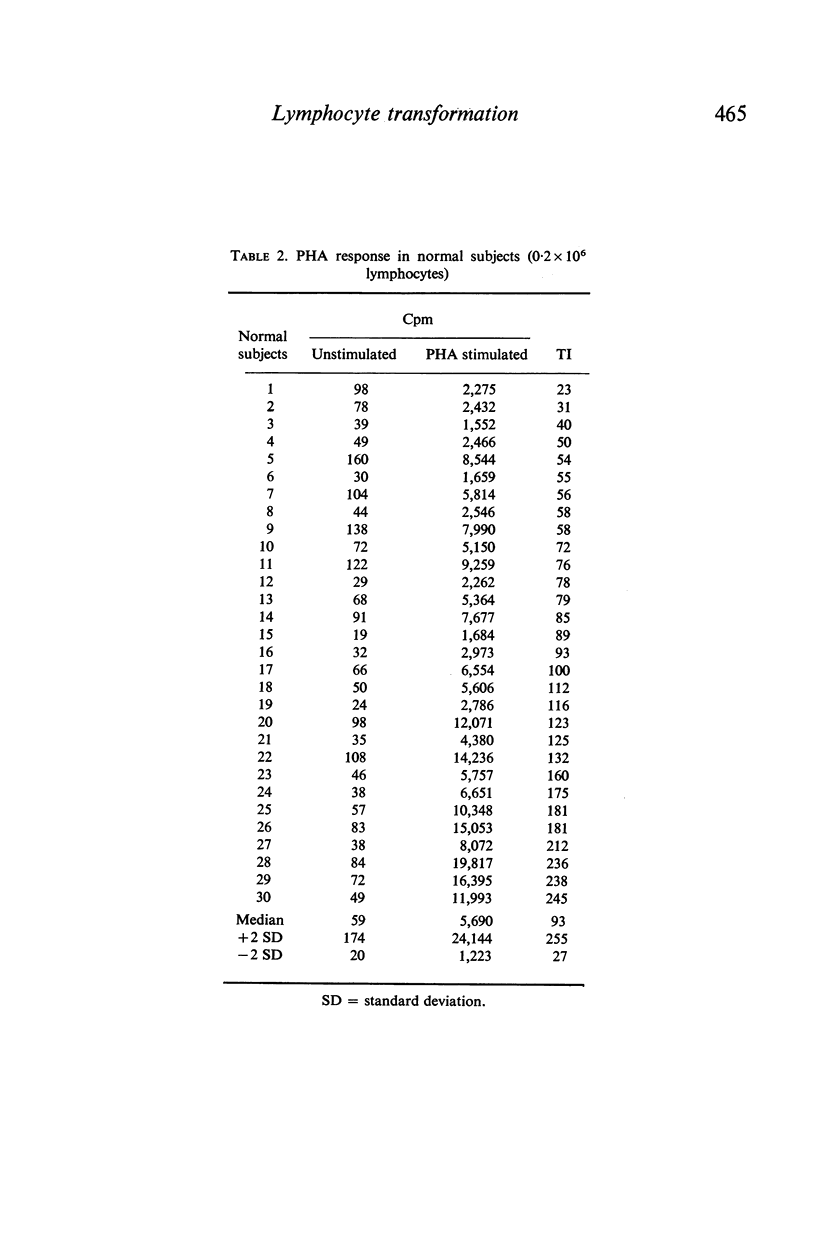
In contrast to the million cells used by many current workers, careful analysis of lymphocyte transformation to phytohaemagglutinin determined optimum conditions as (i) 200,000 lymphocytes in 1 ml of culture fluid in 100 × 12-mm round-bottomed glass tubes (ii) phytohaemagglutinin at 1 μg/tube (iii) 3H-thymidine at 1 μCi/tube (iv) with a convenient incorporation time 4 hr (v) starting 44 hr after adding phytohaemagglutinin. It was then found 10% autologous serum was the optimum concentration in the culture fluid. The log–normal range of transformation indices for young adults was 27–93–255.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Caron G. A., Sarkany I., Williams H. S., Todd A. P., Gell H. M. Radioactive method for the measurement of lymphocyte transformation in vitrol. Lancet. 1965 Dec 18;2(7425):1266–1268. doi: 10.1016/s0140-6736(65)92282-8. [DOI] [PubMed] [Google Scholar]
- Chalmers D. G., Cooper E. H., Evans C., Topping N. E. Quantitation of the response of lymphocytes in culture to specific and non-specific stimulation. Int Arch Allergy Appl Immunol. 1967;32(2):117–130. doi: 10.1159/000229922. [DOI] [PubMed] [Google Scholar]
- Coulson A. S., Chalmers D. G. Lack of demonstrable IgG in phytohaemagglutinin-stimulated human transformed cells cultured in a serum-free medium. Transplantation. 1967 May;5(3):547–548. doi: 10.1097/00007890-196705000-00017. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. G. The establishment of a normal human population dose-response curve for lymphocytes cultured with PHA (phytohaemagglutinin). Clin Exp Immunol. 1971 Mar;8(3):421–425. [PMC free article] [PubMed] [Google Scholar]
- Hartog M., Cline M. J., Grodsky G. M. The response of human leucocyte cultures to stimulation by tuberculin and phytohaemagglutinin measured by the uptake of radioactive thymidine. Clin Exp Immunol. 1967 Mar;2(2):217–228. [PMC free article] [PubMed] [Google Scholar]
- Havemann K., Dosch H. M., Bürger S. Phythämagglutinin (PHA) und Serumproteine in der Lymphocytenkultur. I. Nachweis von fördernden und hemmenden Serumfaktoren. Z Gesamte Exp Med. 1970;153(4):297–307. [PubMed] [Google Scholar]
- Knight S., Ling N. R., Sell S., Oxnard C. E. The transformation in vitro of peripheral lymphocytes of some laboratory animals. Immunology. 1965 Dec;9(6):565–574. [PMC free article] [PubMed] [Google Scholar]
- Mattsson A., Lindahl-Kiessling K. Lymphocyte culture in serum-free medium. Lancet. 1971 Apr 3;1(7701):704–704. doi: 10.1016/s0140-6736(71)92716-4. [DOI] [PubMed] [Google Scholar]
- Sample W. F., Chretien P. B. Thymidine kinetics in human lymphocyte transformation: determination of optimal labelling conditions. Clin Exp Immunol. 1971 Sep;9(3):419–427. [PMC free article] [PubMed] [Google Scholar]
- Schellekens P. T., Eijsvoogel V. P. Lymphocyte transformation in vitro. I. Tissue culture conditions and quantitative measurements. Clin Exp Immunol. 1968 Jul;3(6):571–584. [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Thomson A. E. Death and division of lymphocytes. Neglected factors in assessment of P.H.A.-induced transformation. Lancet. 1968 Nov 23;2(7578):1120–1123. doi: 10.1016/s0140-6736(68)91583-3. [DOI] [PubMed] [Google Scholar]



