Abstract
The cytoplasmic membrane protein CcdA and its homologues in other species, such as DsbD of Escherichia coli, are thought to supply the reducing equivalents required for the biogenesis of c-type cytochromes that occurs in the periplasm of gram-negative bacteria. CcdA-null mutants of the facultative phototroph Rhodobacter capsulatus are unable to grow under photosynthetic conditions (Ps−) and do not produce any active cytochrome c oxidase (Nadi−) due to a pleiotropic cytochrome c deficiency. However, under photosynthetic or respiratory growth conditions, these mutants revert frequently to yield Ps+ Nadi+ colonies that produce c-type cytochromes despite the absence of CcdA. Complementation of a CcdA-null mutant for the Ps+ growth phenotype was attempted by using a genomic library constructed with chromosomal DNA from a revertant. No complementation was observed, but plasmids that rescued a CcdA-null mutant for photosynthetic growth by homologous recombination were recovered. Analysis of one such plasmid revealed that the rescue ability was mediated by open reading frame 3149, encoding the dithiol:disulfide oxidoreductase DsbA. DNA sequence data revealed that the dsbA allele on the rescuing plasmid contained a frameshift mutation expected to produce a truncated, nonfunctional DsbA. Indeed, a dsbA ccdA double mutant was shown to be Ps+ Nadi+, establishing that in R. capsulatus the inactivation of dsbA suppresses the c-type cytochrome deficiency due to the absence of ccdA. Next, the ability of the wild-type dsbA allele to suppress the Ps+ growth phenotype of the dsbA ccdA double mutant was exploited to isolate dsbA-independent ccdA revertants. Sequence analysis revealed that these revertants carried mutations in dsbB and that their Ps+ phenotypes could be suppressed by the wild-type allele of dsbB. As with dsbA, a dsbB ccdA double mutant was also Ps+ Nadi+ and produced c-type cytochromes. Therefore, the absence of either DsbA or DsbB restores c-type cytochrome biogenesis in the absence of CcdA. Finally, it was also found that the DsbA-null and DsbB-null single mutants of R. capsulatus are Ps+ and produce c-type cytochromes, unlike their E. coli counterparts, but are impaired for growth under respiratory conditions. This finding demonstrates that in R. capsulatus the dithiol:disulfide oxidoreductases DsbA and DsbB are not essential for cytochrome c biogenesis even though they are important for respiration under certain conditions.
The c-type cytochromes (Cyts) are electron transport proteins that contain heme molecules as prosthetic groups. In these proteins, heme is covalently attached to the polypeptide via thioether linkages formed between the heme vinyl groups and the thiol side chains of the cysteine residues in the conserved CXXCH heme-binding motif of the apoprotein. In gram-negative bacteria, synthesis of holo-Cyt c (heme-bound Cyt c) is an elaborate process that occurs on the periplasmic face of the cytoplasmic membrane and requires multiple membrane-associated components (e.g., products of ccmABCDEFGH in Escherichia coli) (20, 30, 53, 54). These components act in a coordinated fashion to transport heme across the membrane, to chaperone the apo-Cyts from the secretion translocons to c-type Cyt maturation sites, and to allow efficient stereo-specific thioether bond formation followed by subsequent folding of holo-Cyt c.
The periplasm is a more oxidizing environment than the cytoplasm; thus, keeping the apo-Cyts and the heme moieties in a reduced state conducive for thioether bond catalysis is crucial. Apo-Cyts are also subject, after secretion and prior to heme attachment, to the action of the disulfide bond formation pathways of the periplasm. In E. coli, these pathways involve the DsbA, DsbB, DsbC, DsbD, and DsbG components (8, 12, 17, 42) and, of these, DsbA, DsbB, and DsbD are required for the biogenesis of c-type Cyts (9, 34, 43). Upon the entrance of the apo-Cyts into the periplasm, the cysteines in their heme-binding site are first thought to be oxidized by the DsbA-DsbB couple and subsequently reduced by the thioreduction pathway that comprises DsbD, CcmG, and CcmH to allow heme ligation (54). Like the disulfide bond isomerases DsbC and DsbG, CcmG receives reducing equivalents from the integral membrane protein DsbD (7, 25, 31) and is believed to convey electrons through CcmH to the apo-Cyts (18, 41).
The gram-negative facultative photosynthetic bacterium Rhodobacter capsulatus synthesizes a number of different c-type Cyts, including the Cyts c1, c2, cy, co, and cp, which are required for either photosynthetic or cbb3-type Cyt c oxidase-dependent respiratory growth (11, 21, 29, 56). In R. capsulatus, 10 components (the products of helABCDX, ccl1-2, cycJ, cycH, and ccdA) involved in various aspects of cyt c biogenesis have thus far been characterized (3, 4, 6, 13, 19, 32). Of these components, HelABCD (homologues of CcmABCD) (19) and CycJ (homologue of CcmE) (13) are thought to deliver heme, while CycH (partly homologous to the carboxyl-terminal part of CcmH) is proposed to chaperone the apo-Cyt (32) to Ccl1 (homologue of CcmF), considered to provide a heme ligation platform. Electrons required for reduction of the heme-binding cysteines are shuttled across the membrane via CcdA (14, 27). R. capsulatus CcdA is an integral membrane protein homologous to the central (β) domain of E. coli DsbD (13, 25), and in its absence, biogenesis of c-type Cyts is abolished unless the growth medium is supplemented with reducing chemicals such as dithiothreitol (13). In R. capsulatus, reducing equivalents are believed to be relayed during heme ligation from CcdA via HelX (homologue of CcmG) to Ccl2 (homologous to the amino-terminal part of CcmH) and then to the apo-Cyts (26).
We have previously shown that R. capsulatus CcdA-null mutants are unable to grow photosynthetically (Ps−) and do not produce any active Cyt c oxidase due to a pleiotropic Cyt c deficiency (13). Such mutants are unable to catalyze the reaction α-naphthol + dimethylphenylenediamine → indophenol blue + H2O (Nadi−) (28). On the other hand, they grow well by respiration (Res+) via an alternate respiratory pathway that does not contain any c-type Cyts and that uses a hydroquinone oxidase. Interestingly, CcdA-null mutants revert frequently to the Ps+ Nadi+ phenotype under both photosynthetic and respiratory growth conditions, indicating that they regain the ability to synthesize c-type Cyts in the absence of CcdA. In this work, in order to gain further insights into the biogenesis of c-type Cyts, we analyzed Ps+ revertants of a CcdA-null mutant of R. capsulatus. Our findings revealed that inactivation of either dsbA or dsbB restores the inability of a CcdA-null mutant to produce c-type Cyts, presumably by reinstating periplasmic redox homeostasis. Moreover, R. capsulatus mutants lacking either DsbA or DsbB are proficient in photosynthesis and able to produce c-type Cyts, unlike their E. coli counterparts. However, they are impaired in respiration, especially in enriched growth medium, pointing out a need for the DsbA-DsbB pathway during the respiratory growth of R. capsulatus.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this work are described in Table 1. R. capsulatus strains were grown on enriched medium (MPYE) (10) or in Sistrom's minimal medium A (Med A) (50) supplemented when appropriate with antibiotics (spectinomycin, 10 μg/ml; tetracycline, 2.5 μg/ml; kanamycin, 10 μg/ml) at 35°C chemoheterotrophically (respiratory growth conditions) or photoheterotrophically (photosynthetic growth conditions) in anaerobic jars with H2- and CO2-generating gas packs from BBL Microbiology Systems (Cockeysville, Md.). E. coli strains were grown on Luria broth supplemented with appropriate antibiotics (ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; spectinomycin, 50 μg/ml; tetracycline, 12.5 μg/ml; gentamicin, 15 μg/ml) as described previously (23).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Relevant phenotypeb | Source or reference |
|---|---|---|---|
| Strains | |||
| E. coli | |||
| HB101 | F− Δ(gpt-proA)62leuB6 supE44 ara-14 galK2 lacY1 Δ(mcrC-mrr) rpsL20 (Strr) xyl-5 mtl-1 recA13 | 44 | |
| XL1-Blue | F′::Tn10 proA+B+lacIq Δ(lacZ)M15/recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rK− mK+) supE44 relA1 lac | Stratagene | |
| RP4182 | dam dcm | A. Bhagwat | |
| R. capsulatus | |||
| MT1131a | crtD121 Rifr | Wild type, Nadi+ Ps+ | 47 |
| Y262 | GTA overproducer | 55 | |
| MD1 | Δ(ccdA::spe) | Nadi− Ps− | 13 |
| MD1-Rev1A | Ps+ revertant of MD1 obtained on MYPE | Res+ Psslow on Med A, Res− Ps+ on MPYE | This work |
| MD20 | Δ(dsbA::kan) | Res+ Ps+ on Med A | This work |
| Res− Ps+ on MPYE | |||
| MD21 | Δ(ccdA::spe dsbA::kan) | Res+ Psslow on Med A, Res− Ps+ on MPYE | This work |
| MD22 | Δ(dsbB::kan) obtained on Med A under respiratory growth conditions | Res+ Ps+ on Med A, Res+ Ps+ on MPYE | This work |
| MD23 | Δ(dsbB::kan) obtained on Med A under photosynthetic growth conditions | Res+ Ps+ on Med A, Res− Ps+ on MPYE | This work |
| MD24 | Δ(ccdA::spe dsbB::kan) obtained on Med A under respiratory growth conditions | Res+ Psslow on Med A, Res+ Psslow on MPYE | This work |
| ST1, ST6, and ST7 | MD1 revertants selected on Med A under photosynthetic growth conditions | Res+ Ps+ on Med A | This work |
| ST11, ST12, ST14, and ST18 | MD1 revertants selected on MPYE under photosynthetic growth conditions | Res+ Ps+ on Med A | This work |
| MD1R1.13C | Ps+ revertant selected from MD1/pDsbAWT and subsequently cured of its plasmid | Sper Res+ | This work |
| Plasmids | |||
| pRK2013 | Kanr, helper | 15 | |
| pRK404 | Tetr | 15 | |
| pRK415 | Tetr | 27 | |
| pCHB500 | Tetr | 5 | |
| pHPΩ45-Km | Kanr | 40 | |
| pMA117 | Kanr | 10 | |
| pBluescript | pBluescript II KS(+) | Ampr | Stratagene |
| pTC4-1 | 6.2-kb noncontiguous chromosomal DNA fragments containing R. capsulatus dsbA in pRK415 | Tetr | This work |
| pTC4-1A | 4.5-kb EcoRI fragment of pTC4-1 in EcoRI site of pRK415 | Tetr | This work |
| pTC4-1B | pTC4-1A without the 4.0-kb BamHI fragment | Tetr | This work |
| pTC4-1C4 | 2.5-kb PstI fragment of pTC4-1 in PstI site of pRK415 | Tetr | This work |
| pTC4-1D | pTC4-1 without the 2.5-kb PstI fragment | Tetr | This work |
| pTC4-1E | pTC4-1C4 without the 1,045-bp BamHI-BglII fragment | Tetr | This work |
| pTC4-1G | 2.5-kb PstI fragment of pTC4-1C4 in the PstI site of pBluescript | Ampr | This work |
| pTC4-1H | 521-bp BglII-SfiI fragment of pTC4-1G replaced with the 2.2-kb kanamycin resistance cassette | Ampr Kanr | This work |
| pTC4-1K | 4.2-kb insert of pTC4-1H in XbaI and KpnI sites of pRK415 | Tetr Kanr | This work |
| pBS-dsbAWT | 700-bp PCR product containing wild-type dsbA cloned into the SmaI site of pBluescript | Ampr | This work |
| pDsbAWT | 700-bp XbaI and KpnI fragment of pBS-dsbAWT cloned into the corresponding sites of pCHB500 | Tetr | This work |
| pBS-dsbBWT | 1.4-kb PCR product containing wild-type dsbB cloned into the XbaI and KpnI site of pBSII | Ampr | This work |
| pDsbBWT | XbaI and KpnI fragment of pBS-dsbBWT cloned into the respective sites of pRK415 | Tetr | This work |
| pDA2 | The Kanr cartridge of pMA117 inserted in the unique PstI site of pBS-dsbBWT; Kanr and dsbB transcribed in opposite directions | Ampr | This work |
| pDA4 | XbaI and KpnI fragment of pDA2 cloned into sites of pRK4151 | Tetr | This work |
R. capsulatus MT1131 is referred to as the wild type because it is the wild type with respect to its Cyt c profile and growth properties. All R. capsulatus strains, except Y262, are derivatives of MT1131, which was originally isolated as a green derivative of R. capsulatus SB1003 (47).
Psslow, slow growth under photosynthetic conditions.
Molecular genetic techniques.
Standard molecular genetic techniques were performed as described by Sambrook et al. (44). Conjugal transfer of plasmids from E. coli to R. capsulatus and interposon mutagenesis via the gene transfer agent (GTA) (55) by using the gene cassette (from pHP45Ω [40] or pMA117 [10]) conferring resistance to kanamycin (Kanr) were performed as described by Daldal et al. (10).
For the preparation of a genomic library, EcoRI-digested chromosomal DNA of R. capsulatus strain MD1-Rev1A, grown under photosynthetic conditions in enriched medium, was conjugated into MD1 to yield pTC4-1, which contained two noncontiguous EcoRI fragments of 4.5 and 1.7 kb (Table 1). The 4.5-kb EcoRI fragment of pTC4-1 was subcloned into the EcoRI site of pRK415 to generate pTC4-1A. The 4.0-kb BamHI fragment of pTC4-1 was deleted to yield pTC4-1B, and the removal of the 2.5-kb PstI fragment from pTC4-1A produced pTC4-1D. The 2.5-kb PstI fragment was ligated to a PstI-cut pRK415 to generate pTC4-1C4, and the 1,045-bp BamHI-BglII fragment of pTC4-1C4 was removed to generate pTC4-1E. The 2.5-kb PstI fragment of pTC4-1C4 was subcloned into the PstI site of pBluescript to yield pTC4-1G. To create a deletion-insertion allele of dsbA, the 521-bp BglII-SfiI fragment of dsbA on pTC4-1G was removed and the ends of the remaining 5-kb fragment were made blunt and ligated to the 2.2-kb BamHI-cut and blunt-ended kanamycin resistance gene cassette from pHP45ΩKan, yielding plasmid pTC4-1H. The 4.2-kb XbaI-KpnI insert of pTC4-1H carrying the dsbA::kan allele was then cloned into the corresponding sites of pRK415 to give pTC4-1K. Finally, a 713-bp PCR product containing a wild-type dsbA was amplified from a wild-type chromosomal library of R. capsulatus MT1131 (24) by using the primers DsbA-Seq-F (5′-CCA GAC GGC GGG ACG AGC-3′) and DsbA-Seq-R (5′-GCA CCG CTT CTC AAG GCC-3′). The PCR product was cloned into the SmaI site of pBluescript to yield pBS-dsbAWT and then excised with XbaI and KpnI and cloned into the same sites of pCHB500 (5) to yield pDsbAWT.
A 1.5-kb DNA fragment containing the entire dsbB gene was PCR amplified similarly by using the primers RRC01454F (5′-CAC CGG TAC CAG CTC GTT CTT TCC GAT CCC GG-3′) and RRC01454R (5′-AAG TCT AGA CCA GCG CGT CAT AGA TCG CCG CGT CG-3′) containing XbaI and KpnI sites, respectively, engineered at their 5′ ends. The PCR product was digested with XbaI and KpnI and cloned into the respective sites of pBSII and pCHB500 to yield pBS-dsbBWT and pDsbBWT, respectively. To create a null allele of the dsbB gene, pBS-dsbBWT was cut at the unique PstI site within dsbB and the 1.6-kb SalI fragment of pMA117 carrying the kanamycin resistance cartridge was ligated, after the ends of these fragments were blunted with T4 DNA polymerase, to yield pDA2. The XbaI-KpnI fragment of pDA2, in which kan and dsbB were oriented in opposite directions, was cloned into the respective sites of pRK415 to yield pDA4, which was then used for GTA-mediated interposon mutagenesis as described above to create a DsbB-null mutant.
Chromosomal DNA of dsbA-independent revertant strains was isolated by using the Qiagen DNEasy kit, and dsbA and dsbB were amplified by PCR by using the primers DsbA-Seq-F and DsbA-Seq-R and DsbB-F (5′-CCC CGA TCG CCA GCT TTG TC-3′) and DsbB-R (5′-ACG GCG AAT GGT CTG TCC GG-3′), respectively. The PCR products were cleaned with the Qiagen PCR purification kit and either directly sequenced by using appropriate primer pairs or cloned into a plasmid by using Invitrogen's TOPO cloning kit and sequenced by using universal primers.
DNA sequence analysis.
Automated DNA sequencing with the Big-Dye terminator cycle sequencing kit (AmpliTaq FS; Applied Biosystems) was performed as specified by the manufacturer by using the primers DsbA-Seq-F and DsbA-Seq-R, DsbA-Seq-F1 (5′-TGA GCC GCG AAA AGA TCC-3′), and DsbB-F and DsbB-R as well as the M13 forward and reverse primers. DNA sequence analyses and homology searches were done by using MacVector (IBI; Kodak) and BLAST (1) softwares. The programs TmPred (22), SignalP (38), and Clustal W (52) were used to predict the positions of the transmembrane helices and signal peptidase cleavage sites and align the amino acid sequences, respectively.
Biochemical techniques.
Cells were grown in Med A under respiratory growth conditions, and intracytoplasmic membrane vesicles (chromatophores) were prepared in 10 mM Tris buffer (pH 7.5) by using a French pressure cell as described earlier (21). Protein concentrations were determined by the method of Lowry and Rosebrough (33). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 16.5% acrylamide gels as described by Schägger and von Jagow (45) to separate various c-type Cyts which were subsequently revealed via their endogenous peroxidase activity by using either 3,3′,5,5′-tetramethylbenzidine (TMBZ) (51) or O-dianisidine (46) as the substrate. Cyt c oxidase activity of the colonies was detected with the reaction α-naphthol + dimethylphenylenediamine → indophenol blue stain as described previously (28).
Chemicals.
All chemicals were of reagent grade and obtained from commercial sources.
RESULTS
Ps+ revertants of R. capsulatus CcdA-null mutant MD1.
The R. capsulatus CcdA-null mutant MD1 [Δ(ccdA::spe)] is Ps− Nadi− due to its inability to synthesize multiple c-type Cyts. However, it reverts frequently to Ps+ or Nadi+ phenotypes under either photosynthetic or respiratory growth conditions on either enriched (MPYE) or minimal (Med A) growth medium (e.g., at a frequency of about 10−5 to 10−6 for the Ps+ phenotype on MPYE medium) (13). To gain further insight into the biogenesis of c-type Cyts, Ps+ revertants were isolated on enriched medium under photosynthetic growth conditions. These revertants grew, though less vigorously than the wild-type strain MT1131, under photosynthetic growth conditions on both media (Ps+ on MPYE and Ps+ on Med A) and under respiratory growth conditions on minimal medium (Res+ on Med A). They exhibited a weaker Nadi+ phenotype (Nadislow) than the wild-type strain MT1131 (Table 2). Moreover, unlike those of MD1, their respiratory growth abilities were compromised on enriched medium (Res− on MPYE), and an additional mutation(s) (at a frequency of about 10−6) that improved their respiratory growth abilities was also observed. One of the revertants, MD1-Rev1A, was characterized further.
TABLE 2.
Growth phenotypes of the Ps+ revertants of a CcdA-null mutant
| Strain | Medium for Ps+ selectiona | Growth under photosynthetic conditions inb:
|
Growth under respiratory conditions inb:
|
||
|---|---|---|---|---|---|
| MPYE | Med A | MPYE | Med A | ||
| MT1131 | NA | ++ | ++ | ++, Nadi+ | ++, Nadi+ |
| MD1 | NA | − | − | ++, Nadi− | ++, Nadi− |
| MD1-Rev1A | MPYE | + | ± | − | +, Nadislow |
| MD1-Rev1B | MPYE | + | ± | − | +, Nadislow |
| MD21 | NA | + | ± | − | +, Nadislow |
NA, not applicable.
++, +, ±, and − indicate semiquantitatively the strength of growth under these conditions.
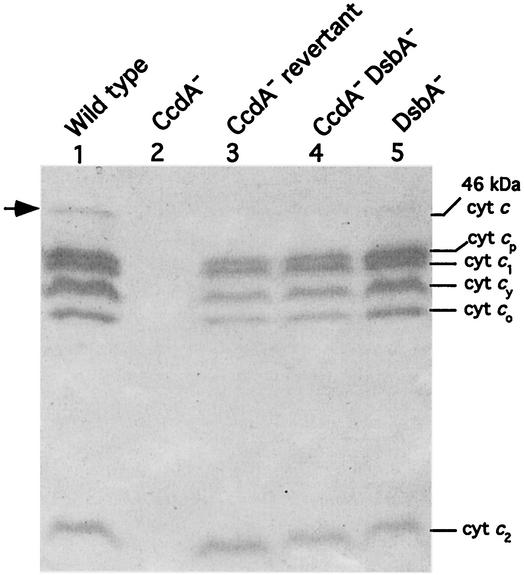
The c-type Cyt profiles of chromatophore membranes from appropriate strains grown by respiration in minimal medium were analyzed by TMBZ staining and SDS-PAGE (Fig. 1). Under these conditions, six distinct heme-staining bands, with molecular masses of 46, 32, 31, 29, 28, and 12 kDa, were readily detected in chromatophore membranes of the wild-type strain MT1131 (Fig. 1, lane 1). Of these, the 31-kDa protein is the Cyt c1 subunit of the Cyt bc1 complex (24), the 29-kDa protein is the membrane-associated electron carrier Cyt cy (23), the 12-kDa protein is the soluble Cyt c2 (10), and the remaining 32- and 28-kDa Cyt cp and Cyt co, respectively, correspond to the heme c-containing subunits of the Cyt cbb3 oxidase (21). The remaining 46-kDa band may correspond to the pentaheme c-type Cyt DorC, involved in electron transfer to the dimethyl sulfoxide (DMSO) reductase required for DMSO-dependent anaerobic dark growth of R. capsulatus (48, 49). Indeed, none of these c-type Cyts were present in the CcdA-null mutant MD1 (Fig. 1, lane 2) (13), but almost all of them (with the exception of the 46-kDa c-type Cyt) were detectable in MD1-Rev1A (Fig. 1, lane 3). Noticeably, the amounts of the c-type Cyts produced in the CcdA-null revertant MD1-Rev1A were lower than those seen in the wild-type strain MT1131, in agreement with its less vigorous Ps+ and Nadislow phenotypes.
FIG. 1.
c-type Cyt profiles of the revertant of MD1 and dsbA mutants. Chromatophore membranes of R. capsulatus strains grown in minimal medium Med A under respiratory conditions were subjected to SDS-PAGE as described by Schägger and von Jagow (45). Approximately 70 μg of protein was loaded in each lane, and after electrophoresis, c-type Cyts were detected by TMBZ staining (51). The heme-staining band at 46 kDa probably corresponds to the pentaheme c-type Cyt DorC (48, 49), involved in electron transfer to the DMSO reductase. Lanes 1 to 5 correspond to the R. capsulatus strain MT1131 (wild type), the CcdA− mutant MD1 [Δ(ccdA::spe)], the Ps+ revertant of MD1 (MD1-Rev1A), the CcdA− DsbA− double mutant MD21 [Δ(ccdA::spe) Δ(dsbA::kan)], and the DsbA− mutant MD20 [Δ(dsbA::kan)], respectively.
Genetic complementation of MD1 with plasmid library constructed by using MD1-Rev1A chromosomal DNA.
In order to uncover directly the molecular nature of the reversion mutation in MD1-Rev1A, complementation of MD1 with a transferable genomic library constructed by using chromosomal DNA of MD1-Rev1A was attempted. However, despite repeated efforts, no clone that could complement MD1 for the Ps+ phenotype was obtained, suggesting that the reversion mutation in MD1-Rev1A might be recessive with respect to its wild-type allele. We reasoned that if this is the case, then a plasmid carrying the suppressor allele might increase the frequency of Ps+ revertants of MD1 by allele exchange via homologous recombination between the plasmid-borne mutant allele and the chromosomal wild-type allele. The transconjugants were screened for the increased Ps+ reversion phenotype by being shifted from respiratory to photosynthetic growth conditions, and three such derivatives were identified. Remobilization of the plasmids from the Ps+ transconjugants into MD1 confirmed the observed phenotype, and restriction analyses indicated that these plasmids contained identical inserts composed of two EcoRI fragments of 4.5 and 1.7 kb. One of these plasmids, pTC4-1, was then retained for further analyses (Fig. 2).
FIG. 2.
Plasmid pTC4-1 and its derivatives. The two EcoRI fragments of pTC4-1 are noncontiguous and originate from two different regions of the R. capsulatus chromosome, with the 1.7- and 4.5-kb EcoRI fragments being from the contig 1D09-1F02 and the contig 1F12-2G06, respectively, and plasmid pTC4-1A carries the latter EcoRI fragment only. The abilities of various subclones to confer to MD1 increased Ps+ reversion phenotype, as described in the text, are indicated on the right. Plasmids pTC4-1K and pDsbAWT were generated as described in Materials and Methods, and they contain a dsbA::kanr allele and a wild-type dsbA expressed from PcycA (indicated as Pc2), which is the promoter of the structural gene of Cyt c2, respectively. The identities of the ORFs are as follows: ORF1860, ORF1862, and ORF1863 are gvpF, gvpJ, and gvpO, respectively, encoding proteins involved in the synthesis of gas vesicles; ORF1861 encodes a 144-residue polypeptide with no similarity to known proteins; ORF3148 is kdtA and encodes deoxy-d-manno-octulosonic acid transferase; ORF3149 is dsbA and encodes dithiol:disulfide oxidoreductase; ORF3150 is ycaH and encodes tetraacyldisaccharide 4′-kinase; ORF3151 encodes a hypothetical cytosolic protein with no homology to known proteins; ORF3152 is mutY and encodes A/G-specific adenine glycosylase; ORF3153 is alkB and encodes alkylated DNA repair protein AlkB; and ORF3154 is dnaK and encodes DnaK (Hsp70). E, EcoRI; B, BamHI; P, PstI; Bg, BglII; H, HindIII; D, Δ; KmR, kanamycin resistance gene.
DNA sequence analysis of pTC4-1.
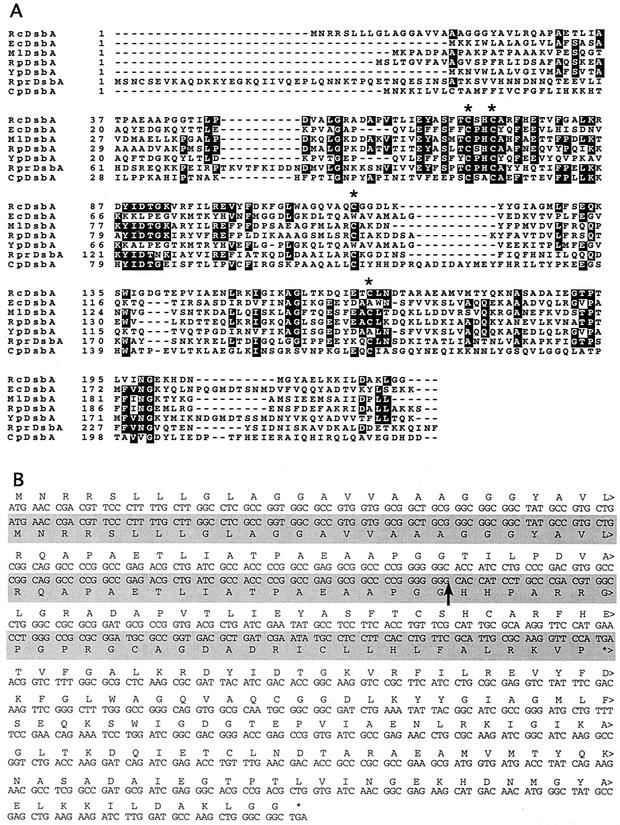
Determination of the DNA sequences of the ends of the EcoRI fragments and comparison to the R. capsulatus genome sequence (Integrated Genomics, Inc.) revealed that these fragments were not contiguous on the genome. The 4.5-kb fragment was part of the 1F12-2G06 contig and contained five complete (ORF3149 through ORF3153) and two incomplete (ORF3148 and ORF3154) open reading frames (ORFs). On the other hand, the 1.7-kb fragment belonged to the 1D09-1F02 contig and had three complete ORFs (ORF1860 to ORF1862) and one incomplete ORF (ORF1863) (Fig. 2). To determine which of the two regions mediated the increased Ps+ reversion phenotype, subclones of pTC4-1 retaining either the 4.5-kb region (pTC4-1A) or the 1.7-kb region (pTC4-1B) were obtained and mobilized into MD1. Only pTC4-1A conferred the increased Ps+ reversion phenotype, and subsequent subclonings (Fig. 2) established that pTC4-1E, which carried only ORF3149 and ORF3151, was sufficient for this phenotype. ORF3151 corresponded to a hypothetical protein with no known homologue, but interestingly, ORF3149 was homologous to dsbA, encoding a dithiol:disulfide oxidoreductase present in various bacteria and known to be involved in the biogenesis of c-type Cyts in E. coli (35). The R. capsulatus DsbA homologue is a 220-amino-acid-long polypeptide with a putative signal sequence and a signal peptidase cleavage site, suggesting that it is also a periplasmic protein like its E. coli counterpart (Fig. 3).
FIG. 3.
R. capsulatus DsbA and its mutant allele. (A) The deduced protein sequence of R. capsulatus DsbA is aligned with those of DsbA proteins from E. coli (Ec), Mesorhizobium loti (Ml), Rhodopseudomonas palustris (Rp), Yersinia pestis (Yp), Rickettsia prowazekii (Rpr), and Chlamydia pneumoniae (Cp). The conserved cysteines in the thioredoxin fold as well as the two additional cysteines are indicated by asterisks. Black and grey boxes correspond to completely and partially conserved residues, respectively. (B) The nucleotide sequences of the dsbA alleles cloned from the R. capsulatus wild-type strain and a Ps+ revertant of the CcdA-null mutant, MD1-Rev1A, are shown. The sequence obtained from MD1-Rev1A is shaded in grey. The deduced protein sequence is also indicated either above (for the sequence obtained from the wild-type strain) or below (for the sequence obtained from MD1-Rev1A) the respective DNA sequence. Note that the dsbA allele cloned from a Ps+ revertant of the CcdA-null mutant, MD1-Rev1A, contains an additional guanine (G) residue (indicated by an arrow) after bp 135 in the coding region of dsbA. This insertion produces a frameshift mutation after amino acid residue 44 of DsbA to yield a 77-residue-long truncated product.
The dsbA allele on pTC4-1 has a frameshift mutation.
The DNA fragment carried by pTC4-1E was sequenced completely, and the sequence was compared with that available on the R. capsulatus genome database to define the nature of the mutation responsible for the Ps+ phenotype of MD1-Rev1A. This comparison revealed that the dsbA allele on pTC4-1E encoded an extra guanine residue at position 135, predicted to cause a frameshift leading to the production of a truncated, nonfunctional DsbA with only 44 N-terminal amino acid residues (Fig. 3). This finding suggested that replacement of the chromosomal wild-type dsbA allele by a plasmid-borne inactive dsbA allele via homologous recombination might be the basis for the increased Ps+ reversion phenotype. To prove that this is indeed responsible for the increased Ps+ phenotype, plasmid pTC4-1K carrying an insertion-deletion allele of dsbA [Δ(dsbA::kan)] was constructed (Fig. 2), and, as expected, pTC4-1K was shown to confer a similar increased Ps+ phenotype upon its introduction into MD1.
A CcdA-null DsbA-null double mutant is proficient in the biogenesis of c-type Cyts.
To demonstrate that inactivation of dsbA is responsible for the Ps+ Nadi+ phenotype of MD1-Rev1A, a ccdA dsbA double mutant was constructed. Introduction of the Δ(dsbA::kan) allele into the chromosome of the CcdA-null mutant MD1 via GTA yielded MD21 [Δ(dsbA::kan) Δ(ccdA::spe)], which exhibited the same phenotypes as MD1-Rev1A (i.e., Ps+ Res− on MPYE and Ps+ Res+ Nadislow on Med A). Furthermore, the c-type Cyt profile of MD21 as revealed by TMBZ and SDS-PAGE analyses was identical to that of MD1-Rev1A in that it exhibited lower amounts of all c-type Cyts than the wild type and lacked the 46-kDa c-type Cyt (Fig. 1, lane 4). These data therefore clearly established that loss of DsbA activity overcomes the defect inflicted by the absence of CcdA on the biogenesis of c-type Cyts in R. capsulatus.
A DsbA-null mutant of R. capsulatus is also proficient in the biogenesis of c-type Cyts but is impaired in respiration especially on enriched medium.
A DsbA-null mutant of R. capsulatus was obtained by introducing the Δ(dsbA::kan) allele into the chromosome of the wild-type strain MT1131 by selecting for Kanr colonies under both photosynthetic and respiratory growth conditions and on both enriched and minimal growth media. In all but enriched medium under respiratory growth conditions, Kanr derivatives were obtained, and one such derivative, MD20 [Δ(dsbA::kan)], was analyzed further. The DsbA-null mutant MD20 was Ps+ Nadi+ Res− on enriched medium and produced all c-type Cyts like MD1-Rev1A and MD21 (Fig. 1, lane 5). Moreover, all DsbA− strains (MD1-Rev1A, MD20, and MD21) reverted (at a frequency of roughly 10−6) to overcome the respiratory growth impairment. These findings indicated that the Res− growth defect encountered in MD1-Rev1A and MD21 was apparently due to the loss of dsbA, indicating that DsbA activity must be important for the growth of R. capsulatus under respiratory conditions on enriched medium. Unlike that in R. capsulatus, DsbA in E. coli is required for production of c-type Cyts (34, 43), and apparently E. coli DsbA− mutants do not exhibit any significant growth defect.
dsbA can suppress the Ps+ Res− phenotypes of MD1-Rev1A and MD21.
To further confirm that the Ps+ Res− phenotype seen in MD1-Rev1A, MD20, and MD21 is due to the absence of dsbA, the wild-type allele of R. capsulatus dsbA was cloned as described in Materials and Methods. Plasmid pDsbAWT thus obtained (Fig. 2) was conjugated into the Ps+ revertants, MD1-Rev1A and MD1-Rev1B, and the CcdA-null DsbA-null double mutant MD21 (Table 2). The transconjugants thus obtained were found to be Ps− Res+ Nadi− on enriched medium, like MD1. Thus, dsbA reversed fully the Ps+ Res− phenotype of both MD1-Rev1A and MD21, further confirming that these phenotypes encountered in the ccdA suppressor strains, and also in MD20 [Δ(dsbA::kan)], were consequences of the inactivation of dsbA.
dsbA-independent suppressors of a CcdA-null mutant.
The high frequency of reversion of the CcdA-null mutant MD1 [Δ(ccdA::spe)] hinted that other suppressors of ccdA in addition to dsbA might also exist. Moreover, as the reduction-oxidation cycle of DsbA involves its membrane-bound partner DsbB (8), it seemed reasonable that mutations in dsbB might also bypass the lack of CcdA. Thus, the ability of the wild-type dsbA to suppress the Ps+ phenotype of ccdA dsbA double mutants was exploited to isolate dsbA-independent Ps+ revertants of MD1 via two different approaches. First, 16 Ps+ revertants of MD1 on minimal medium Med A and 16 on enriched medium MPYE were isolated and screened for their ability to retain the Ps+ phenotype upon receiving pDsbAWT. This yielded seven revertants (ST1, ST6, and ST7, initially isolated on Med A, and ST11, ST12, ST14, and ST18 on MPYE medium) that still retained their Ps+ phenotype upon receiving pDsbAWT (Table 3). In the second approach, Ps+ revertants were isolated directly from MD1/pDsbAWT that carried multiple copies of dsbA in order to increase the chance of obtaining dsbA-independent suppressors. Several such revertants were found, and one of them, MD1R1.13C, that retained the Ps+ phenotype upon curing of pDsbAWT was retained.
TABLE 3.
Characterization of dsbA-independent revertants of a CcdA-null mutanta
| Strainb | Phenotype on Med A | Mutation in dsbB |
|---|---|---|
| ST1 | Ps+ Res+ | 12-bp deletion |
| ST6 | Ps+ Res+ | 1-bp insertion |
| ST7 | Ps+ Res+ | 1-bp insertion |
| ST11 | Ps+ Res+ | 88-bp deletion |
| ST12 | Ps+ Res+ | 88-bp deletion |
| ST14 | Ps+ Res+ | 90-bp deletion |
| ST18 | Ps+ Res+ | Deletion of undefined length |
| MD1R1.13C | Ps+ Res+ | 1-bp deletion |
| MD24 (ccdA dsbB) | Ps+ Res+ | dsbB::kan |
Ps+ revertants of the CcdA-null mutant MD1 were obtained as described in Materials and Methods and tested for their ability to have their Ps+ phenotype suppressed by pDsbAWT or pDsbBWT carrying wild-type alleles of dsbA or dsbB, respectively. pDsbAWT did not suppress the phenotype and pDsbBWT did so only transiently for all the strains listed except MD24, which was not tested in this experiment.
The nucleotide sequences of the dsbA loci in all strains listed have also been determined and found to be identical to that of the wild-type locus.
Characterization of dsbA-independent suppressors of MD1.
First, the dsbB locus was amplified by PCR by using chromosomal DNA from these suppressors and appropriate primers as described in Materials and Methods. The PCR fragments thus obtained showed differences in size (data not shown) indicating that some of the revertants carried deletions within the amplified region. DNA sequence analysis of the PCR products established that all dsbA-independent Ps+ revertants carried insertion or deletion mutations in dsbB and had unaltered dsbA alleles (Table 3).
Next, the ability of dsbB to suppress the Ps+ phenotype of the dsbA-independent revertants of MD1 was tested by cloning dsbB as described in Materials and Methods and conjugating pDsbBWT thus obtained into these revertants. While pDsbAWT suppressed the Ps+ phenotype of DsbA− revertants of CcdA− mutants, pDsbBWT suppressed the phenotype of the dsbA-independent suppressors tested (Table 3). However, the suppression mediated by pDsbBWT was transient, unlike that mediated by pDsbAWT, and upon prolonged incubation these strains exhibited Ps+ growth ability.
A CcdA-null DsbB-null double mutant is proficient in the biogenesis of c-type Cyts.
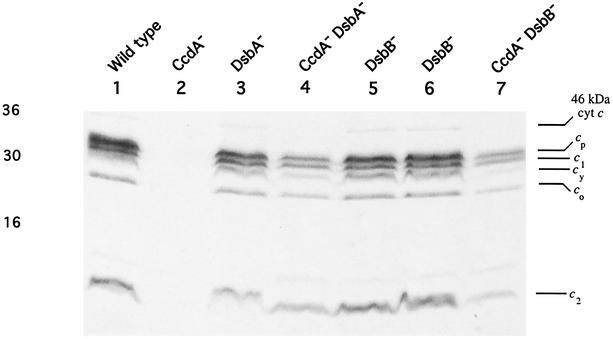
To demonstrate that the absence of DsbB also suppresses the lack of CcdA during the biogenesis of c-type Cyts, a ccdA dsbB double mutant was constructed by introduction of the Δ(dsbB::kan) allele into MD1 [Δ(ccdA::spe)], as described in Materials and Methods. Kanr colonies were obtained under respiratory growth conditions, on both Med A and MPYE media, and a ccdA dsbB double mutant, MD24 (obtained on Med A under respiratory growth conditions, which appeared to be the most permissive conditions), was retained for further studies. MD24 was Ps+ Nadi+, and its c-type Cyt profile determined by heme staining and SDS-PAGE (Fig. 4, lane 7) was similar to that of the ccdA dsbA double mutant MD21 (Fig. 4, lane 4), although the amounts of c-type Cyts, especially Cyt cy and the 46-kDa Cyt c, in the ccdA suppressor strains were noticeably lower than those in a wild-type strain (Fig. 4, lane 1).
FIG. 4.
c-type Cyt profiles of the Ps+ suppressors of CcdA-null mutants and the DsbA-null and DsbB-null mutants of R. capsulatus. Chromatophore membranes of R. capsulatus strains grown in minimal medium Med A under respiratory conditions were subjected to SDS- PAGE as described by Schägger and von Jagow (45). Approximately 70 μg of protein was loaded in each lane, and after electrophoresis, c-type Cyts were detected by heme staining by using O-dianisidine (Sigma) as the substrate (46). The heme-staining band at 46 kDa probably corresponds to the pentaheme c-type Cyt DorC (48, 49), involved in electron transfer to the DMSO reductase. Lanes 1 to 7 correspond to the R. capsulatus strain MT1131 (wild type), the CcdA-null mutant MD1 [Δ(ccdA::spe)], the DsbA-null mutant MD20 [Δ(dsbA::kan)], the CcdA-null DsbA-null double mutant MD21 [Δ(ccdA::spe) Δ(dsbA::kan)], the DsbB-null mutants MD22 and MD23 (both dsbB::kan), and the CcdA-null DsbB-null double mutant MD24 [Δ(ccdA::spe) (dsbB::kan)], respectively.
A DsbB-null mutant of R. capsulatus is also proficient in the biogenesis of c-type Cyts.
Finally, to probe whether or not dsbB is involved in the biogenesis of c-type Cyts, the Δ(dsbB::kan) allele was introduced into the chromosome of the wild-type strain MT1131 by using GTA. The DsbB-null mutants obtained on Med A under respiratory or photosynthetic growth conditions were termed MD22 or MD23, respectively. Like the DsbA-null mutant MD20, both MD22 and MD23 were Ps+ Nadi+ and they produced all c-type Cyts, including Cyt cy and the 46-kDa Cyt c, at near-wild-type amounts as indicated by heme staining and SDS-PAGE analyses (Fig. 4, lanes 1, 5, and 6). Moreover, the DsbB-null mutants were less affected than the DsbA-null mutants under respiratory growth conditions on enriched medium. The overall data therefore established that under the growth conditions tested neither DsbA nor DsbB is required for c-type Cyt biogenesis in R. capsulatus.
DISCUSSION
Our previous work has shown that R. capsulatus CcdA-null mutants are unable to produce c-type Cyts unless an exogenous source of reduced chemicals is provided, yet they can frequently regain the ability to produce c-type Cyts independently of CcdA (13). In this work, we have exploited this observation to further dissect cellular mechanisms that supply reducing equivalents during the biogenesis of c-type Cyts in R. capsulatus. R. capsulatus CcdA and its partial homologue E. coli DsbD are evolutionarily related membrane proteins involved in conveying reducing equivalents from the cytoplasm to the periplasm. However, while R. capsulatus CcdA is required only for the biogenesis of c-type Cyts, E. coli DsbD conveys reducing equivalents both to CcmG for the biogenesis of c-type Cyts and to DsbC for disulfide bond isomerization pathways (25, 31). Indeed, it was recently shown that E. coli dsbD can complement an R. capsulatus CcdA− mutant and that R. capsulatus CcdA can substitute functionally for E. coli DsbD only with respect to c-type Cyt biogenesis (26).
In spite of the functional similarities, many bacterial genomes, including that of R. capsulatus, contain several ccdA- and dsbD-like genes. A search to identify any plausible candidate gene that might act as a suppressor of ccdA in the R. capsulatus genome sequence indicated the presence of a gene (ORF409, herein referred to as ccdA-resembling gene or crgA) whose product, CrgA, is highly homologous to E. coli DsbD and R. capsulatus CcdA (Fig. 5). The alignment of these three integral membrane proteins indicated that they not only share highly similar topologies but also have conserved Pro-Cys doublets in their appropriate transmembrane helices. In particular, the N-terminal part of CrgA aligns well with R. capsulatus CcdA and the β domain of E. coli DsbD (Fig. 5). On the other hand, CrgA does not have an N-terminal α domain similar to that of E. coli DsbD, and its last two transmembrane helices (Fig. 5) and its C-terminal hydrophilic part are clearly different. The presence of both ccdA and crgA in the R. capsulatus genome and their striking homologies to E. coli DsbD raised the question of whether or not CrgA plays a role in the biogenesis of c-type Cyts. However, CrgA-null mutants still produced c-type Cyts (data not shown), indicating that CrgA is not required for the biogenesis of c-type Cyts in R. capsulatus and that apparently ccdA and crgA belong to different physiological redox pathways.
FIG. 5.
Alignment of R. capsulatus CrgA (Rc CrgA) with R. capsulatus CcdA (Rc CcdA) and E. coli DsbD (Ec DsbD). Amino acid alignment shows similarities between R. capsulatus CrgA and DsbD polypeptides from Haemophilus influenzae (hiDsbD), Pseudomonas aeruginosa (paDsbD), and E. coli, as well as between R. capsulatus CrgA and R. capsulatus CcdA. Predicted or experimentally confirmed membrane topologies of R. capsulatus CrgA, R. capsulatus CcdA, and E. coli DsbD are also aligned, showing the location of the transmembrane helices (TM). Amino acid sequences of TM7 and TM8 of R. capsulatus CrgA and E. coli DsbD are nonhomologous and are thus indicated in different shades of grey. The conserved cysteine and proline residues are indicated with filled circles and asterisks, respectively, and the numbers indicate the amino acid residues in each protein shown. Black and grey boxes correspond to completely and partially conserved residues, respectively. C, C terminus; N, N terminus.
Our search for the molecular nature of the extragenic suppressor encountered in a CcdA-null mutant led us to R. capsulatus dsbA and dsbB. Interestingly, these suppressors turned out to be loss-of-function mutations due to insertions or deletions in dsbA or dsbB (Fig. 3 and Table 3). That the inactivation of either DsbA or DsbB can bypass the need for CcdA during the biogenesis of c-type Cyts was directly demonstrated by the construction of bona fide CcdA-null DsbA-null (MD21) and CcdA-null DsbB-null (MD24) double mutants. These double mutants were found to be Ps+ Nadi+ and able to produce almost all c-type Cyts (Fig. 4), albeit at amounts lower than those seen in a wild-type strain of R. capsulatus, except the 46-kDa Cyt. The identity of this latter Cyt c is unknown, but as both MD21 and MD24 mutants grew extremely poorly under anaerobic dark growth conditions in the presence of DMSO, it might be the product of dorC. If indeed this is the case, then this observation suggests that the biogenesis of DorC, which is a structurally complex pentaheme Cyt (48), might be more complicated.
In E. coli, DsbA is required for both disulfide bond formation (2) and c-type Cyt biogenesis in the periplasm (34). Its inactivation can be overcome by the loss of DsbD (36), suggesting that the absence of either DsbD (a reductase) or DsbA (an oxidase) perturbs periplasmic redox homeostasis while that of both activities reestablishes a new redox balance. In R. capsulatus, the converse of the E. coli situation was observed; that is, the loss of CcdA was counterbalanced by the inactivation of DsbA. The strong periplasmic oxidant DsbA is thought to oxidize the cysteine thiols to disulfides as all proteins (2), including apoCyts (43), enter the periplasm. Upon oxidizing its substrate protein, the reduced and hence catalytically inactive DsbA needs to be reoxidized by DsbB, which links the periplasmic disulfide bond formation pathway to the electron transport chain (8). Thus, inactivation of DsbB would also render DsbA nonfunctional by hampering its catalytic turnover. In the absence of CcdA, a disulfide bond at the apoCyt heme-binding site initially catalyzed by the DsbA-DsbB pathway might remain intact and block heme ligation. If so, then this obstacle can be overcome by inactivation of either DsbA or DsbB, which presumably will leave these cysteines reduced and competent for heme ligation (Fig. 6). Consistent with these findings, multiple copies of dsbA or dsbB can suppress only the dsbA-dependent or the dsbB-dependent revertants of the CcdA-null mutants, respectively. Interestingly, while the suppression mediated by dsbA was permanent, that mediated by dsbB was transient, as such merodiploids regained their Ps+ phenotype upon prolonged incubation. Moreover, while a DsbB-null mutant that carried a cycA::phoA fusion formed blue colonies, like a wild-type strain, on plates containing the chromogenic substrate 5-bromo-4-chloro-3-indolylphosphate (XP) under both aerobic and anaerobic growth conditions, a DsbA-null mutant was able to do so only under photosynthetic conditions. These observations suggest that an additional way of recycling reduced DsbA under anaerobic growth conditions might exist in R. capsulatus.
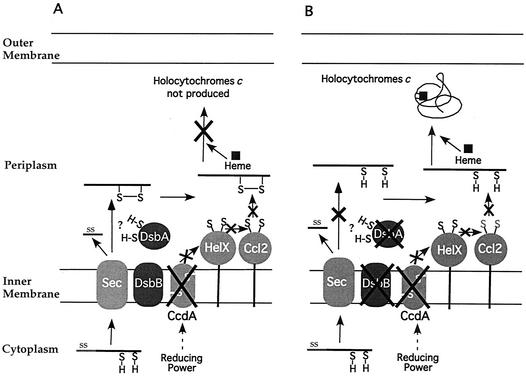
FIG. 6.
A model to illustrate how the inactivation of DsbA or DsbB could restore the biogenesis of c-type Cyts in a CcdA-null mutant of R. capsulatus. Apo-Cyts are translocated across the membrane by the Sec-dependent general secretory pathway (Sec). Upon entry into the periplasm, their signal sequences (SS) are cleaved by the signal peptidase (not shown). A disulfide bond (S—S) between the heme-binding cysteines (S=H) is thought to be formed by the DsbA-DsbB system as the apo-Cyts emerge into the periplasm. This disulfide bond must be subsequently reduced for the apo-Cyts to be competent for heme ligation. The reducing power required for this process is thought to be shuttled across the membrane via CcdA and then relayed through HelX and Ccl2 to the apo-Cyts. In the absence of CcdA and the presence of DsbA-DsbB (panel A), the cysteines in the heme-binding site of the apo-Cyts form a disulfide bond and thus remain inaccessible for heme ligation. When either DsbA or DsbB is absent in addition to CcdA (panel B), the cysteines in the heme-binding site remain reduced and hence available for heme ligation. For the sake of simplicity, other proteins involved in the biogenesis of c-type Cyts in R. capsulatus are not shown. A more complete illustration of this process is presented elsewhere (14).
While this work was in progress, Erlendsson and Hederstedt (16) reported that inactivation of DsbA or DsbB could also suppress the Cyt c deficiency of the CcdA mutants in Bacillus subtilis. Biogenesis of c-type Cyts in the gram-positive bacterium B. subtilis proceeds by system II, unlike that in R. capsulatus, which proceeds by system I (30). However, the finding that in both of these species either dsbA or dsbB can act as a suppressor of ccdA suggests that in both biogenesis systems electron transfer pathways across the cytoplasmic membrane may be more conserved than the other components.
Surprisingly, the DsbA-null (MD20) and DsbB-null (MD22) mutants are Ps+ Nadi+, indicating that dsbA or dsbB is not required for production of the monoheme Cyts c1, c2, cy, and co and the diheme Cyt cp in R. capsulatus. This is unlike the situation in E. coli, which naturally contains only multiheme Cyts (34). In addition, Sambongi and Ferguson (43) have observed that E. coli dsbA or dsbB mutants could not produce an exogenous monoheme c-type Cyt during anaerobic growth. Why DsbA and DsbB activities are required for the biogenesis of c-type Cyts in E. coli and not in R. capsulatus is intriguing. If the presence of a disulfide bond at the heme-binding site of the apo-Cyts is needed for their recognition by the biogenesis apparatus (34), then the R. capsulatus DsbA-null or DsbB-null mutants may form such a disulfide bond via an alternate pathway(s). Transient suppression of the Ps+ phenotype of a CcdA-null DsbB-null mutant upon introduction of multiple copies of dsbB, as well as the Ps+ phenotypes of R. capsulatus DsbA-null or DsbB-null mutants in all growth media, suggests that under anaerobic growth conditions this species might accomplish disulfide bond formation via an alternative pathway. Another possibility may be that the presence of a disulfide bond formed at the heme-binding site of apo-Cyts immediately after their entry into the periplasm is not needed for their recognition by the biogenesis apparatus but is required merely to induce their partial folding to lower their rate of proteolysis. If degradation of apo-Cyts that lack the disulfide bond in their heme-binding region is an extremely efficient process in E. coli, then DsbA and DsbB would be indispensable for Cyt c biogenesis. On the other hand, apo-Cyts might not be as quickly degraded in R. capsulatus as they are in E. coli, allowing them to bind heme in an appropriate redox environment. If so, then a requirement for DsbA and DsbB during the biogenesis of c-type Cyts could simply depend on the structures of the apo-Cyts. Future analyses of the role of the periplasmic degradation pathways in Cyt c biogenesis might shed further light on this issue.
Finally, it was noted that R. capsulatus DsbA-null mutants are Res−, especially on enriched media, and similar growth defects have also been reported for Azotobacter vinelandii mutants (37). The more pronounced respiratory growth defect on enriched media might be due to the accumulation of excess reduced thiol compounds that could aggravate the loss of an oxidizing protein like DsbA. An analogous phenomenon has also been observed with Paracoccus denitrificans ccmG-null mutants, which are postulated to reduce disulfide bonds in the periplasm, as such mutants grow poorly on enriched media containing oxidized thiol compounds (39). Consistent with these observations, the addition of 1 mM oxidized glutathione to enriched media markedly improves the growth of a DsbA-null mutant of R. capsulatus under respiratory conditions while it severely hampers that of the wild-type strain (data not shown). Considering that E. coli DsbA− mutants are defective in several cellular processes (17), the Res− growth defect encountered in R. capsulatus DsbA-null mutants suggests that DsbA may be an important disulfide bond catalyst for various components implicated in diverse cellular functions in this species as well. Interestingly, these mutants revert frequently to overcome their Res− phenotype on enriched media, and their characterization may further progress our understanding of periplasmic redox homeostasis in R. capsulatus. Whether the milder Res− phenotype of DsbB-null mutants and the transient suppression mediated by DsbB as described above indicate the presence of an additional oxidative pathway in R. capsulatus remains to be seen.
In summary, in this work we have demonstrated that in R. capsulatus inactivation of either dsbA or dsbB can allow ccdA mutants to overcome their inability to produce c-type Cyts. The suppression of the deficiency is presumably due to the restoration of a periplasmic redox state adequate for production of c-type Cyts. Furthermore, we have shown for the first time that while the DsbA-DsbB pathway is not required for the biogenesis of various c-type Cyts in R. capsulatus, it appears to be important for efficient growth of this species under respiratory conditions, especially in enriched medium.
Acknowledgments
We thank F. Katzen, J. Cooley, and C. Sanders for critical reading of the manuscript.
This work was supported by grants DOE 91ER20052 and NIH GM38237 to F.D.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1999. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bardwell, J. C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67:581-589. [DOI] [PubMed] [Google Scholar]
- 3.Beckman, D. L., and R. G. Kranz. 1993. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc. Natl. Acad. Sci. USA 90:2179-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman, D. L., D. R. Trawick, and R. G. Kranz. 1992. Bacterial cytochromes c biogenesis. Genes Dev. 6:268-283. [DOI] [PubMed] [Google Scholar]
- 5.Benning, C., and C. R. Sommerville. 1992. Isolation and genetic complementation of a sulfolipid-deficient mutant of Rhodobacter sphaeroides. J. Bacteriol. 174:2352-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biel, S. W., and A. J. Biel. 1990. Isolation of a Rhodobacter capsulatus mutant that lacks c-type cytochromes and excretes porphyrins. J. Bacteriol. 172:1321-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, J., T. Chen, and D. Missiakas. 2000. Transfer of electrons across the cytoplasmic membrane by DsbD, a membrane protein involved in thiol-disulphide exchange and protein folding in the bacterial periplasm. Mol. Microbiol. 35:1099-1109. [DOI] [PubMed] [Google Scholar]
- 8.Collet, J. F., and J. C. Bardwell. 2002. Oxidative protein folding in bacteria. Mol. Microbiol. 44:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Crooke, H., and J. Cole. 1995. The biogenesis of c-type cytochromes in Escherichia coli requires a membrane-bound protein, DipZ, with a protein disulphide isomerase-like domain. Mol. Microbiol. 15:1139-1150. [DOI] [PubMed] [Google Scholar]
- 10.Daldal, F., S. Cheng, J. Applebaum, E. Davidson, and R. Prince. 1986. Cytochrome c2 is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc. Natl. Acad. Sci. USA 83:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson, E., and F. Daldal. 1987. Primary structure of the bc1 complex of Rhodopseudomonas capsulata. Nucleotide sequence of the pet operon encoding the Rieske cytochrome b, and cytochrome c1 apoproteins. J. Mol. Biol. 195:13-24. [DOI] [PubMed] [Google Scholar]
- 12.Debarbieux, L., and J. Beckwith. 1999. Electron avenue: pathways of disulfide bond formation and isomerization. Cell 99:117-119. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh, M., G. Brasseur, and F. Daldal. 2000. Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol. Microbiol. 35:123-138. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh, M., M. May, Y. Zhang, K. K. Gabbert, K. A. Karberg, R. G. Kranz, and F. Daldal. 2002. Overexpression of ccl1-2 can bypass the need for the putative apocytochrome chaperone CycH during the biogenesis of c-type cytochromes. Mol. Microbiol. 46:1069-1080. [DOI] [PubMed] [Google Scholar]
- 15.Ditta, G., T. Schmidhauser, E. Yacobson, P. Lu, X. Liang, D. R. Finlay, D. Guiney, and D. R. Helinski. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149-153. [DOI] [PubMed] [Google Scholar]
- 16.Erlendsson, L. S., and L. Hederstedt. 2002. Mutations in the thiol-disulfide oxidoreductases BdbC and BdbD can suppress cytochrome c deficiency of CcdA-defective Bacillus subtilis cells. J. Bacteriol. 184:1423-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabianek, R. A., H. Hennecke, and L. Thöny-Meyer. 2000. Periplasmic protein thiol:disulfide oxidoreductases of Escherichia coli. FEMS Microbiol. Rev. 24:303-316. [DOI] [PubMed] [Google Scholar]
- 18.Fabianek, R. A., T. Hofer, and L. Thöny-Meyer. 1999. Characterization of the Escherichia coli CcmH protein reveals new insights into the redox pathway required for cytochrome c maturation. Arch. Microbiol. 171:92-100. [DOI] [PubMed] [Google Scholar]
- 19.Goldman, B. S., D. L. Beckman, A. Bali, E. M. Monika, K. K. Gabbert, and R. G. Kranz. 1997. Molecular and immunological analysis of an ABC transporter complex required for cytochrome c biogenesis. J. Mol. Biol. 268:724-738. [DOI] [PubMed] [Google Scholar]
- 20.Goldman, B. S., and R. G. Kranz. 2001. ABC transporters associated with cytochrome c biogenesis. Res. Microbiol. 152:323-329. [DOI] [PubMed] [Google Scholar]
- 21.Gray, K. A., M. Grooms, H. Myllykallio, C. Moomaw, C. Slaughter, and F. Daldal. 1994. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA center. Biochemistry 33:3120-3127. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann, K., and W. Stoffel. 1993. TMbase-A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 347:166. [Google Scholar]
- 23.Jenney, F. E., Jr., and F. Daldal. 1993. A novel membrane-associated c-type cytochrome, cytochrome cy, can mediate the photosynthetic growth of Rhodobacter capsulatus and Rhodobacter sphaeroides. EMBO J. 12:1283-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenney, F. E., Jr., R. C. Prince, and F. Daldal. 1994. Roles of the soluble cytochrome c2 and membrane-associated cytochrome cy of Rhodobacter capsulatus in photosynthetic electron transfer. Biochemistry 33:2496-2502. [DOI] [PubMed] [Google Scholar]
- 25.Katzen, F., and J. Beckwith. 2000. Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell 103:769-779. [DOI] [PubMed] [Google Scholar]
- 26.Katzen, F., M. Deshmukh, F. Daldal, and J. Beckwith. 2002. Evolutionary domain fusion expanded the substrate specificity of the transmembrane electron transporter DsbD. EMBO J. 21:3960-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 28.Keilin, D. 1966. The history of cell respiration and cytochromes. Cambridge University Press, Cambridge, United Kingdom.
- 29.Koch, H.-G., O. Hwang, and F. Daldal. 1998. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J. Bacteriol. 180:969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kranz, R., R. Lill, B. Goldman, G. Bonnard, and S. Merchant. 1998. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 29:383-396. [DOI] [PubMed] [Google Scholar]
- 31.Krupp, R., C. Chan, and D. Missiakas. 2001. DsbD-catalyzed transport of electrons across the membrane of Escherichia coli. J. Biol. Chem. 276:3696-3701. [DOI] [PubMed] [Google Scholar]
- 32.Lang, S. E., F. E. Jenney, and F. Daldal. 1996. Rhodobacter capsulatus CycH: a bipartite gene product with pleiotropic effects on the biogenesis of structurally different c-type cytochromes. J. Bacteriol. 178:5279-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowry, O., and N. Rosebrough. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 34.Metheringham, R., L. Griffiths, H. Crooke, S. Forsythe, and J. Cole. 1995. An essential role for DsbA in cytochrome c synthesis and formate-dependent nitrite reduction by Escherichia coli K-12. Arch. Microbiol. 164:301-307. [DOI] [PubMed] [Google Scholar]
- 35.Metheringham, R., K. L. Tyson, H. Crooke, D. Missiakas, S. Raina, and J. A. Cole. 1996. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol. Gen. Genet. 253:95-102. [DOI] [PubMed] [Google Scholar]
- 36.Missiakas, D., F. Schwager, and S. Raina. 1995. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 14:3415-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng, T. C., J. F. Kwik, and R. J. Maier. 1997. Cloning and expression of the gene for a protein disulfide oxidoreductase from Azotobacter vinelandii: complementation of an Escherichia coli dsbA mutant strain. Gene 188:109-113. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 39.Page, M. D., and S. J. Ferguson. 1997. Paracoccus denitrificans CcmG is a periplasmic protein-disulfide oxidoreductase required for c- and aa3-type cytochrome biogenesis; evidence for a reductase role in vivo. Mol. Microbiol. 24:977-990. [DOI] [PubMed] [Google Scholar]
- 40.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:293-303. [DOI] [PubMed] [Google Scholar]
- 41.Reid, E., J. Cole, and D. J. Eaves. 2001. The Escherichia coli CcmG protein fulfils a specific role in cytochrome c assembly. Biochem. J. 355:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritz, D., and J. Beckwith. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21-48. [DOI] [PubMed] [Google Scholar]
- 43.Sambongi, Y., and S. Ferguson. 1996. Mutants of Escherichia coli lacking disulphide oxidoreductase DsbA and DsbB cannot synthesise an exogenous monohaem c-type cytochrome except in the presence of disulphide compounds. FEBS Lett. 398:265-268. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range form 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 46.Schulz, H., E. C. Pellicioli, and L. Thöny-Meyer. 2000. New insights into the role of CcmC, CcmD and CcmE in the haem delivery pathway during cytochrome c maturation by a complete mutational analysis of the conserved tryptophan-rich motif of CcmC. Mol. Microbiol. 37:1379-1388. [DOI] [PubMed] [Google Scholar]
- 47.Scolnik, P. A., M. A. Walker, and B. L. Marrs. 1980. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J. Biol. Chem. 255:2427-2432. [PubMed] [Google Scholar]
- 48.Shaw, A. L., A. Hochkoeppler, P. Bonora, D. Zannoni, G. R. Hanson, and A. G. McEwan. 1999. Characterization of DorC from Rhodobacter capsulatus, a c-type cytochrome involved in electron transfer to dimethyl sulfoxide reductase. J. Biol. Chem. 274:9911-9914. [DOI] [PubMed] [Google Scholar]
- 49.Shaw, A. L., S. Leimkuhler, W. Klipp, G. R. Hanson, and A. G. McEwan. 1999. Mutational analysis of the dimethylsulfoxide respiratory (dor) operon of Rhodobacter capsulatus. Microbiology 145:1409-1420. [DOI] [PubMed] [Google Scholar]
- 50.Sistrom, W. R. 1960. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J. Gen. Microbiol. 22:778-785. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, P. E., D. Ryan, and W. Levin. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168-176. [DOI] [PubMed] [Google Scholar]
- 52.Thompson, J., D. Higgins, and T. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighing, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thöny-Meyer, L. 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61:337-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thöny-Meyer, L. 2002. Cytochrome c maturation: a complex pathway for a simple task? Biochem. Soc. Trans. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 55.Yen, H. C., N. T. Hu, and B. L. Marrs. 1979. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J. Mol. Biol. 131:157-168. [DOI] [PubMed] [Google Scholar]
- 56.Zannoni, D., and F. Daldal. 1993. The role of c-type cytochromes in catalyzing oxidative and photosynthetic electron transport in the dual functional plasma membrane of facultative phototrophs. Arch. Microbiol. 160:413-423. [DOI] [PubMed] [Google Scholar]