Abstract
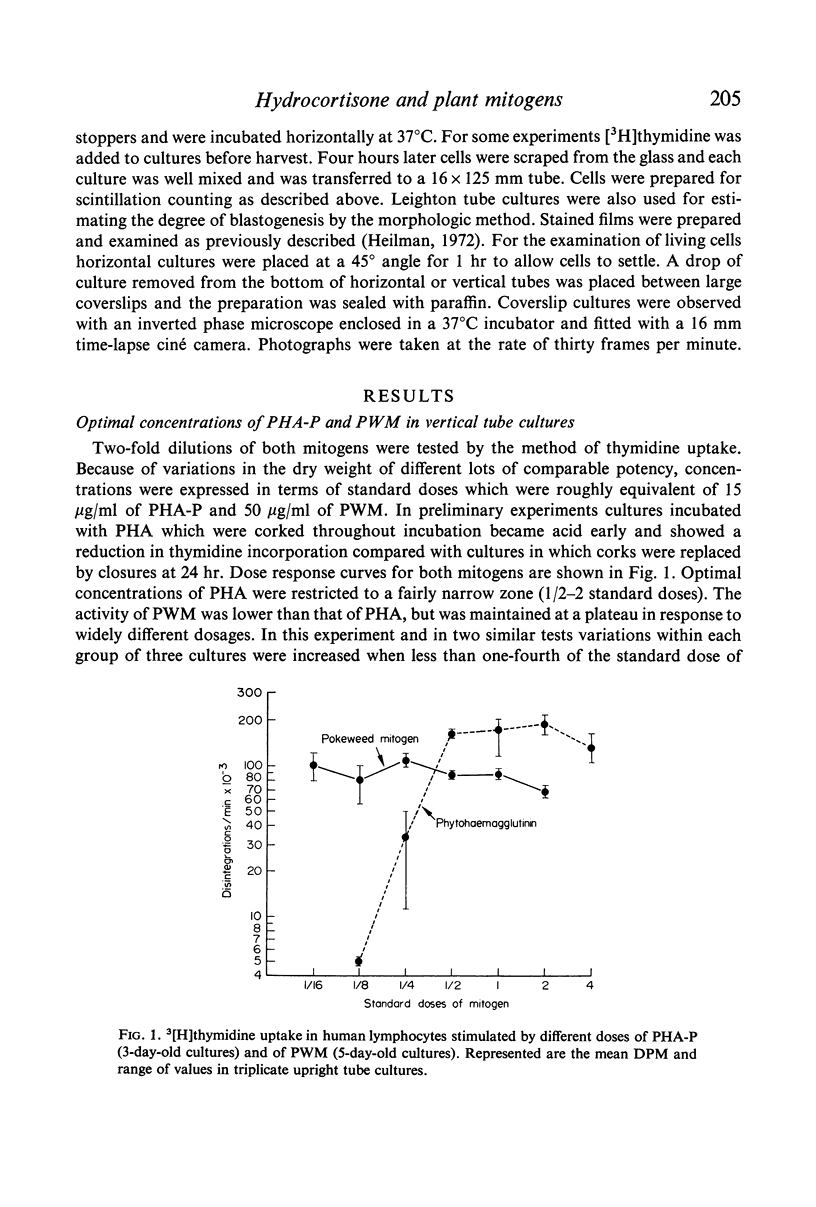
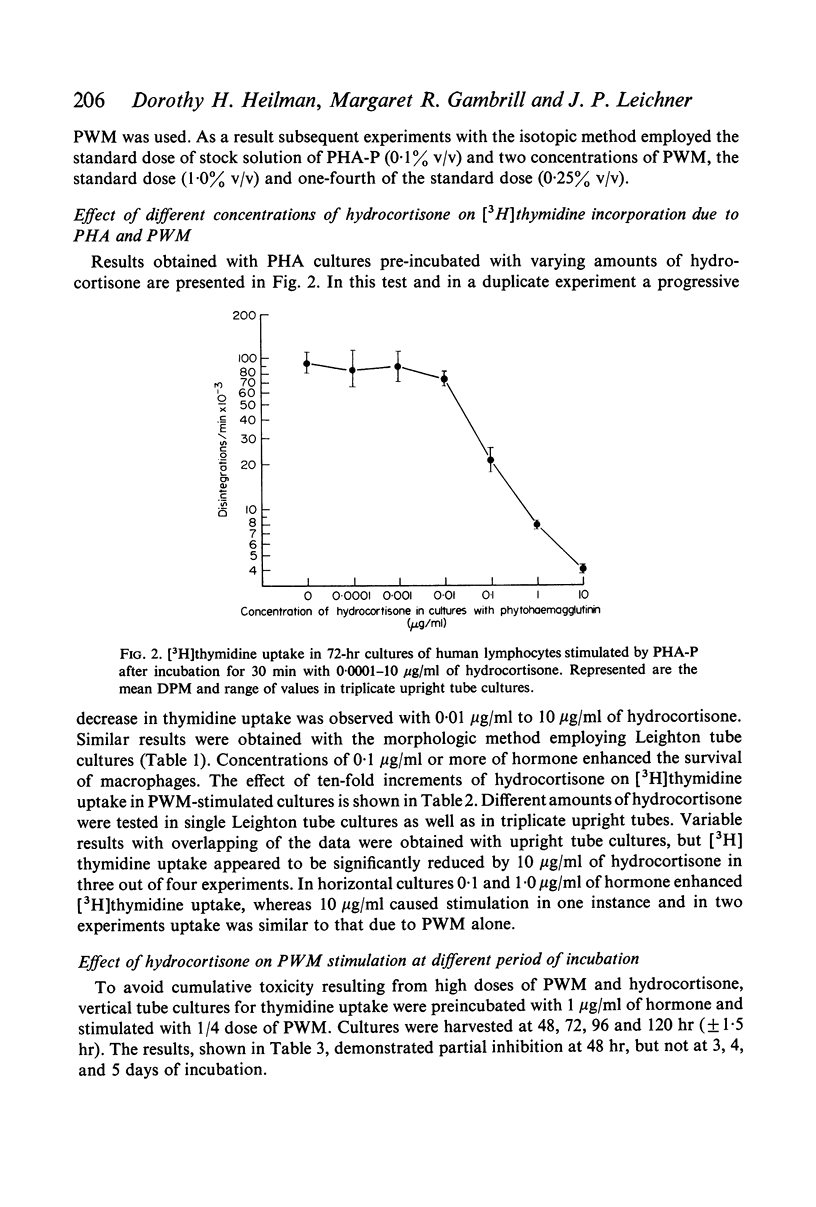
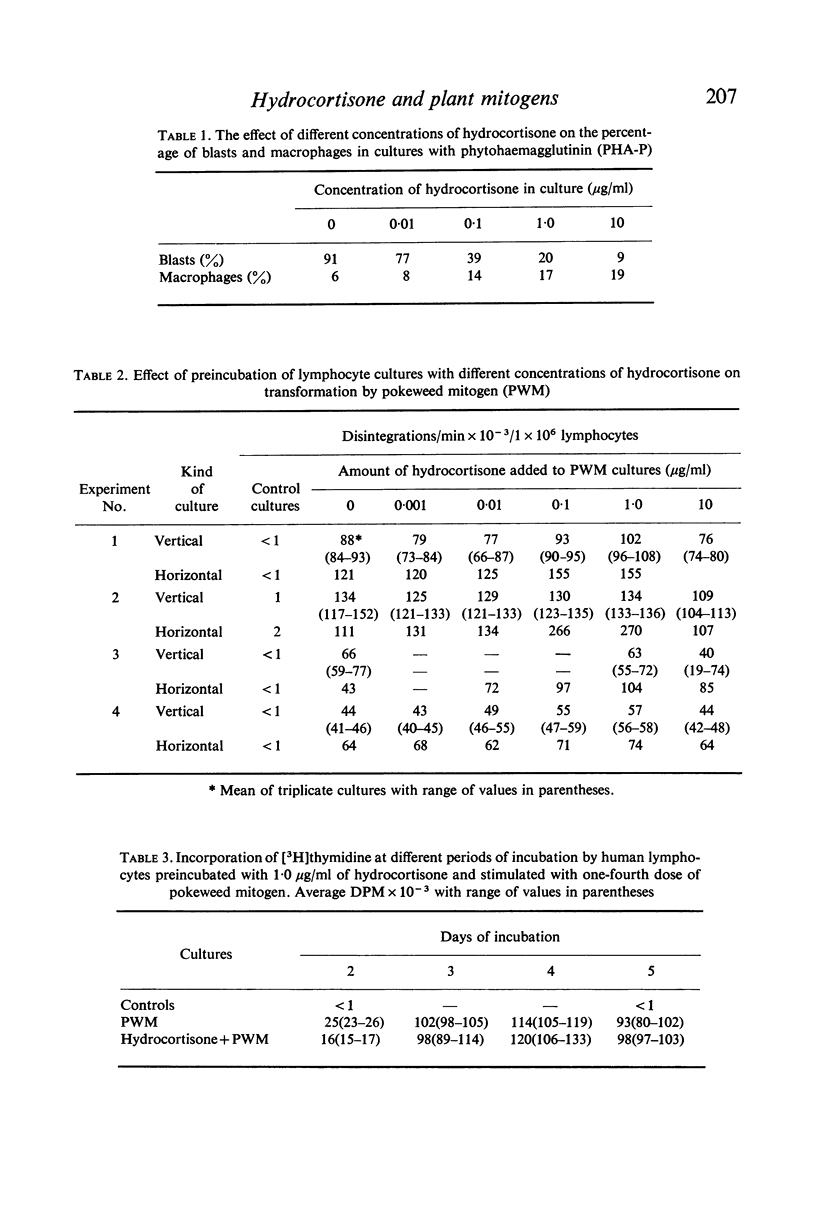
Methods based on [3H]thymidine incorporation and morphology were used for further studies on the effect of hydrocortisone on the transformation of human lymphocytes by phytohaemagglutinin (PHA) and pokeweed mitogen (PWM). Result obtained with both methods showed inhibition of PHA stimulation in cultures preincubated with 0·01–10 μg/ml of hydrocortisone. [3H]thymidine incorporation due to PWM in vertical tube cultures was depressed by 1·0 μg/ml of hydrocortisone in cultures harvested at 48 hr, but not in similar cultures incubated for 3, 4 and 5 days. In vertical cultures 10 μg/ml of hormone depressed the level of uptake in most experiments. By contrast [3H]thymidine incorporation was enhanced in horizontal PWM cultures by 0·1 and 1·0 μg/ml of hydrocortisone, and 10 μg/ml caused stimulation or a return to the normal PWM level.
Microscopic and time-lapse observations on living cells showed that typical PWM blasts first appeared on the third day of incubation and after 5 days were numerous in corticosteroid-treated as well as in untreated cultures. Ten micrograms per millilitre of hydrocortisone increased the fragility of PWM blasts in both kinds of cultures and caused degeneration of variable numbers of blasts in 5-day-old vertical tube cultures. The depression of [3H]thymidine uptake in vertical cultures was thought to be due to a combination of enhanced toxicity of corticosteroid hormone in deep cultures, and loss of incorporated [3H]thymidine due to increased cellular fragility. Results obtained with the isotopic and morphologic methods indicated that the transformation of B lymphocytes by PWM is relatively resistant to the action of hydrocortisone in vitro.
Full text
PDF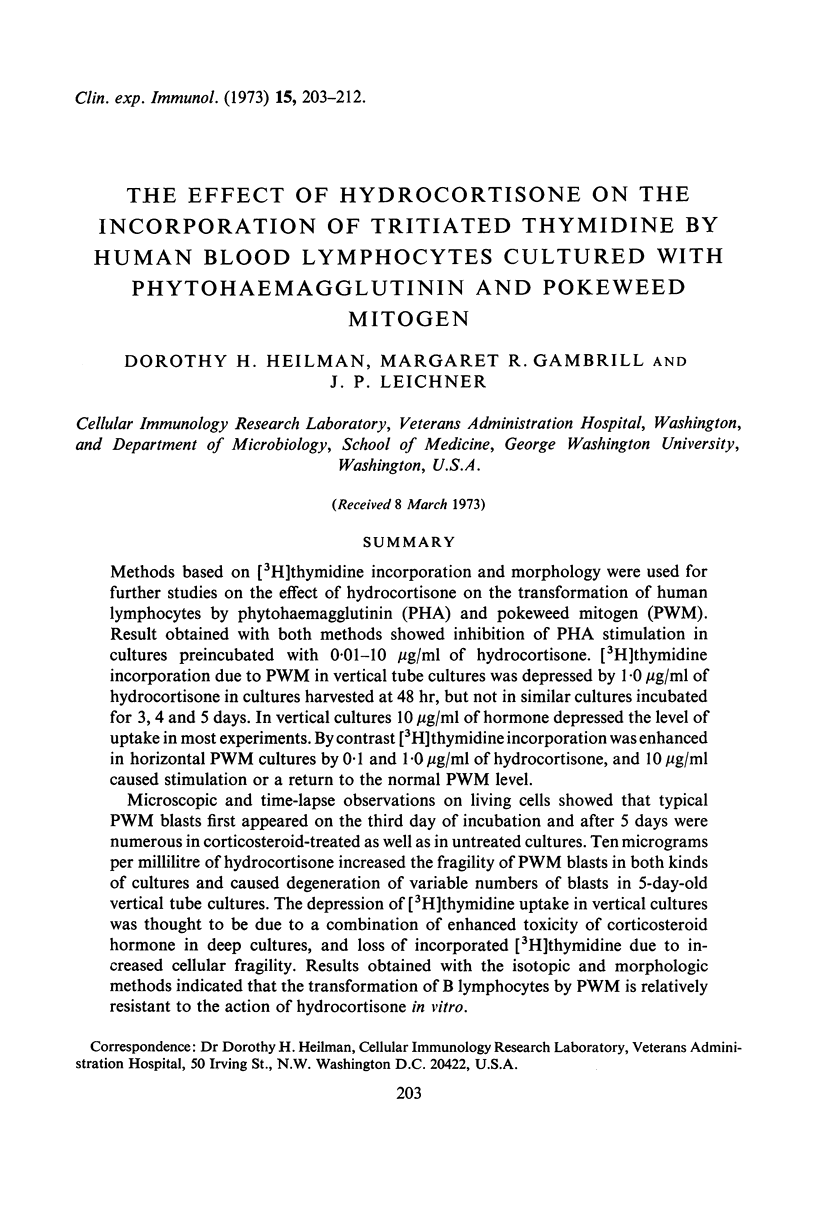





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker B. E., Farnes P. Histochemistry of blood cells treated with pokeweed mitogen. Nature. 1967 May 20;214(5090):787–789. doi: 10.1038/214787a0. [DOI] [PubMed] [Google Scholar]
- Chessin L. N., Börjeson J., Welsh P. D., Douglas S. D., Cooper H. L. Studies on human peripheral blood lymphocytes in vitro. II. Morphological and biochemical studies on the transformation of lymphocytes by pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):873–884. doi: 10.1084/jem.124.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. D., Hoffman P. F., Borjeson J., Chessin L. N. Studies on human peripheral blood lymphocytes in vitro. 3. Fine structural features of lymphocyte transformation by pokeweed mitogen. J Immunol. 1967 Jan;98(1):17–30. [PubMed] [Google Scholar]
- ELVES M. W., GOUGH J., ISRAUELS M. C. THE PLACE OF THE LYMPHOCYTE IN THE RETICULO-ENDOTHELIAL SYSTEM: A STUDY OF THE IN VITRO EFFECT OF PREDNISOLONE ON LYMPHOCYTES. Acta Haematol. 1964 Aug;32:100–107. doi: 10.1159/000209561. [DOI] [PubMed] [Google Scholar]
- Heilman D. H. Failure of hydrocortisone to inhibit blastogenesis by pokeweed mitogen in human leucocyte cultures. Clin Exp Immunol. 1972 Jul;11(3):393–403. [PMC free article] [PubMed] [Google Scholar]
- Heilman D. H., Thornton C., Baetz B. A method for quantitating blastogenesis by tuberculins in cultures of human blood lymphocytes. Am Rev Respir Dis. 1970 Apr;101(4):569–575. doi: 10.1164/arrd.1970.101.4.569. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Jones G. Lymphocyte activation. I. Expression of theta, H-2 and immunoglobulin determinants on lymphocytes stimulated by phytohaemagglutinin, pokeweed mitogen, concanavalin A or histocompatibility antigen. Clin Exp Immunol. 1972 Nov;12(3):391–402. [PMC free article] [PubMed] [Google Scholar]
- Jones G., Roitt I. M. Immunoglobulin determinants on lymphoid cells in culture. Cell Immunol. 1972 Mar;3(3):478–492. doi: 10.1016/0008-8749(72)90253-5. [DOI] [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R. Effects of compounds which inhibit lymphocyte stimulation on the utilization of glucose by leukocytes. J Allergy. 1970 Jul;46(1):21–28. doi: 10.1016/0021-8707(70)90057-2. [DOI] [PubMed] [Google Scholar]
- Moorhead J. F., McFarland W. Efficiency of scintillation counting of tritium labelled DNA-protein in the mixed leucocyte reaction in vitro. Nature. 1966 Sep 10;211(5054):1157–1159. doi: 10.1038/2111157a0. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Inhibition of human leukocyte mitosis by prednisolone in vitro. Cancer Res. 1961 Dec;21:1518–1521. [PubMed] [Google Scholar]
- Naspitz C. K., Richter M. The response of human peripheral lymphocytes to various mitogenic agents in vitro. Int Arch Allergy Appl Immunol. 1968;33(4):411–415. doi: 10.1159/000230055. [DOI] [PubMed] [Google Scholar]
- Rigas D. A., Tisdale V. V., Hecht F. Transformation of blood lymphocytes in ataxia telangiectasia. Dose and time response to phytohemagglutinin. Int Arch Allergy Appl Immunol. 1970;39(2-3):221–233. doi: 10.1159/000230350. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Fudenberg H. H., Kamin R. M. Effect of prednisolone on synthesis of DNA and RNA by human lymphocytes in vitro. Nature. 1967 Jan 21;213(5073):281–282. doi: 10.1038/213281a0. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Mueller G. C. An assay for the mitogenic activity of phytohemagglutinin preparations. Blood. 1965 Nov;26(5):569–578. [PubMed] [Google Scholar]
- Wu A. Y., Waksman B. H. Cellular differentiation in the thymus. IV. Response of rat thymic subpopulations to anti-thymus serum and other nonspecific mitogens. Cell Immunol. 1972 Mar;3(3):516–528. doi: 10.1016/0008-8749(72)90256-0. [DOI] [PubMed] [Google Scholar]



