Abstract
Highly purified blood lymphocytes from patients with plasma cell myeloma were tested in different in vitro systems. The patients were untreated or had received a standardized 4-day treatment with melphalan and prednisolone every sixth week. They were tested 5 weeks after the last treatment to minimize the acute toxic effects of the drugs. Lymphocytes from healthy controls were included in each experiment.
Stimulation of lymphocytes was measured by incorporation of 14C-thymidine into DNA after activation with phytohaemagglutinin (PHA), concancavalin A (Con A) and pokeweed mitogen (PWM). Their cytotoxicity was tested against Chang cells (human cell line) and chicken red blood cells in presence of PHA or heat-inactivated rabbit antibodies to target cell antigens. Lysis was quantitated as release of radio-activity from target cells labelled with 51Cr-chromate.
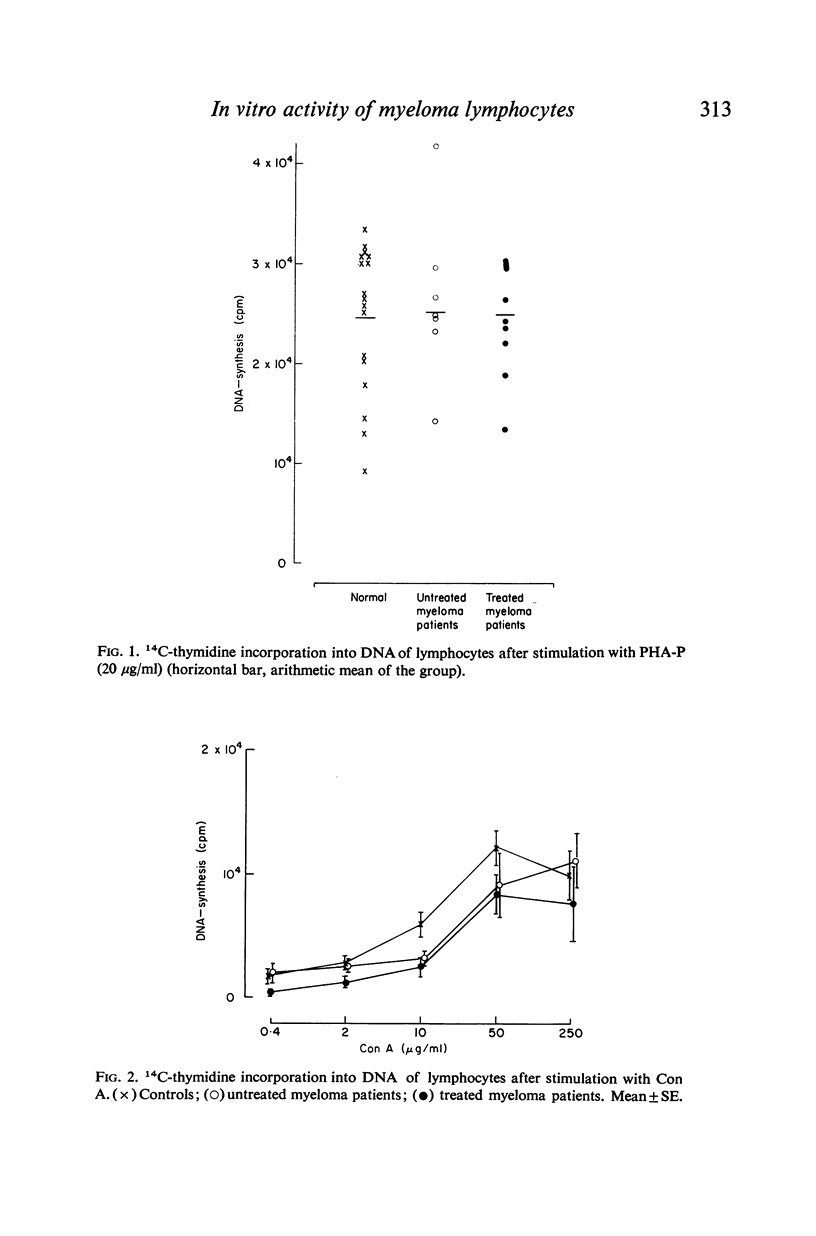
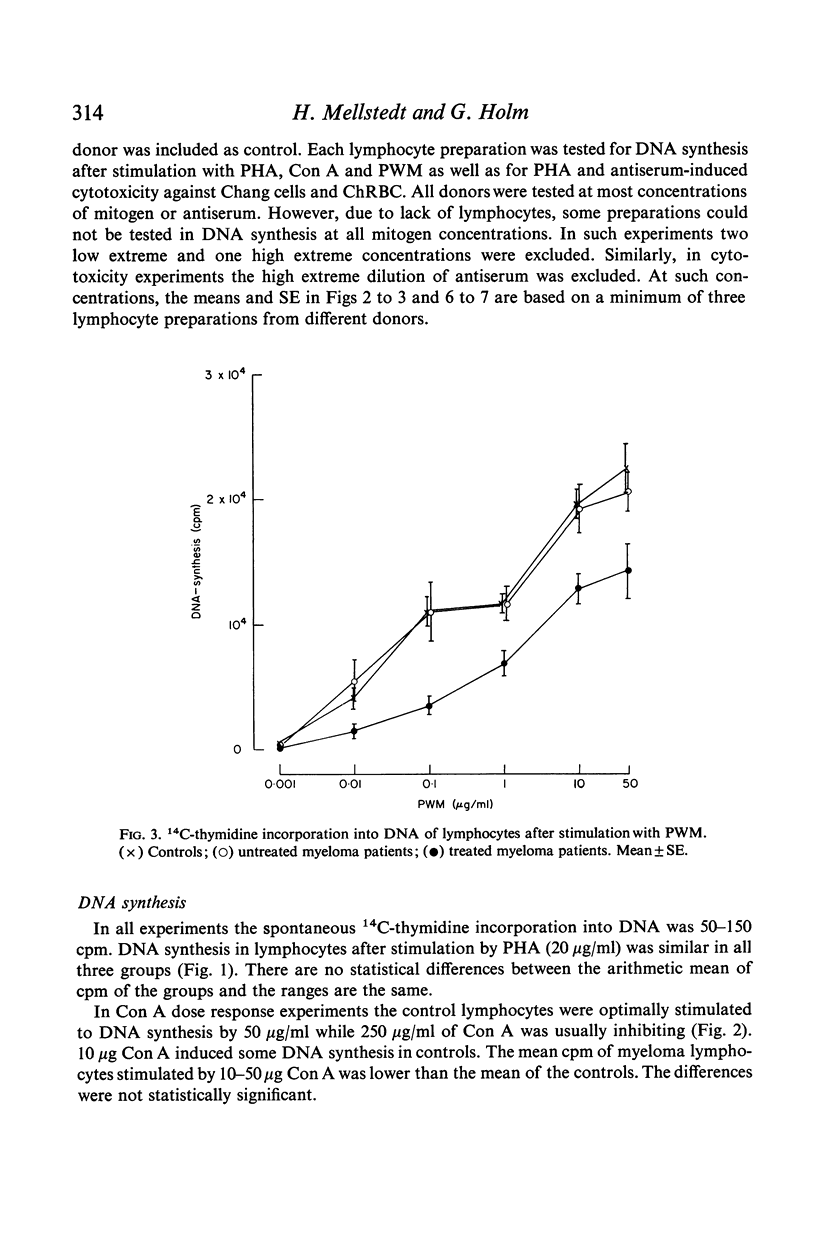
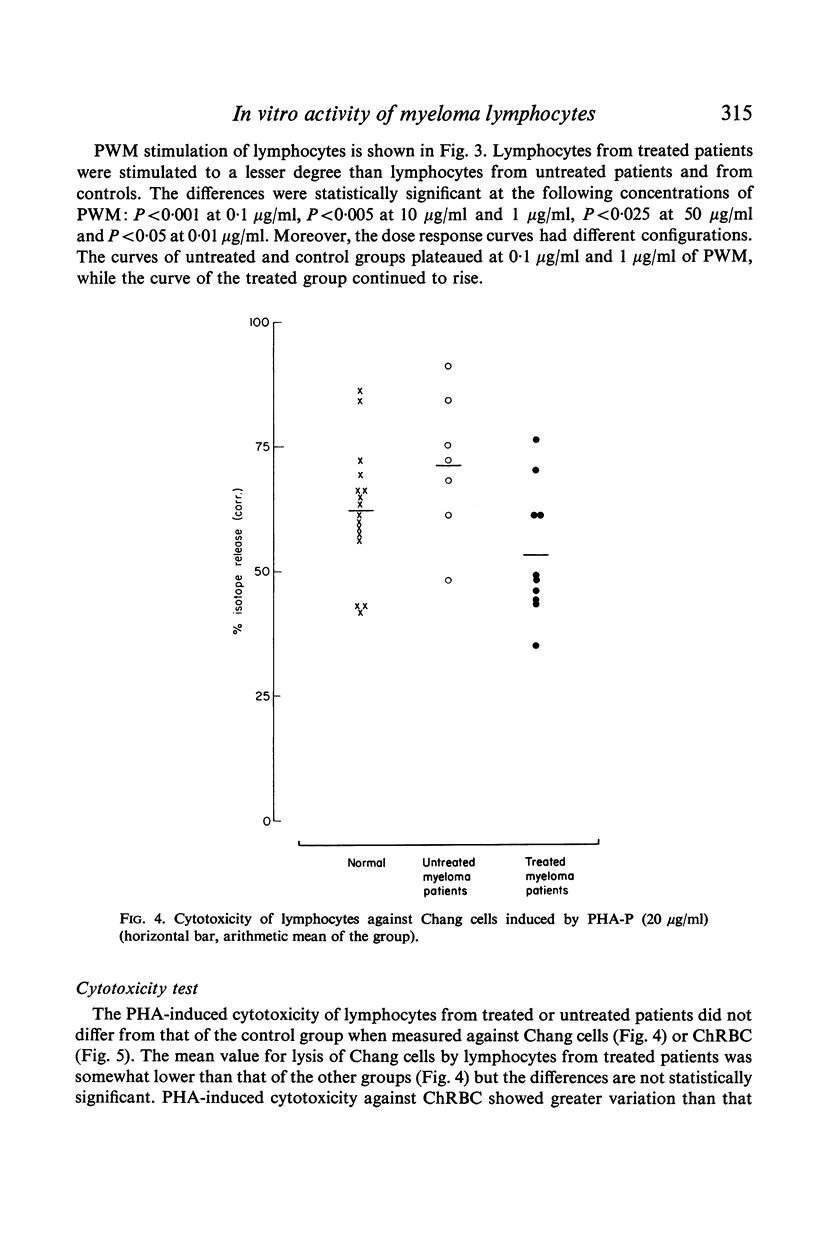
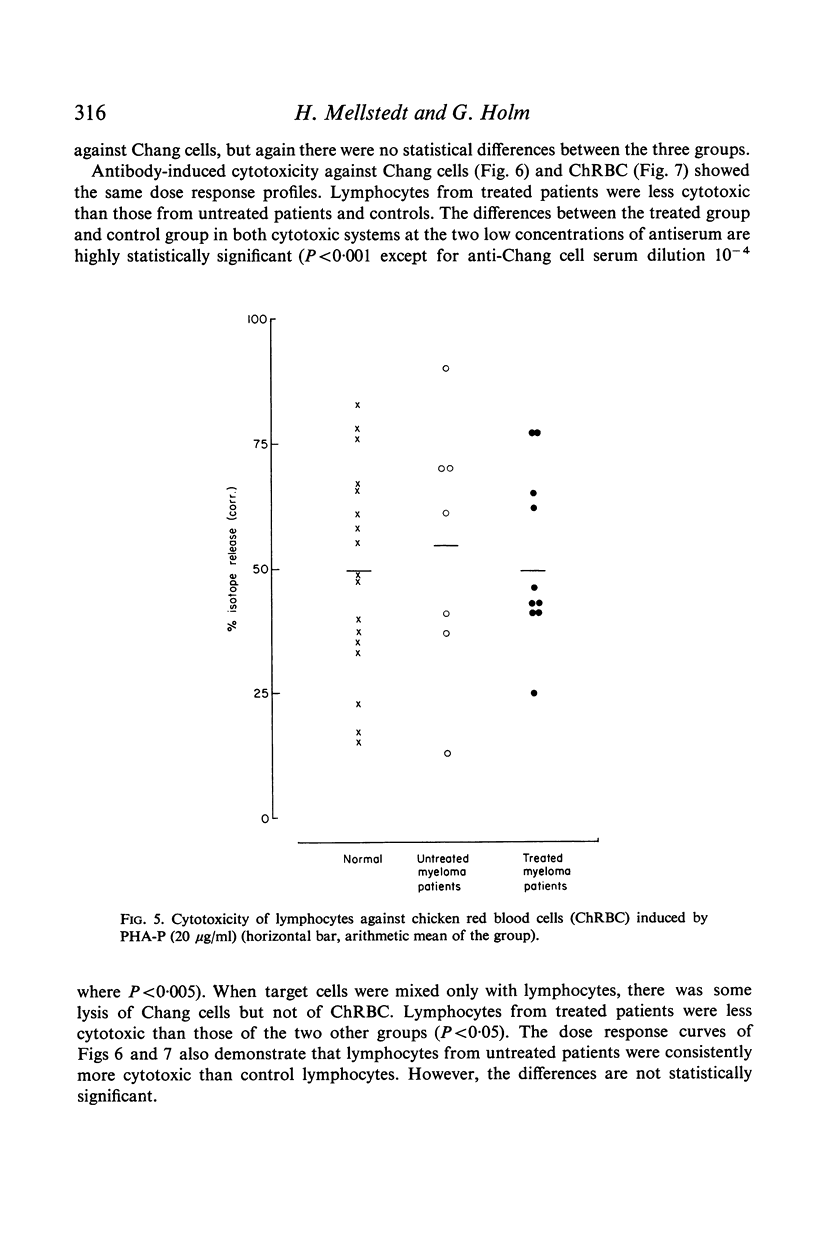
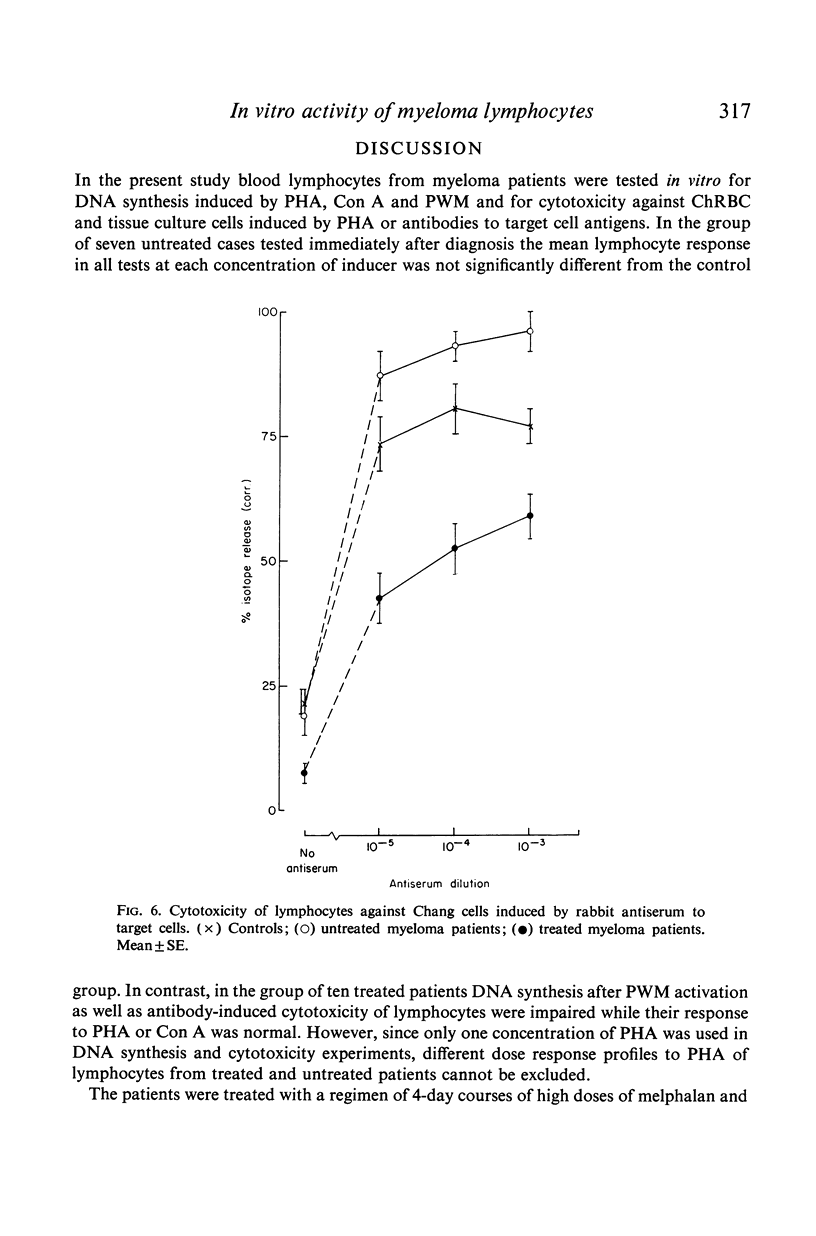
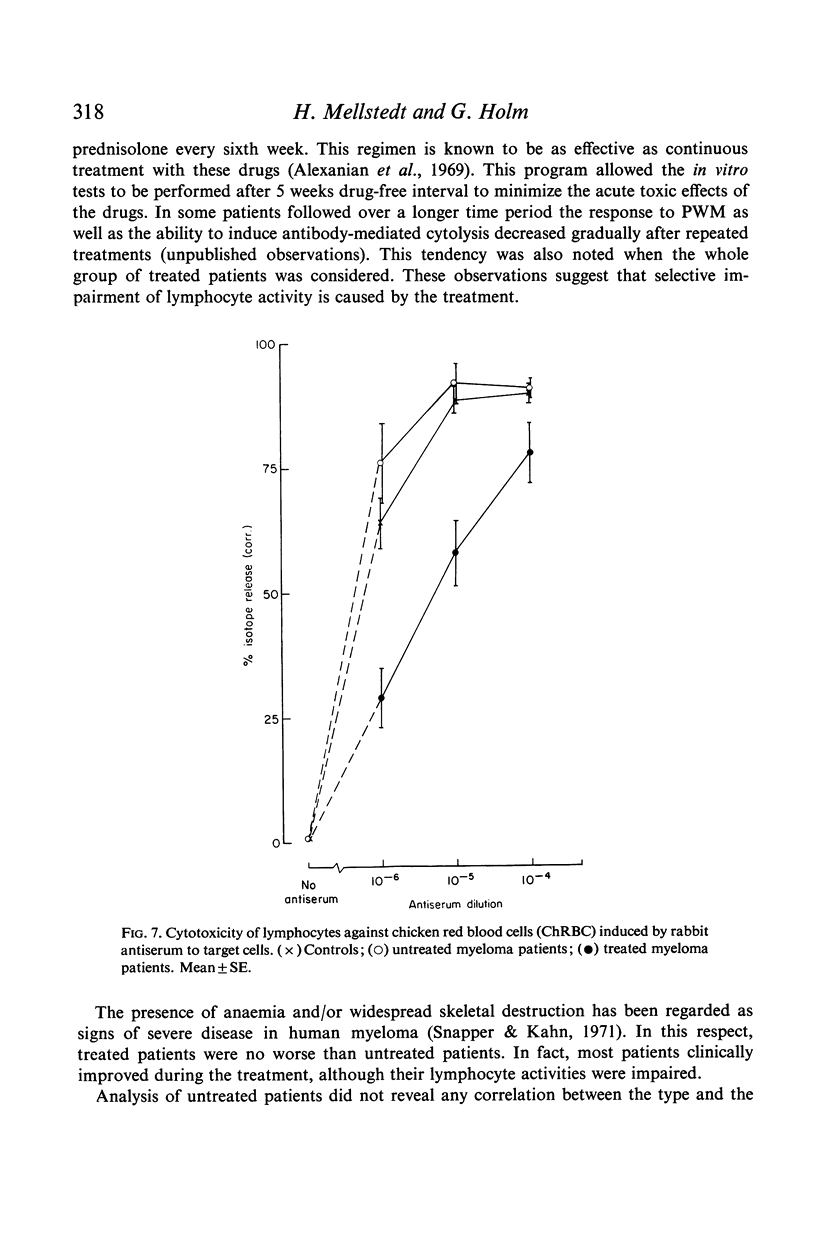
Antibody-induced cytotoxicity of lymphocytes from untreated patients was normal or slightly elevated while that of treated patients was severely depressed. Also, lymphocytes from treated patients were significantly less stimulated to DNA synthesis by PWM than were control lymphocytes. PHA-induced cytotoxicity and stimulation of lymphocytes by Con A or PHA were normal in all groups.
These results suggest that treatment of myeloma patients with melphalan and cortisone selectively impairs lymphocytes which respond to PWM by DNA synthesis and which participate in antibody-mediated cytolysis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexanian R., Haut A., Khan A. U., Lane M., McKelvey E. M., Migliore P. J., Stuckey W. J., Jr, Wilson H. E. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969 Jun 2;208(9):1680–1685. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- Alexanian R., Migliore P. J. Normal immunoglobulins in multiple myeloma: effect of melphalan chemotherapy. J Lab Clin Med. 1970 Feb;75(2):225–233. [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONE L., UHR J. W. IMMUNOLOGICAL DEFICIENCY DISORDERS ASSOCIATED WITH CHRONIC LYMPHOCYTIC LEUKEMIA AND MULTIPLE MYELOMA. J Clin Invest. 1964 Dec;43:2241–2248. doi: 10.1172/JCI105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R. Immunocytoma o' mice an' men. Br Med J. 1971 Apr 10;2(5753):67–72. doi: 10.1136/bmj.2.5753.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. R. Paraproteins, benign or malignant? Br Med J. 1967 Sep 16;3(5567):699–704. doi: 10.1136/bmj.3.5567.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Cytotoxicity of lymphocytes and its suppression. Antibiot Chemother. 1969;15:295–309. doi: 10.1159/000386787. [DOI] [PubMed] [Google Scholar]
- Holm G., Perlmann P., Johansson B. Impaired phytohaemagglutinin-induced cytotoxicity in vitro of lymphocytes from patients with Hodgkin's disease or chronic lymphatic leukaemia. Clin Exp Immunol. 1967 May;2(3):351–360. [PMC free article] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Quantitative studies on phytohaemagglutinin-induced cytotoxicity by human lymphocytes against homologous cells in tissue culture. Immunology. 1967 May;12(5):525–536. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Lundgren G., Zukoski C. F., Möller G. Differential effects of human granulocytes and lymphocytes on human fibroblasts in vitro. Clin Exp Immunol. 1968 Oct;3(8):817–836. [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Jondal M., Holm G. In vitro studies of lymphocytes from patients with plasma cell myeloma. II. Characterization by cell surface markers. Clin Exp Immunol. 1973 Nov;15(3):321–330. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Smith R. T. Possibilities and problems of immunologic intervention in cancer. N Engl J Med. 1972 Aug 31;287(9):439–450. doi: 10.1056/NEJM197208312870905. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Stobo J. D., Paul W. E., Green I. Antibody-dependent lymphoid cell-mediated cytotoxicity: no requirement for thymus-derived lymphocytes. Science. 1972 Jan 14;175(4018):194–196. doi: 10.1126/science.175.4018.194. [DOI] [PubMed] [Google Scholar]


