Abstract
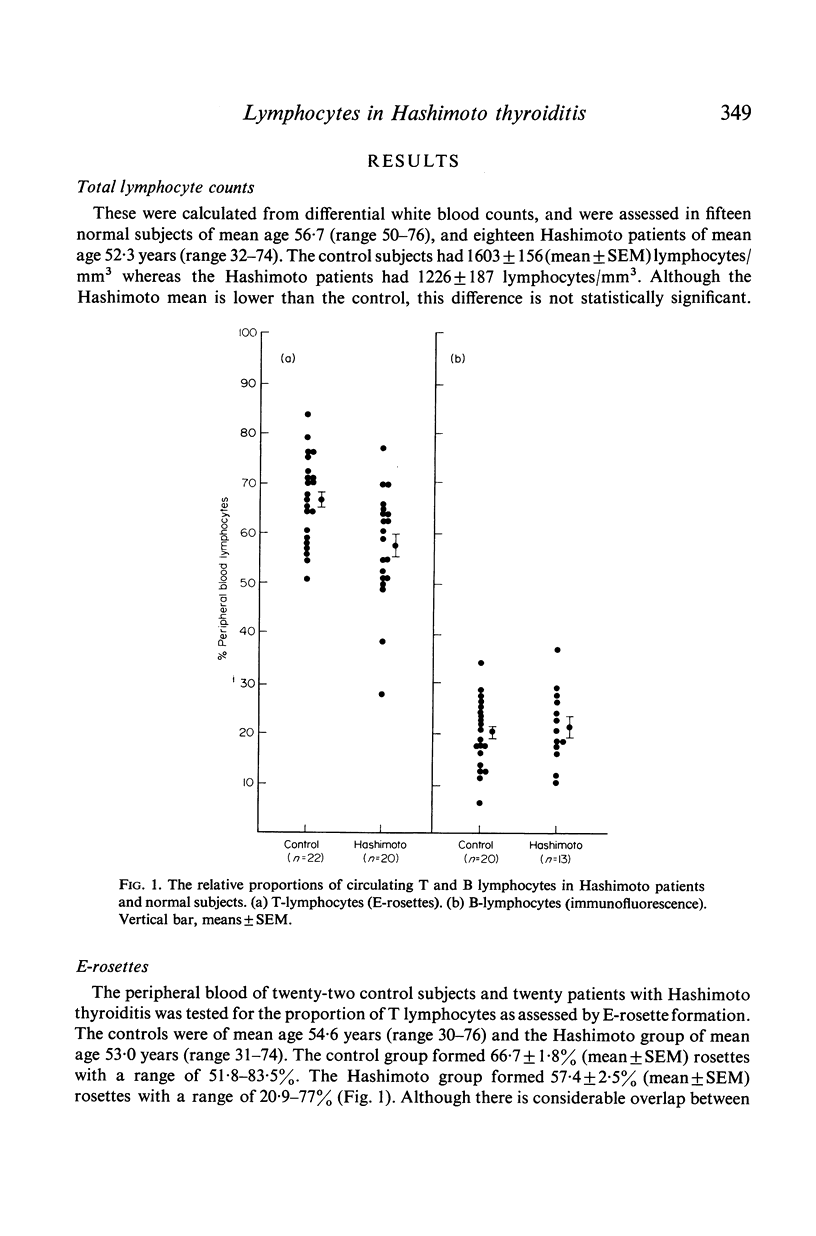
Peripheral blood and T and B lymphocytes and [125I]thyroglobulin-binding lymphocytes were investigated in twenty-two euthyroid Hashimoto thyroiditis patients and in twenty-two age- and sex-matched normal subjects. Although the total lymphocyte count in Hashimoto patients (mean±SEM = 1226±187/mm3) was lower than in normal subjects (1603±156/mm3) this difference was not statistically significant. There was, however, a statistically significant reduction in the proportion of circulating T lymphocytes in the Hashimoto patients (mean±SEM = 57·4±2·5%) as assessed by the sheep red-cell rosette method when compared with the normal controls (mean±SEM = 66·7±1·8%). The proportion of B lymphocytes in the peripheral blood as assessed by indirect immunofluorescence, was not significantly different being 21·6±2·1% in the Hashimoto patients and 20·2±1·1% in normal subjects.
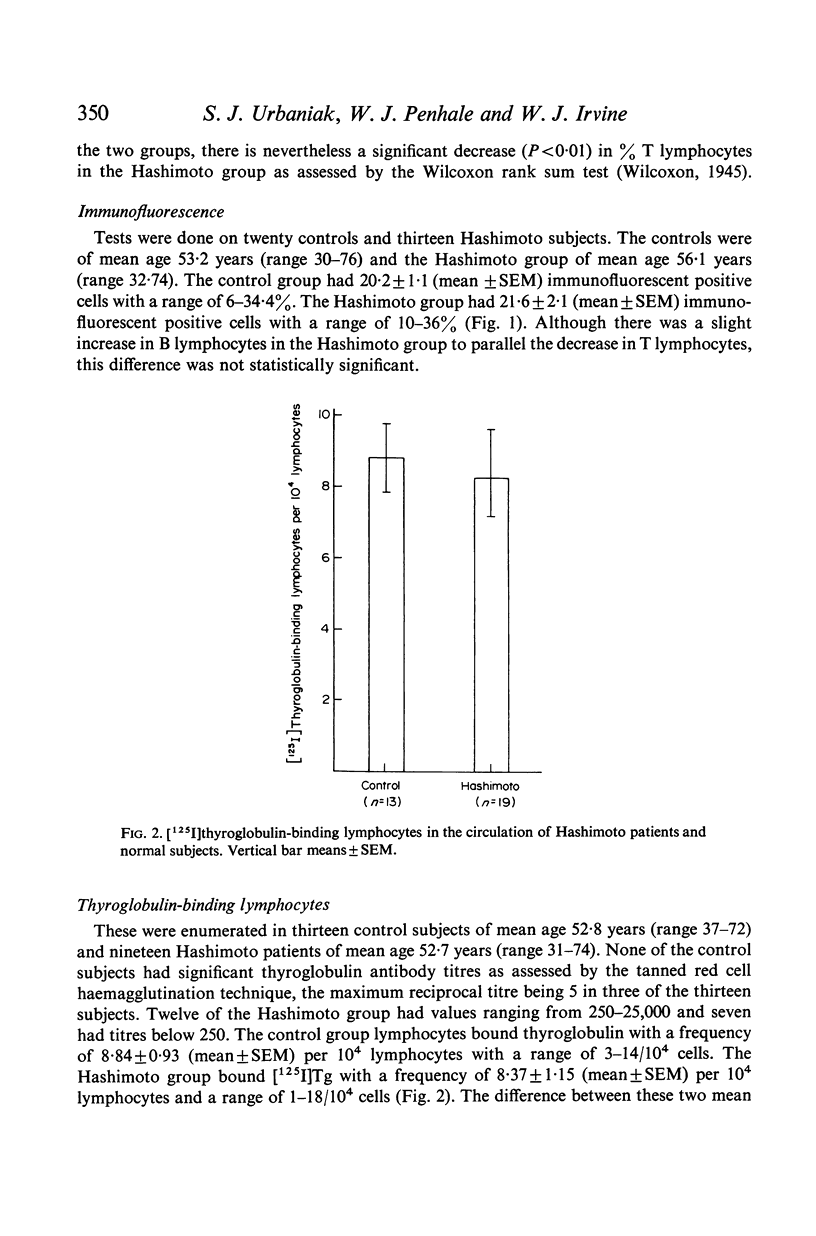
[125I]thyroglobulin-binding lymphocytes, as assessed by autoradiography were present in the circulation of nineteen Hashimoto patients with a mean frequency of 8·37±1·15/104 lymphocytes and in thirteen normal subjects with a mean of 8·84±0·93/104 lymphocytes. There was no difference in the degree of [125I]thyroglobulin binding between the two groups as determined by grain count analysis. There was no apparent correlation between age or thyroglobulin antibody titres and the frequency of [125I]thyroglobulin-binding lymphocytes. Thyroglobulin-binding lymphocytes were increased 100-fold in a Hashimoto thyroid biopsy in comparison to the patient's peripheral blood.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L. Antigen binding cells in tolerance and immunity. Transplant Rev. 1970;5:105–129. doi: 10.1111/j.1600-065x.1970.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Aoki N., Wakisaka G., Nagata I. Increase of T cells in Graves' disease. Lancet. 1973 Jul 7;2(7819):49–50. doi: 10.1016/s0140-6736(73)91993-4. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Bankhurst A. D., Torrigiani G., Allison A. C. Lymphocytes binding human thyroglobulin in healthy people and its relevance to tolerance for autoantigens. Lancet. 1973 Feb 3;1(7797):226–230. doi: 10.1016/s0140-6736(73)90066-4. [DOI] [PubMed] [Google Scholar]
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- Calder E. A., Penhale W. J., McLeman D., Barnes E. W., Irvine W. J. Lymphocyte-dependent antibody-mediated cytotoxicity in Hashimoto thyroiditis. Clin Exp Immunol. 1973 Jun;14(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Janeway C. A., Jr, Wilson A. B., Gell P. G., Kelus A. S. Immunoglobulin determinants on the lymphocytes of normal rabbits. I. Demonstration by the mixed antiglobulin reaction of determinants recognized by anti-gamma, anti-mu, anti-Fab and anti-allotype sera, anti-As4 and anti-As6. Immunology. 1970 Mar;18(3):417–429. [PMC free article] [PubMed] [Google Scholar]
- Farid N. R., Munro R. E., Row V. V., Volpé R. Peripheral thymus-dependent (T) lymphocytes in Graves's disease and Hashimoto's thyroiditis. N Engl J Med. 1973 Jun 21;288(25):1313–1317. doi: 10.1056/NEJM197306212882502. [DOI] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- Lamki L., Row V. V., Volpé R. Cell-mediated immunity in Graves' disease and in Hashimoto's thyroiditis as shown by the demonstration of migration inhibition factor (MIF). J Clin Endocrinol Metab. 1973 Feb;36(2):358–364. doi: 10.1210/jcem-36-2-358. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Holborow E. J., Keith H. I., Currey H. L. Subpopulations of human peripheral blood lymphocytes distinguished by combined rosette formation and membrane immunofluorescence. Lancet. 1972 Jul 8;2(7767):64–66. doi: 10.1016/s0140-6736(72)91553-x. [DOI] [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., McKenna R. P., Irvine W. J. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin Exp Immunol. 1973 Oct;15(2):225–236. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Stjernswärd J., Jondal M., Vánky F., Wigzell H., Sealy R. Lymphopenia and change in distribution of human B and T lymphocytes in peripheral blood induced by irradiation for mammary carcinoma. Lancet. 1972 Jun 24;1(7765):1352–1356. doi: 10.1016/s0140-6736(72)91091-4. [DOI] [PubMed] [Google Scholar]
- Teague P. O., Friou G. J. Antinuclear antibodies in mice. II. Transmission with spleen cells; inhibition or prevention with thymus or spleen cells. Immunology. 1969 Nov;17(5):665–675. [PMC free article] [PubMed] [Google Scholar]
- Thivolet J., Monier J. C., Ruel J. P., Richard M. H. Antinuclear autoantibodies in Swiss mice thymectomized at birth. Nature. 1967 Jun 10;214(5093):1134–1136. doi: 10.1038/2141134a0. [DOI] [PubMed] [Google Scholar]
- Welch P., Rose N. R., Kite J. H., Jr Neonatal thymectomy increases spontaneous autoimmune thyroiditis. J Immunol. 1973 Feb;110(2):575–577. [PubMed] [Google Scholar]


