Abstract
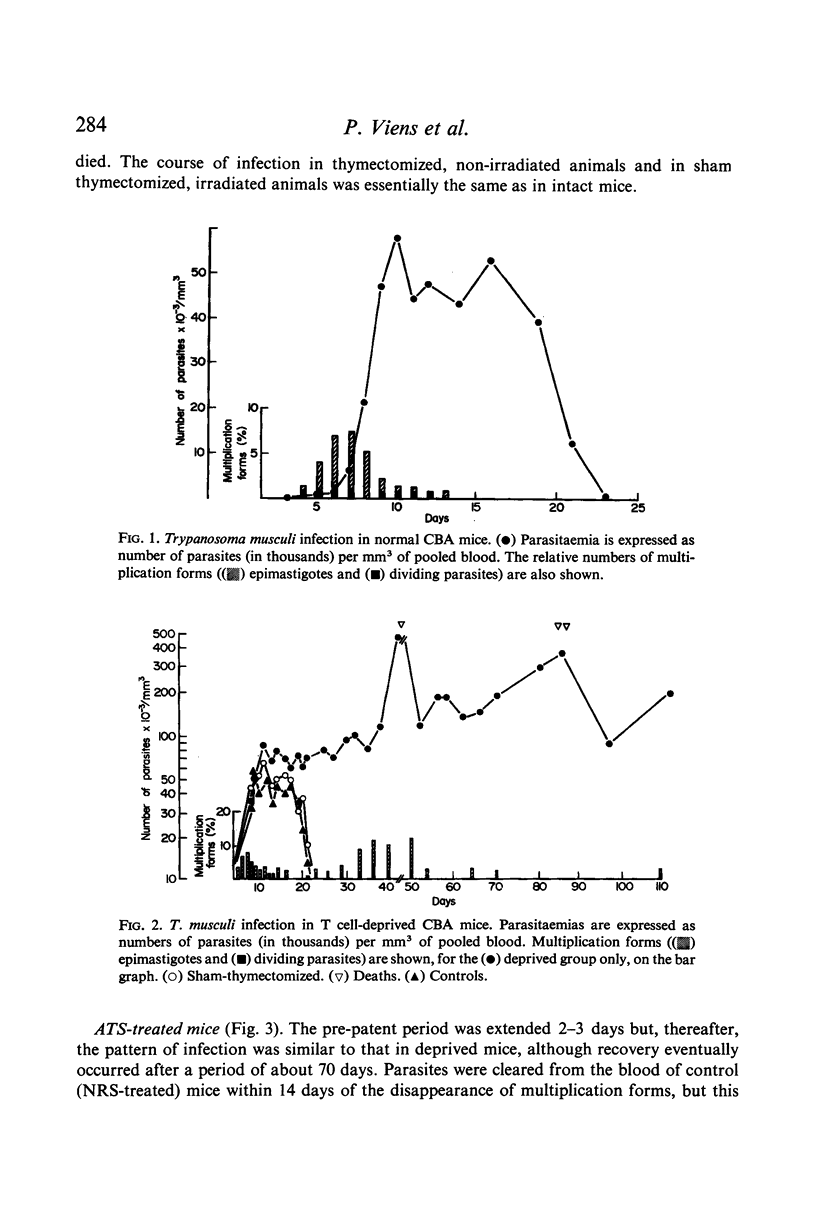
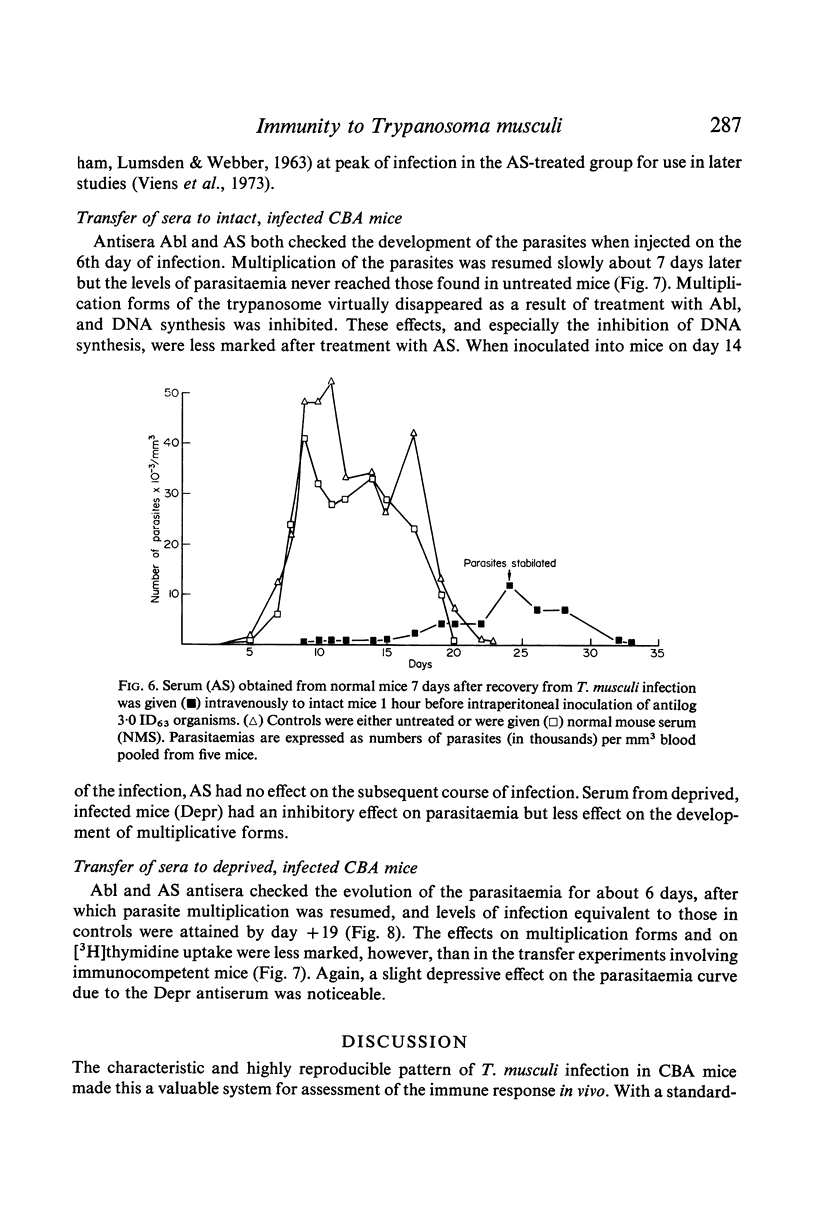
Trypanosoma musculi produced a self-limiting infection in CBA mice which was characterized by a phase of increasing parasitaemia, during which dividing forms of the parasite were present in the blood, and a more stable plateau phase, when only non-dividing `adult' parasites are seen. Blood parasitaemia then rapidly regressed and subsequently blood was non-infective on sub-inoculation. Infection of normal mice in this manner apparently conferred a strong and lasting immunity. Fluorescent antibody titres rose rapidly during infection and IgM, IgGl and IgG2 antibodies were synthesized simultaneously. Total immunoglobulin and IgG2 antibody titres fell following recovery from infection but relatively high and constant antibody levels were detectable for many months.
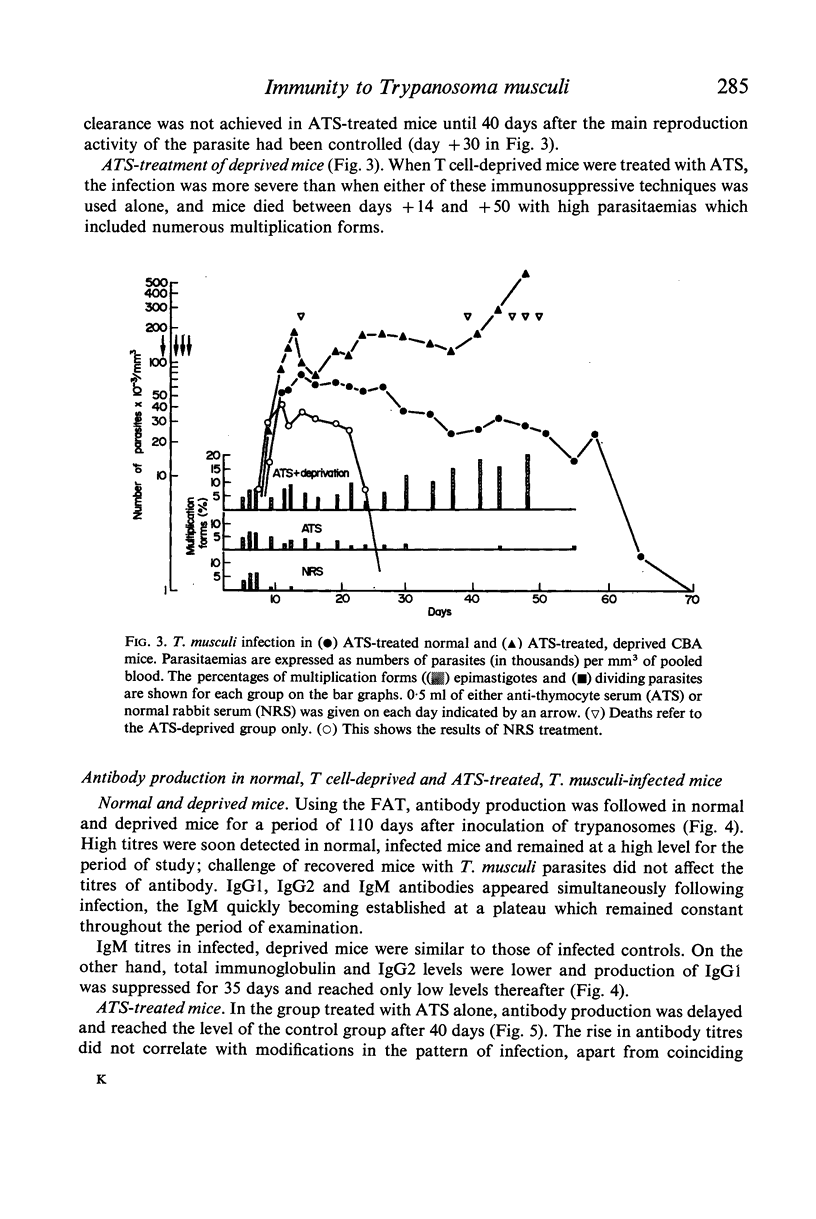
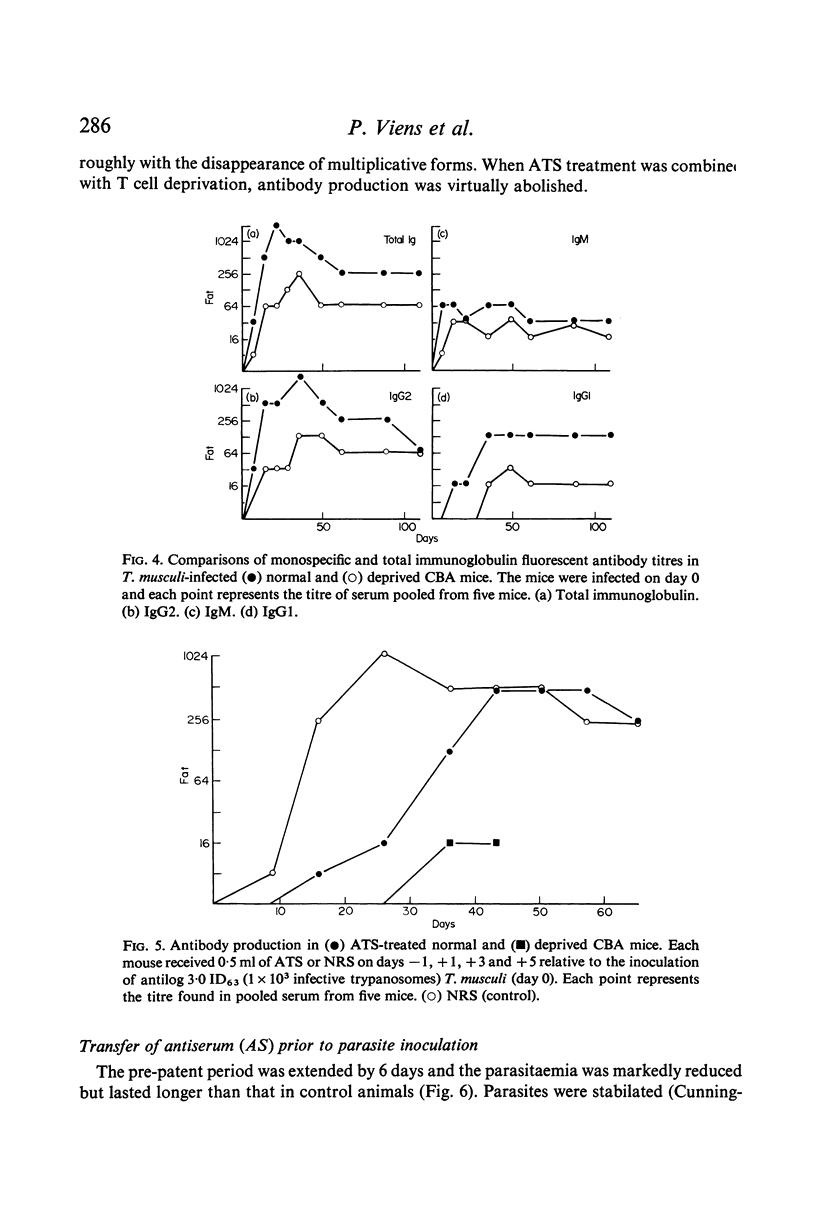
The parasitaemia in infected, T cell-deprived mice also rose rapidly and plateaued, but at a higher level than in normal mice, and deprived mice did not recover; multiplicative forms of the parasite persisted throughout the infection (up to 300+ days). Production of IgGl antibodies was markedly inhibited in the deprived mice but IgM antibody levels were normal. The effects of anti-thymocyte antisera (ATS) administration on the course of infection in normal mice were similar to those seen in thymectomized mice but the ATS-treated mice eventually recovered. Both antibody production and the elimination of parasites from the blood was delayed by ATS treatment.
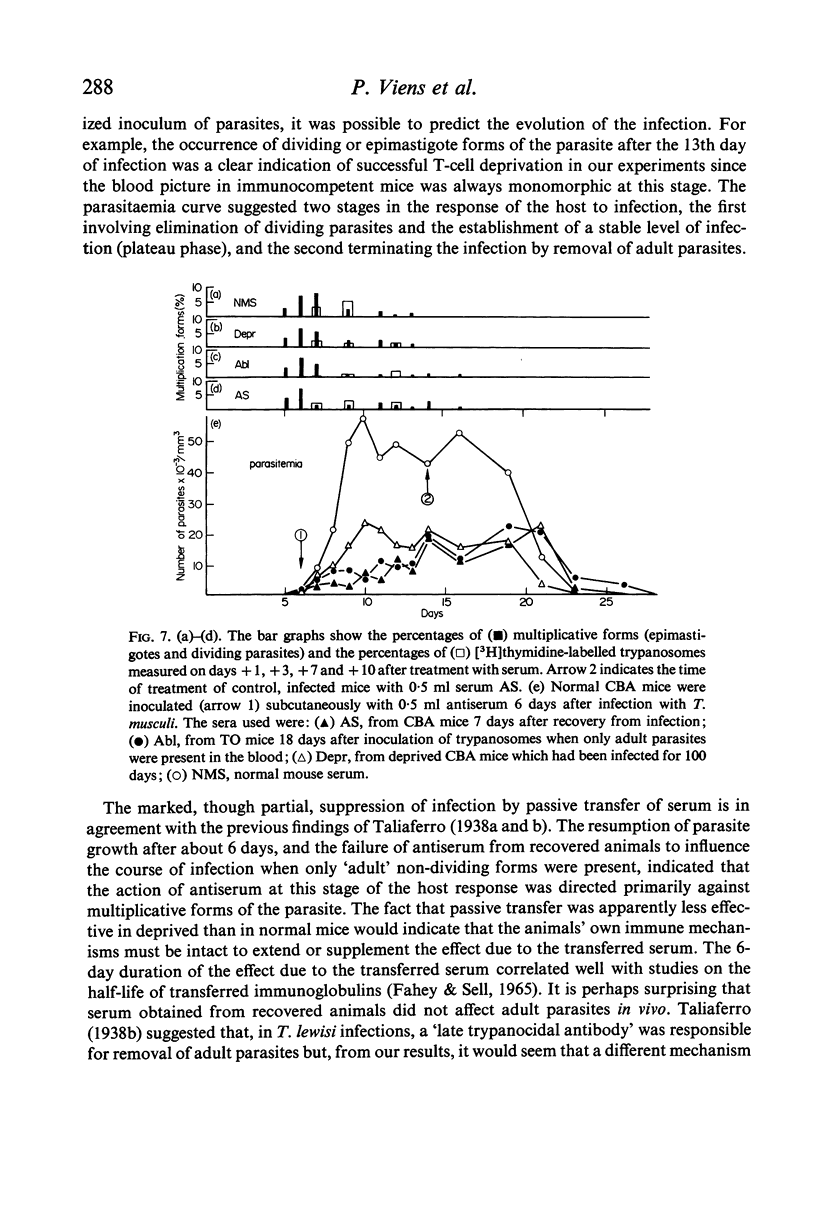
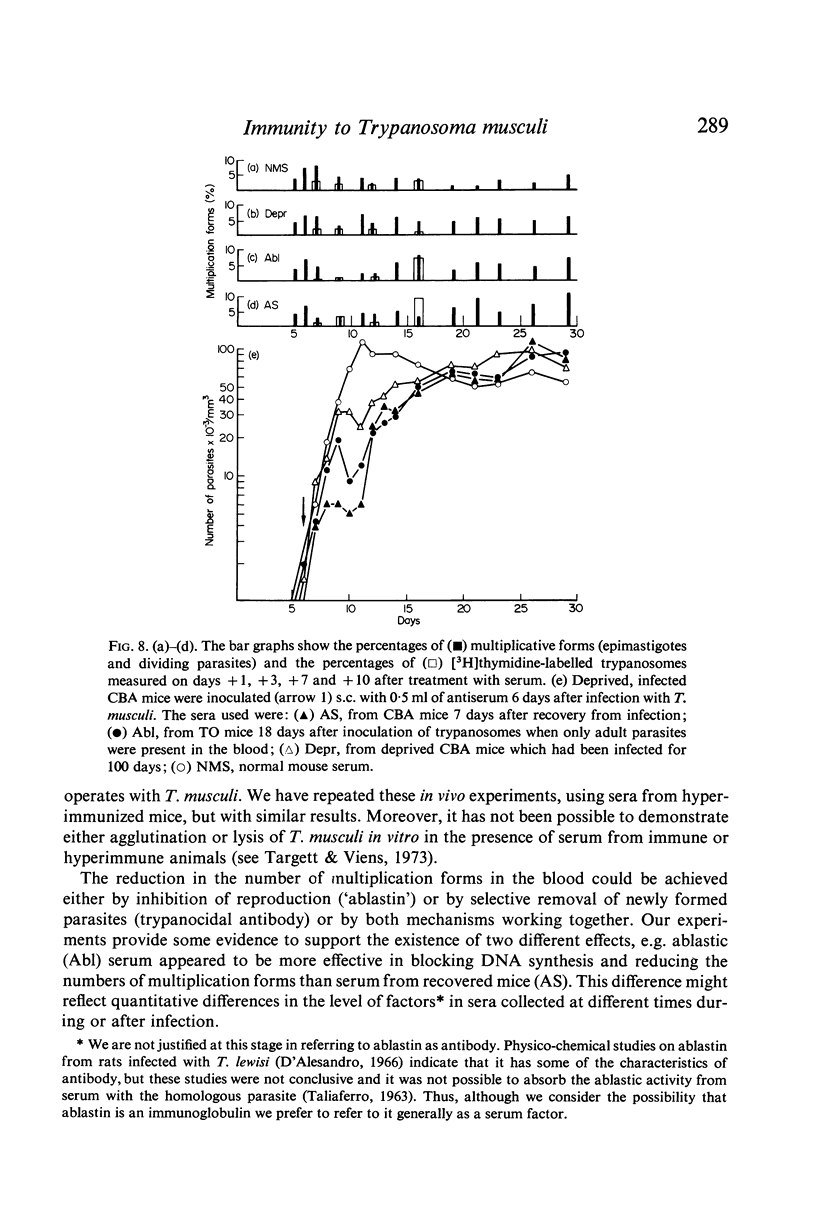
Passive transfer of antiserum just before infection prolonged the pre-patent period, and the subsequent parasitaemia was markedly reduced. If intact mice were treated on the 6th day of infection with serum collected during the plateau phase of an infection the rise in parasitaemia was checked, the number of multiplicative forms in the blood was reduced and DNA synthesis was inhibited. Antiserum from recovered mice had a similar though less marked effect on the parasitaemia. Transfer of these sera to infected, T cell-deprived mice produced similar though less dramatic effects.
These results suggest that the initial control of the infection, at the `first crisis', may be due to the joint action of a thymus-dependent ablastin, which inhibits parasite reproduction, and a thymus-independent `first' trypanocidal antibody which removes newly-formed parasites.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Lichtenstein E. P. Effects of various soil fungi and insecticides on the capacity of Mucor alternans to degrade DDT. Can J Microbiol. 1972 May;18(5):553–560. doi: 10.1139/m72-088. [DOI] [PubMed] [Google Scholar]
- BAILEY D. W., USAMA B. A rapid method of grafting skin on tails of mice. Transplant Bull. 1960 Apr;7:424–425. doi: 10.1097/00006534-196004000-00045. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM M. P., LUMSDEN W. H., WEBBER W. A. PRESERVATION OF VIABLE TRYPANOSOMES IN LYMPH TUBES AT LOW TEMPERATURE. Exp Parasitol. 1963 Dec;14:280–284. doi: 10.1016/0014-4894(63)90032-8. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Entner N. Further studies on antigenic changes in Trypanosoma lewisi. J Protozool. 1968 Nov;15(4):638–640. doi: 10.1111/j.1550-7408.1968.tb02185.x. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt C. L., Tyroler E. Trypanosoma lewisi: in vitro behavior of rat spleen cells. Exp Parasitol. 1971 Dec;30(3):363–374. doi: 10.1016/0014-4894(71)90100-7. [DOI] [PubMed] [Google Scholar]
- Hudson K. M. Further studies on the cotton rat, and the action of its serum on Trypanosoma vivax. Trans R Soc Trop Med Hyg. 1972;66(2):345–345. doi: 10.1016/0035-9203(72)90229-5. [DOI] [PubMed] [Google Scholar]
- Jooste S. V., Lance E. M., Levey R. H., Medawar P. B., Ruszkiewicz M., Sharman R., Taub R. N. Notes on the preparation and assay of anti-lymphocytic serum for use in mice. Immunology. 1968 Nov;15(5):697–705. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- Kloetzel J., Deane M. P. Adherence of sensitized trypanosomes to peritoneal cells. Rev Inst Med Trop Sao Paulo. 1970 Nov-Dec;12(6):383–387. [PubMed] [Google Scholar]
- Krampitz H. E. Verbreitung, Wirt-Parasit-Beziehungen und Vermehrung sizilianischer Stämme von Trypanosoma (H erpetosoma) duttoni Thiroux, 1905 (Protozoa, Trypanosomatidae) Z Parasitenkd. 1969;32(4):297–315. doi: 10.1007/BF00259643. [DOI] [PubMed] [Google Scholar]
- LUMSDEN W. H., CUNNINGHAM M. P., WEBBER W. A., VANHOEVE K., WALKER P. J. A METHOD FOR THE MEASUREMENT OF THE INFECTIVITY OF TRYPANOSOME SUSPENSIONS. Exp Parasitol. 1963 Dec;14:269–279. doi: 10.1016/0014-4894(63)90031-6. [DOI] [PubMed] [Google Scholar]
- Lumsden W. H. Principles of viable preservation of parasitic Protozoa. Int J Parasitol. 1972 Sep;2(3):327–332. doi: 10.1016/0020-7519(72)90070-7. [DOI] [PubMed] [Google Scholar]
- Medawar P. Review lecture. Immunosuppressive agents, with special reference to antilymphocytic serum. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):155–172. doi: 10.1098/rspb.1969.0086. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- ORMEROD W. E. A study of cytoplasmic inclusions in Trypanosoma lewisi and their relationship to the formation of antibody. J Gen Microbiol. 1959 Oct;21:287–294. doi: 10.1099/00221287-21-2-287. [DOI] [PubMed] [Google Scholar]
- Pike R. M. Antibody heterogeneity and serological reactions. Bacteriol Rev. 1967 Jun;31(2):157–174. doi: 10.1128/br.31.2.157-174.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheagren J. N., Monaco A. P. Protective Effect of Antilymphocyte Serum on Mice Infected with Plasmodium berghei. Science. 1969 Jun 20;164(3886):1423–1425. doi: 10.1126/science.164.3886.1423. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Ruble J. A. Virulent Trypanosoma lewisi infections in cortisone-treated rats. J Parasitol. 1967 Apr;53(2):258–262. [PubMed] [Google Scholar]
- Targett G. A., Wilson V. C. The blood incubation infectivity test as a means of distinguishing between Trypanosoma brucei brucei and T. brucei rhodesiense. Int J Parasitol. 1973 Jan;3(1):5–11. doi: 10.1016/0020-7519(73)90003-9. [DOI] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Torrigiani G. Quantitative estimation of antibody in the immunoglobulin classes of the mouse. II. Thymic dependence of the different classes. J Immunol. 1972 Jan;108(1):161–164. [PubMed] [Google Scholar]
- Viens P., Chevalier J. L., Sonea S., Yoeli M. The effect of reticulocytosis on Plasmodium vinckei infection in white mice. Action of phenylhydrazine and of repeated bleedings. Can J Microbiol. 1971 Feb;17(2):257–261. doi: 10.1139/m71-043. [DOI] [PubMed] [Google Scholar]
- Walker P. J. Reproduction and heredity in trypanosomes. A critical review dealing mainly with the African species in the mammalian host. Int Rev Cytol. 1964;17:51–98. doi: 10.1016/s0074-7696(08)60405-2. [DOI] [PubMed] [Google Scholar]


